Continuous-Flow Enantioselective Hydroacylations under Heterogeneous Chiral Rhodium Catalysts
Abstract
Transition metal-catalyzed enantioselective C−H bond functionalizations have become efficient methods for the synthesis of complex optically active molecules. Heterogeneous catalysts for this chemistry remain largely unexplored despite the advantages they offer in terms of ease of separation and reuse of catalysts. Herein, we report the development of heterogeneous chiral Rh catalysts for continuous-flow enantioselective hydroacylations. Heterogeneous catalysts could be prepared simply by mixing supports and Rh complexes. The prepared catalysts exhibited excellent activity and enantioselectivity affording optically active ketones in quantitative yields with 99 % ee's. Under the optimized reaction conditions, a turnover number >300 was achieved without the leaching of Rh species. The catalysts exhibited a wide substrate scope and in sequential-flow reactions with other heterogeneous catalysts, the syntheses of biologically active molecules and functional materials were demonstrated.
Introduction
In modern organic chemistry, transition metal-catalyzed enantioselective C−H bond functionalization has become a new, powerful, and environmentally benign synthetic strategy for optically active molecules providing novel transformations that are otherwise impossible.1-5 This method allows for the efficient construction of C−C and C-heteroatom bonds from simple and readily available molecules in enantioselective manners and has been applied in the synthesis of complex molecules such as natural products and active pharmaceutical ingredients (APIs).6-9 Over the past decades, the development of a new class of ligands and metal complexes has enabled various types of catalytic enantioselective transformations and, even today, this is being extensively studied.10 However, major challenges remain, particularly associated with increasing the efficiency of this chemistry. Because most of the reported catalysis require the use of precious and expensive transition metal complexes such as Pd,11-13 Ir,14-16 Rh,14, 15, 17, 18 and Ru19 with high catalyst loading, recovery and reuse of catalysts are in high demand for the practical use of this methodology.20, 21 Catalyst immobilization can overcome these problems, and in particular, continuous-flow reactions using heterogeneous catalysts offer great advantages over conventional batch reactions because catalysts can be separated and reused continuously along with the continuous production of target compounds in high efficiency.22
Significant progress in continuous-flow reactions with chiral heterogeneous catalysis has been made in recent years.23 However, transition metal-catalyzed continuous-flow C−H bond functionalization is significantly limited to achiral and homogeneous catalysis due to the lack of an efficient immobilization method.24, 25 To the best of our knowledge, only one example of heterogeneous enantioselective catalysis has been reported by Davies, Jones, and co-workers, where a SiO2-supported chiral RhII carboxylate dimer catalyst is utilized for the insertion of diazo compounds into C−H bonds.26, 27 Whereas the chiral catalysts immobilized on SiO2 via covalent bonds demonstrated high enantioselectivity, chemical modification of chiral ligands was necessary. However, this required additional synthetic costs and potentially caused negative effects in terms of catalyst performance. Therefore, a novel, efficient and versatile immobilization method of chiral transition metal catalysts without chemical modification of chiral ligands is essential for the further development of enantioselective C−H bond functionalization.
Herein, we describe continuous-flow enantioselective hydroacylations with heterogeneous chiral Rh catalysts to synthesize various types of optically active ketones in excellent yield and enantioselectivity as a model reaction of enantioselective C−H bond functionalizations.30-33 Moreover, sequential-flow reactions with several heterogeneous catalysts to derivatize obtained optically active ketones into several compounds including biologically active molecules were achieved without the isolation of intermediates (Figure 1).
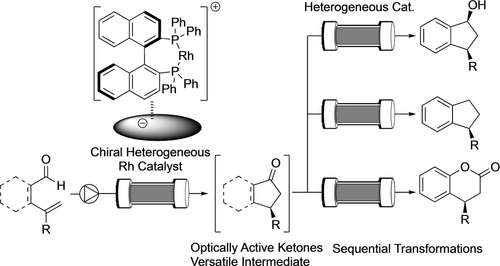
Continuous-flow enantioselective hydroacylations.
