Synthesis of 2-Azanorbornanes via Strain-Release Formal Cycloadditions Initiated by Energy Transfer
Abstract
Rigid bicycles are becoming more popular in the pharmaceutical industry because they allow for expansion to new and unique chemical spaces. This work describes a new strategy to construct 2-azanorbornanes, which can act as rigid piperidine/pyrrolidine scaffolds with well-defined exit vectors. To achieve the synthesis of 2-azanorbornanes, new strain-release reagent, azahousane, is introduced along with its photosensitized strain-release formal cycloaddition with alkenes. Furthermore, new reactivity between a housane and an imine is disclosed. Both strategies lead to various substituted 2-azanorbornanes with good selectivities.
The concept of escaping flatland has had a growing impact on medicinal chemistry in recent years.1 In concert with these efforts, the efficient synthesis of bicyclic scaffolds is of high interest.2, 3 For example, bicycle[1.1.1]pentanes, bicycle[2.1.1]hexanes, and cubanes have been shown to act as bioisosteres for substituted aryl groups. Thus, these structures have all emerged as useful building blocks to enable medicinal chemistry.4 Despite these advances, a variety of scaffolds with diverse substitution patterns are not available. Along these lines, we became interested in 2-azanorbornanes (Scheme 1A). These scaffolds are important for two reasons. First, the rigid bicyclic motifs have defined exit vectors. Second, they can act as rigidified piperidines/pyrrolidines.5 Piperidines are common to many pharmaceuticals and thus providing a rigidification strategy is of high value.6 Despite the value of 2-azanorbornanes, synthesis of this scaffold is limited (Scheme 1B). Perhaps the most common approach involves the [4+2]-cycloaddition of cyclopentadiene with either an imine or isocyanate.7 While effective, diverse substitution patterns are not easily achieved with this method. It should be noted that a common point of departure for 2-azanorbornanes synthesis, is the Vince lactam, which is derived from cyclopentadiene and chlorosulfonyl isocyanate.8 An alternative approach involves intramolecular substitution.9 While this method is effective, the starting material requires 8–10 steps to make, and synthesis of other substitution patterns is challenging. Very recently, Stephenson and co-workers disclosed an intramolecular radical cyclization initiated by photoredox catalysis to prepare 2-azanorbornanes with geminal substitution at C6.10 Herein, we describe a new approach toward the synthesis of these motifs by a [2π+2σ] formal cycloadditions by two different strategies (Scheme 1C).11 The first involves the formal cycloaddition of azahousanes with alkenes, whereas the second involves the formal cycloaddition of housanes with imines.12
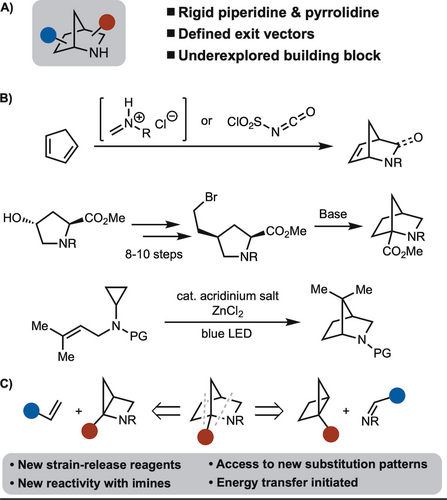
Synthetic approaches towards 2-azanorbornanes (A) 2-azanorboranes. (B) Established syntheses of 2-azanorbornanes. (C) This work: Intermolecular [2π+2σ] formal cycloaddition.
Inspired by our previous work on naphthyl ketone-enabled strain release [2π+2σ] formal cycloaddition of bicyclo[1.1.0]butanes and alkenes,2n we were curious if an azahousane could undergo a related process. In these reactions, the naphthyl group acts as an energy transfer antenna to drive the homolysis of the strained C−C bond to ultimately lead to a stepwise cycloaddition. The primary challenges/questions to address are, 1) as azahousane reagents are not known, a scalable synthesis needs to be established. 2) Will the strained C−C bond undergo homolysis prior to another photochemical reaction (e.g., dearomative [4+2]-cycloaddition),13 and how can this be tuned? 3) For reactions with housane, can a suitable imine be identified?