Results and Discussion
Heterogeneous catalysts were prepared in three steps, according to our previous reports (see Scheme S1).28, 29 First, the surface of SiO2 was functionalized with amines using silane coupling reagents to afford amine-functionalized SiO2 (SiO2−NR2). Four types of amines primary, secondary, tertiary, and dihydroimidazole were employed for the functionalization. Second, SiO2−NR2 was treated with heteropoly acids (HPAs) in EtOH to form the acid-base salts of the surface of SiO2 (HPA/SiO2−NR2). H3PW12O40 (PTA) and H4SiW12O40 (STA) were used as HPAs based on our previous investigations. Finally, the HPA/SiO2−NR2 was treated with a chiral Rh complex prepared from Rh(nbd)2BF4 and (R)-BINAP according to homogeneous catalysis34 in dichloromethane (DCM) solution for 30 min to afford the heterogeneous chiral Rh catalysts (Rh-BINAP/HPA/SiO2−NR2). The loading of Rh was determined by inductively coupled plasma (ICP) analysis: >99.5 % of the Rh complex was immobilized on the support.
The prepared heterogeneous catalysts were characterized by several analytical techniques including solid-state NMR, N2 adsorption/desorption isotherm, and scanning transmission electron microscopy (STEM) (see Figs. S1, S2, Table S3 in Supporting Information). The 13C and 29Si NMR analyses indicated the existence of surface amine anchored on the surface of SiO2. The N2 ads./des. isotherms of each material showed type IV indicating that the mesoporous structure was maintained during the surface functionalization and immobilization of the Rh complex. The decrease in inner surface area and pore volume indicated that the surface modification took place inside the pores and the active catalyst located inside the pores. STEM analysis of the catalysts also revealed the mesoporous structure of the catalyst. The energy-dispersive-spectroscopy (EDS) mapping indicated that all the components were highly dispersed on the surface and that no aggregated species were found. The area distributions of the surface amine, STA, and the Rh complex matched well, which indicated that they located close to each other. All the characterization data were consistent with our target structure, in which chiral cationic Rh complexes are immobilized inside mesopores by ionic interactions with STA.
The activity of the prepared catalyst and the effects of each component were examined by control experiments. For this purpose, two other heterogeneous catalysts were prepared. One catalyst was prepared using a support without surface amine functionalization (Rh-BINAP/STA/SBA-15), while the other was prepared without STA salt formation (Rh-BINAP/SBA-15-Im). First, the immobilization efficiency of the supports was evaluated by ICP analysis (see Table S1 in SI). While a standard catalyst (Rh-BINAP/STA/SBA-15-Im) demonstrated almost quantitative immobilization, the lack of a surface amine or an HPA resulted in a significant loss of immobilization efficiency. These results indicated that both surface amine and HPAs are essential for the immobilization of the Rh complex. This supports the existence of electrostatic interaction between Rh and STA and acid-base interaction between STA and the surface amine. The performance of these heterogeneous catalysts was also evaluated under batch conditions. The control experiments also suggest that both HPA and amine are necessary to achieve high activity and to prevent leaching of Rh (see Table S2 in SI).
Next, the prepared heterogeneous catalysts were evaluated for enantioselective hydroacylation of 1 a under continuous-flow conditions. A solution of 1 a in DCM was pumped into a column reactor packed with a heterogeneous catalyst and the resulting solution was analyzed hourly. The yield of the target product 2 a was plotted against the operation time of the continuous-flow reaction (Figure 2). Initially, STA/SiO2−NH2, which showed good performance for continuous-flow enantioselective hydrogenation in our previous study, was employed as the support. The catalyst exhibited excellent activity at the initial stage of the flow reaction, but it was quickly deactivated after 2 h of a flow reaction. We assumed that an aldehyde moiety in 1 a reacted with the NH2 group on the surface of the support before interacting with a Rh catalyst to form an imine, which caused the deactivation of the catalyst. To prevent such undesired side reactions, the amine structure of the surface of the support was investigated. While secondary and tertiary amines did not show any improvement in catalyst durability (STA/SiO2−NHBu, STA/SiO2−NMe2), to our delight, the dihydroimidazole structure was found to be effective and the high yield could be maintained for 4 h (STA/SiO2−Im). Next, the structure of HPA was examined using dihydroimidazole-functionalized SiO2. A Si-containing acid demonstrated higher activity than P-containing one, and STA was found to be the best acid among those examined. It is likely that a soft RhI cation favorably interacts with softer HPA.35 Finally, a longer catalyst lifetime was realized when using mesoporous SiO2 instead of using amorphous SiO2. In particular, SBA-15 demonstrated the best catalyst durability. Under the optimized reaction conditions, the target product 2 a could be obtained in >90 % yield for 6 h with >98 % ee. The turnover number (TON) reached to >300 under these flow reaction conditions and the leaching of Rh, determined by ICP analysis, was under the detection limit. The space time yield (STY) was 22.34 gL−1 h−1. It is noted that the original reactions with homogeneous catalysts (batch conditions) employed 2 mol % of Rh (TON=<50)(see Scheme S2 as well).34 Although the cause of the deactivation could not be identified, it is likely that the irreversible formation of an inactive Rh-carbonyl complex generated by decarbonylation of the acyl-Rh intermediate reported in many homogeneous catalyses is a major reason for the deactivation in both homogeneous and heterogeneous catalysts.36-38
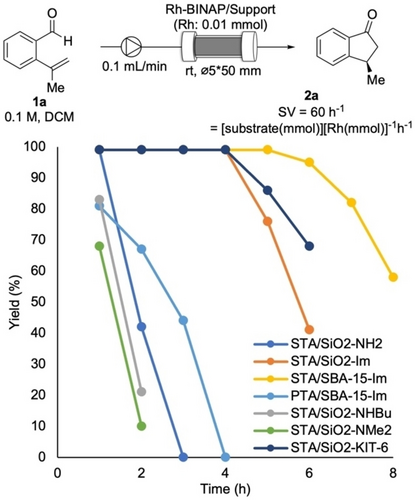
Continuous-flow hydroacylation.
With the optimized conditions in hand, the scope of substrates was examined under continuous-flow conditions (Figure 3). First, the effect of substituents on aromatic rings was investigated. Electron-donating and -withdrawing groups at the 4-position did not affect the reaction, and excellent reactivity and enantioselectivities were obtained (2 b, c). A substrate with a Cl substituent at the 5-position also demonstrated excellent yield and enantioselectivity (2 d), while a 6-Me substituted substrate resulted in a moderate yield under standard reaction conditions, presumably because of the steric hindrance. The yield could be improved by simply decreasing the concentration to 0.05 M and Space Velocity (SV) value to 30 h−1, and the target ketone 2 e was obtained in excellent yield with excellent enantioselectivity. Next, the effect of substituents on alkenes was studied. Liner primary alkyl groups were applicable to this reaction without loss of reactivity and enantioselectivity (2 f, g). On the other hand, primary alkyl substituents with branched structures required lower SV conditions to obtain target products in high yields, while enantioselectivities were maintained at excellent levels (2 h–j). The functional group compatibility was also examined; ester (2 k), alcohol (2 l), and ether (2 m) were compatible in this reaction. On the other hand, low conversions were observed when sterically demanding secondary alkyl groups were introduced to an alkene. The conversion issue was addressed by decreasing both concentration and flow rate to half to prolong the residence time, and the target products were obtained in high yields whilst maintaining enantioselectivities regardless of the ring size (2 n–p). An acyclic substituent could also be applicable under the same reaction conditions but with a slight loss of enantioselectivity (2 q). Finally, a series of aromatic substituents on alkenes was evaluated. A simple Ph substituent afforded the corresponding ketone (2 r) in quantitative yield with 93–94 % ee. The reaction could be scaled up to afford 1.5 g of the target product without any affect on yield and selectivity. The effect of substitution position was examined with tolyl substrates; comparable yields and enantioselectivities were observed for o-, m-, and p- substituents (2 s–u). The electronic effects were also examined with p-substituted substrates. While the electron-donating OMe group afforded the product 2 v in slightly decreased enantioselectivity, electron-deficient groups such as halogens (2 w–y), CF3 (2 z), ester (2 aa), and NO2 (2 ab) gave higher enantioselectivities. Meanwhile, the yields were unaffected by the electronic nature of substrates and the products were obtained in 88->99 % yields. 1- and 2-Naphthyl substituents also gave the products in high yields and enantioselectivities without modification of the reaction conditions (2 ac, 2 ad). This method could be applicable to the continuous-flow synthesis of an intermediate of a biologically active molecule. Optically active ketone 2 ae could be synthesized from the corresponding aldehyde in high yield with excellent enantioselectivity, it can be converted into D-1 and D-2 dopamine receptor tefludazine by following the reported procedure.39
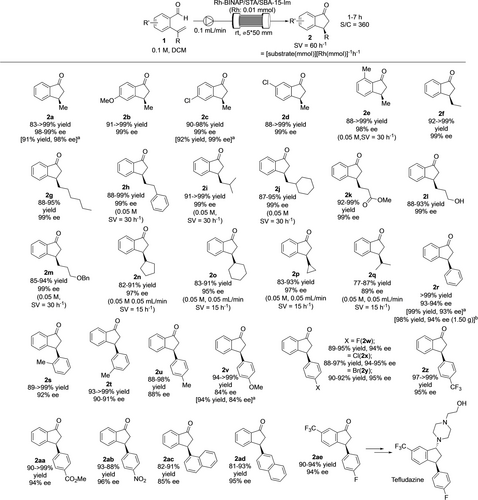
Continuous-flow hydroacylation.[a] Combined isolated yield and ee between 1–7 h.[b] Combined isolated yield and ee between 1–7 h. The amount of catalyst and concentration of the substrate was increased by twice for scale up with keeping SV.