The synthesis of the azahousane started from the commercially available Boc-Hyp-OMe (1) ($ 0.2/g). The hydroxyl group was first substituted for bromine via the Appel reaction. Subsequently, intramolecular nucleophilic substitution occurred upon the addition of LHMDS to generate the azahousane methyl ester 3.14 Finally, the naphthyl ketone 4 was prepared via the Weinreb amide. This route provides access to multigram quantities of 4 in >99 : 1 er (Scheme 2). For the housane, starting from cyclopentene carboxylic acid (5), bromination and esterification allowed for the synthesis of ester 6. A similar sequence of intramolecular substitution and naphthyl ketone synthesis via the Weinreb amide allowed for the preparation of gram quantities of 8 (Scheme 2).
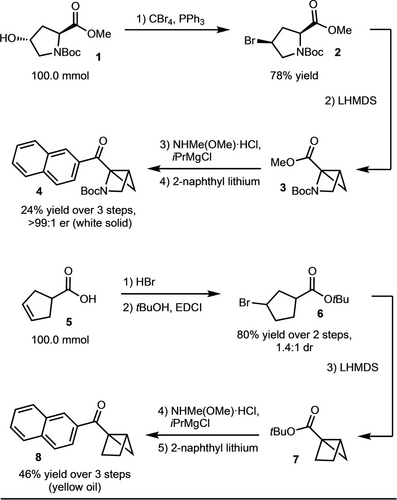
Gram scale synthesis of azahousane and housane.
With the strain-release reagents in hand, we first investigated the reactivity of azahousane 4 with alkenes (Table 1). Based on previous studies with bicyclobutanes, isopropylthioxanthone (ITX), 2,7-Dimethoxy-9H-thioxanthen-9-one (2,2′-OMe-TX) and Ir[(dFCF3ppy)2(dtbbpy)](PF6) provided similar outcomes in the strain-release formal cycloadditions; however, the reaction of styrene with these sensitizers did not lead to formation of 9 (Table 1, entries 1–3). However, use of electron-deficient alkenes such as ethyl acrylate with 2,2′-OMe-TX as the sensitizer and MeCN as the solvent, the desired product 10 was obtained with 65 % NMR yield and greater than 20 : 1 dr (Table 1, entry 4). Further optimization revealed that MeOH was a superior solvent and allowed for the synthesis of 10 in 79 % NMR yield (Table 1, entry 7). Reducing the amount of ethyl acrylate did not affect the yield (Table 1, entry 8). Moreover, control experiments indicate that the naphthalene group is essential to the reactivity as the phenyl group only provides 19 % yield (Table 1, entry 9). No product is observed in the presence of Lewis acid such as Sc(OTf)3 (Table 1, entry 10). Finally, the direct excitation under 365 nm also worked, but in reduced yield (Table 1, entry 11). The reaction likely proceeds via the mechanism illustrated at the bottom of Table 1. After Dexter energy transfer or direct excitation followed by intersystem crossing (ISC) (4 to 11),15 homolysis of the strained C−C bond occurs to generate diradical 12. Stepwise radical addition then follows to form the product 10 via 13. The high level of diastereoselectivity is likely due to the minimization of adverse steric interactions between the NBoc group and the CO2Et substituent.
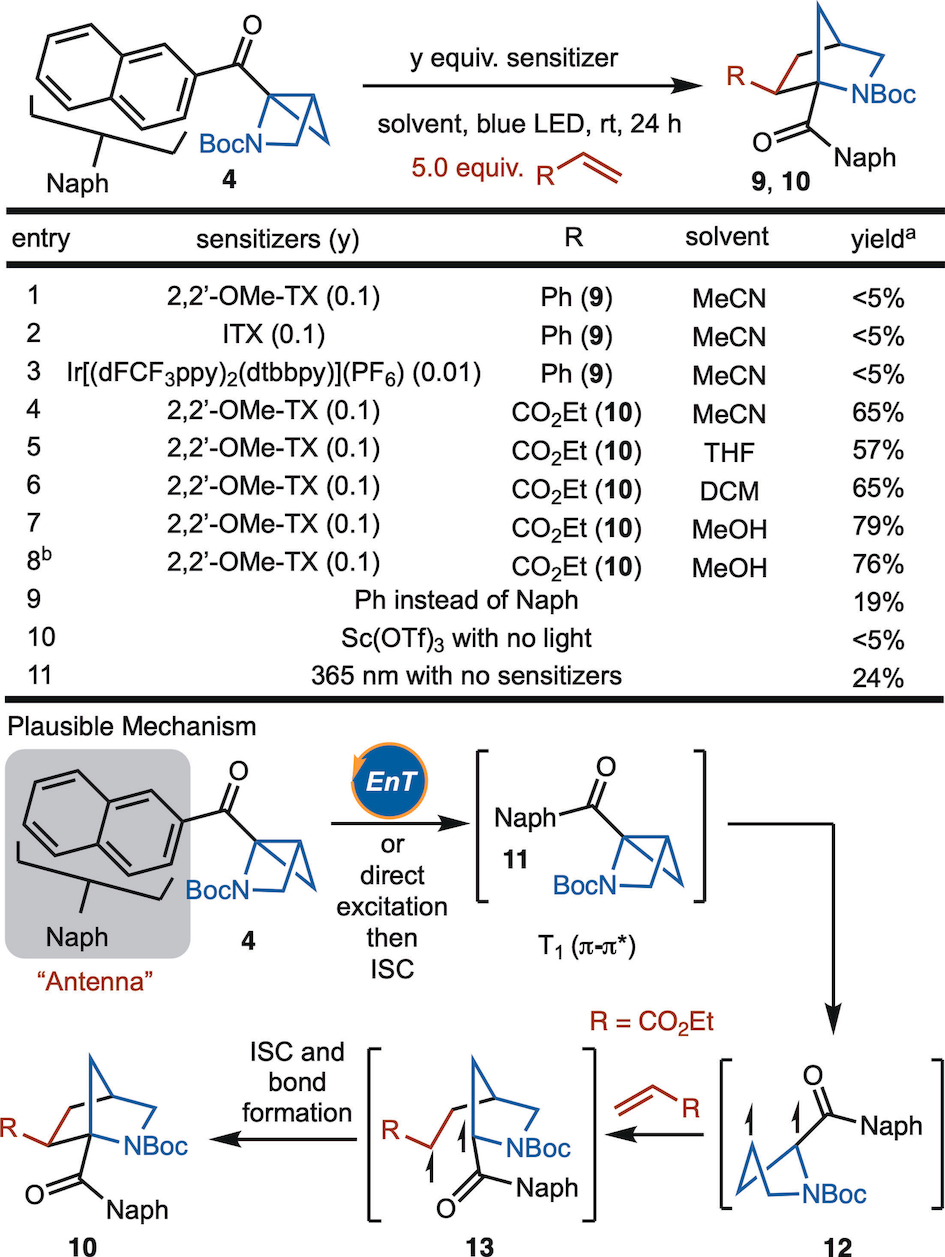
- [a] All reactions are performed on 0.1 mmol scale. Yield was determined by 1H NMR analysis of the unpurified reaction mixture using CH2Br2 as internal standard. [b] 3.0 equiv. of ethyl acrylate used.
Under the optimized reaction conditions, a variety of alkenes were investigated (Scheme 3). In addition to an ester group (product 10), acid (product 14), aldehyde (product 15) and amides (products 16 and 17) functionality were tolerated. Acrylonitrile (product 18) and vinyl acetate (product 19) both worked well. The use of vinylBpin under standard conditions resulted in formation of 20, which arises from an in situ protodeborylation. A slight change of conditions to use of diethyl ether as solvent suppressed the formation of 20 and allowed for the isolation of 21. Furthermore, vinyl sulfone led to the desired product 22 with excellent yield. In addition to monosubstituted alkenes, 1,1- and 1,2-disubstituted alkenes were also examined. With 1,2-disubstituted alkenes, the target product 23 is formed with 1 : 1 dr. The reaction of methylmethacrylate also worked in good yield and selectivity (product 24). Moreover, dihydro-pyrrol-2-one (product 26) reveals good reactivity but low diastereoselectivity. Though the reaction showed poor reactivity with aromatic systems, the use of 4-methyl ester substituted arene worked in 29 % yield (product 27). For this example, slight modification was necessary to the use of thioxanthone (TX) in ethyl acetate.16 Finally, while enantiomerically enriched azahousane was used, the products were generated as racemates due to the formation of achiral intermediate 12. To address this issue, an auxiliary-based approach was used. Reaction with phenylalanine-derived oxazolidinone 28 with azahousane 4 led to the formation of a mixture of diastereomers. Separation and subsequent hydrolysis allowed for 14 to be isolated in greater than 99 : 1 er and 36 % yield over 3 operations.
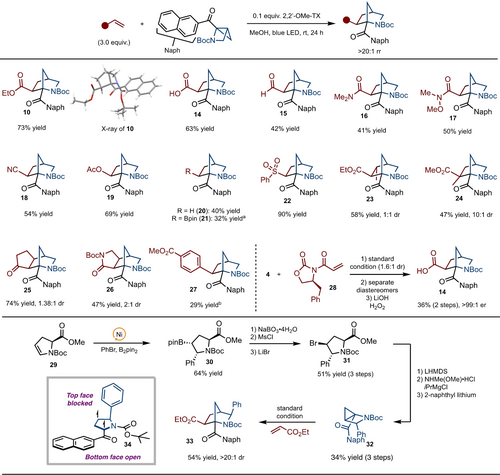
Substrate scope of azahousane with alkenes. Reaction run on 0.2 mmol scale. Yield represents the average of two separate experiments. [a] Reaction run in diethyl ether, see the Supporting Information for details. [b] Reaction run with TX and EtOAc. See the Supporting Information for additional details.
To prepare a substituted azahousane, we relied on a Ni-catalyzed arylboration method recently developed in our lab.17 Arylboration of ene-carbamate 29 occurred to deliver 30 in 64 % yield and >20 : 1 dr. Oxidation and conversion to bromide 31 set the stage for azahousane 32 synthesis in accordance with the standard route shown in Scheme 2. Finally, strain-release promoted formal cycloaddition occurred to form 33 in 54 % yield and >20 : 1 dr. The high levels of diastereoselectivity are likely due to the approach of the alkene from the bottom face as opposed to the top face as illustrated with intermediate 34.
At this stage, we turned our attention to the reactivity between housanes and imines. It was envisioned that the housanes could act as a radical donor and attack an electrophilic imine (Scheme 4). Based on this hypothesis, a variety of imines were investigated. While reactions of NBoc (36) and NTs imines (35) did not lead to product formation, the use of highly electrophilic glyoxyl-derived NOMe imine 39 led to product formation in 6 % yield and 1 : 1 dr with an Ir-based sensitizer (Scheme 4A).
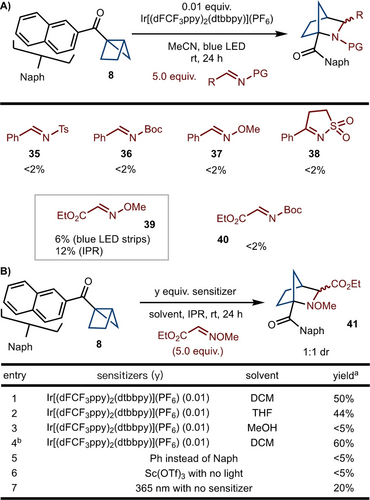
Housane formal cycloadditions. (A) Initial investigations. (B) Reaction optimization. [a] All reactions are performed on 0.1 mmol scale. Yield was determined by 1H NMR analysis of the unpurified reaction mixture using CH2Br2 as internal standard. [b] 10.0 equiv. of imine were used.
It was found that the yield was improved to 12 % when the reaction was conducted in an integrated photoreactor (IPR).18 Further optimization led to the finding that when the reaction was performed in CH2Cl2 the yield improved to 50 % (Scheme 4B, entry 1). Finally, use of 10 equivalents of imine 39 allowed for 41 to be generated in 60 % NMR yield (Scheme 4B, entry 4). In addition, the replacement of naphthalene to phenyl group shut down the reactivity (Scheme 4B, entry 5). Poor reactivity in the presence of Lewis acid states a different mechanism from Leitch's strategy (Scheme 4B, entry 6).2j Finally, direct irradiation with 365 nm LED allows for product formation, albeit in 20 % yield (Scheme 4B, entry 7).
With the optimized condition in hand, the scope of the reaction was then investigated (Scheme 5). The method is specific to reaction with α-imino glyoxylates, however variation to t-Bu esters (product 42) and amides (product 43 and 44) are tolerated (Scheme 5). The reaction of aryl oxmines did not allow for product formation (See the SI). Regardless, the strategy remains useful for the synthesis of polysubstituted 2-azanorbornanes. Finally, strain release reaction of bicyclo[1.1.0]butane 45 with α-imino glyoxylate provides access to aza-bicyclo[2.1.1]hexane 46 in 41 % yield.19
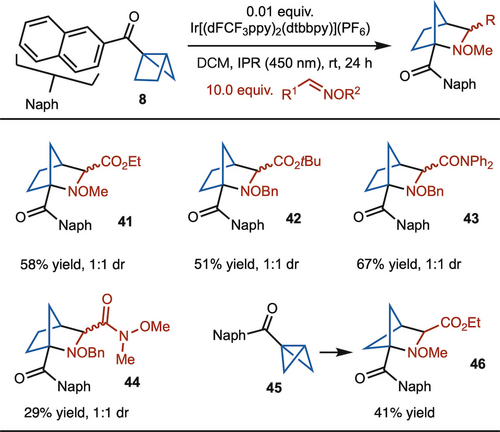
Substrate scope of housane with imines. Reaction run on 0.2 mmol scale. Yield represents the average of two separate experiments.
The reaction is also amenable to gram scale, as shown in Scheme 6A (products 14 and 41). With access to the carboxylic acid building block 14 in hand, introduction of a phenyl group was easily accomplished by nickel-catalyzed decarboxylative cross-coupling of a TCNHPI ester (product 48) (Scheme 6B).20 These products could be subject to regioselective Baeyer–Villiger oxidation to generate the naphthyl ester (product 49). In addition, starting from aldehyde 15, reduction, benzylation, and regioselective Baeyer–Villiger oxidation led to formation of 50. Under the Baeyer–Villiger oxidation conditions, the Boc is concomitantly deprotected. In addition, the amine product 41 could also be functionalized. The methoxy group of 41 b could be removed in presence of SmI2 and reprotected at a Boc group (product 51). Regioselective Baeyer–Villiger oxidation was also carried out to provide 52. Finally, hydrolysis of the ester of 41 a led to synthesis of 53 in 88 % yield and the structure confirmed by X-Ray.
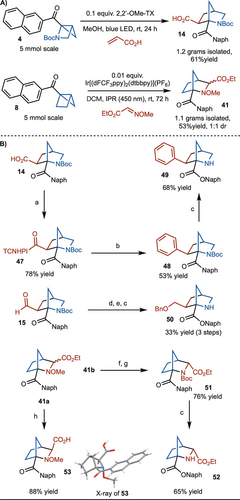
Gram scale synthesis and further functionalizations. Reaction conditions: a) i) TCNHPI, EDC, 0.01 equiv. DMAP, DCM, rt, 12 h. b) 0.2 equiv. Ni(DME)Cl2, 0.4 equiv. dtbbpy, ArZnCl⋅LiCl, THF/DMF, rt, 12 h. c) mCPBA, TFA, DCM, rt, 24 h. d) NaBH4, CH2Cl2/MeOH, −78 °C, 5 h. e) NaH, BnBr, DMF, 12 h. f) SmI2, THF, rt, 1 h. g) Boc2O, DCM, 12 h. h) LiOH⋅H2O, THF/MeOH/H2O, rt, 15 h.
In conclusion, a new strategy to construct 2-azanorbornanes is described. The reaction conditions are mild, simple, and, importantly, amenable to gram-scale synthesis. It is notable that the reaction is intermolecular and provides new substitution patterns that address key challenges in azanorbornane synthesis. We expect that this strategy will allow access to new chemical space.
Acknowledgments
We thank Indiana University and the NIH (R35GM131755). This project was partially funded by the Vice Provost for Research through the Research Equipment Fund and the NSF MRI program, CHE-1726633 and CHE-1920026. Support for the acquisition of the Bruker Venture D8 diffractometer through the Major Scientific Research Equipment Fund from the President of Indiana University and the Office of the Vice President for Research is gratefully acknowledged.
Conflict of interest
The authors declare no conflict of interest.
Open Research
Data Availability Statement
The data that support the findings of this study are available in the supplementary material of this article.