Once we have established the continuous-flow enantioselective intramolecular hydroacylation with aromatic aldehydes, we then investigated more challenging transformations. First, two types of intramolecular hydroacylation with aliphatic aldehydes via desymmetrization were examined. Using the same Rh-BINAP/STA/SBA-15-Im catalyst, γ,δ-unsaturated aliphatic aldehyde 3 underwent stereoselective hydroacylation to afford optically active cyclohexanone 4 in high yield with excellent diastereo- and enantioselectivity (Scheme 1(a)).40 Then, a new heterogeneous catalyst with a different chiral ligand was prepared in efforts to achieve another type of intramolecular hydroacylation. We focused on the ligand-controlled stereoselective isomerization/hydroacylation of aliphatic aldehydes developed by Dong and co-workers.41 Because a catalyst with a BINAP ligand results in low yield and enantioselectivity, the chiral ligand of a heterogeneous catalyst has to be exchanged. Unlike conventional immobilization methods that require chemical modification of chiral ligands, the current method allows to use original chiral ligands for immobilization. Therefore, a supported Rh-(S)-DTBM-MeOBIPHEP catalyst was readily prepared by following the same procedure as with Rh-BINAP catalysts (Rh-DTBM-MeOBIPHEP/STA/SBA-15-Im). To our delight, the prepared heterogeneous catalyst exhibited high activity and excellent enantioselectivity for the continuous-flow isomerization/hydroacylation of aliphatic aldehyde 5 to afford optically pure cyclopentanone 6 having all-carbon-substituted quaternary carbon stereogenic center (Scheme 1(b)). Finally, intermolecular hydroacylation between an aliphatic aldehyde and a methacrylamide originally developed by Tanaka and co-workers using a homogeneous Rh-QuinoxP* catalyst was investigated.42 For this purpose, another new heterogeneous catalyst was prepared from Rh(nbd)2BF4 and (R, R)-QuinoxP* (Rh-QuinoxP*/STA/SBA-15-Im). Using this heterogeneous catalyst, continuous-flow enantioselective intermolecular hydroacylation between aldehyde 7 and methacrylamide 8 was achieved in good yield with excellent enantioselectivity (Scheme 1(c)). It is noteworthy that the enantioselectivity of heterogeneous catalysis was maintained at the same level as that of homogeneous catalysis, which proves the versatility in the ligand structure of the current immobilization method.

Continuous-flow hydroacylation of aliphatic aldehydes.
To expand the utility of this methodology, we then examined sequential-flow reactions42, 43 because optically active ketones produced in this flow reaction can be useful synthetic intermediates. In particular, this flow hydroacylation is suitable for sequential-flow reactions because it does not produce any byproducts and heterogeneous catalysts are readily separated in a reactor. Initially, selective hydrogenation of ketones to alcohols was chosen as a target reaction. Early investigation of various supported metal nanoparticle catalysts for hydrogenation of ketone rac-2 r under batch conditions revealed that an alkane was obtained as a major product. To suppress such overreaction, deactivated Pd catalysts such as Pd/C(en)45 and Pd/C prepared from Pd(OAc)2 and NaBH4 were found to be effective for high selectivity to alcohol 10 with high syn-selectivity (see Table S4 in SI). Further investigations were performed under continuous-flow conditions (Table 1). To improve the chemoselectivity, a continuous-flow reaction was performed under higher SV conditions. While increasing SV twofold had a small impact on the selectivity (entries 1, 2), increasing the SV by tenfold almost suppresses the formation of alkane 10′, and the target alcohol 10 was obtained in moderate yield (entry 3). Further fine-tuning of SV revealed that 7.6 h−1 was the best value to achieve both high conversion and chemoselectivity (entry 5), and both a decrease and increase of SV resulted in an inferior yield of the product (entries 4, 6).
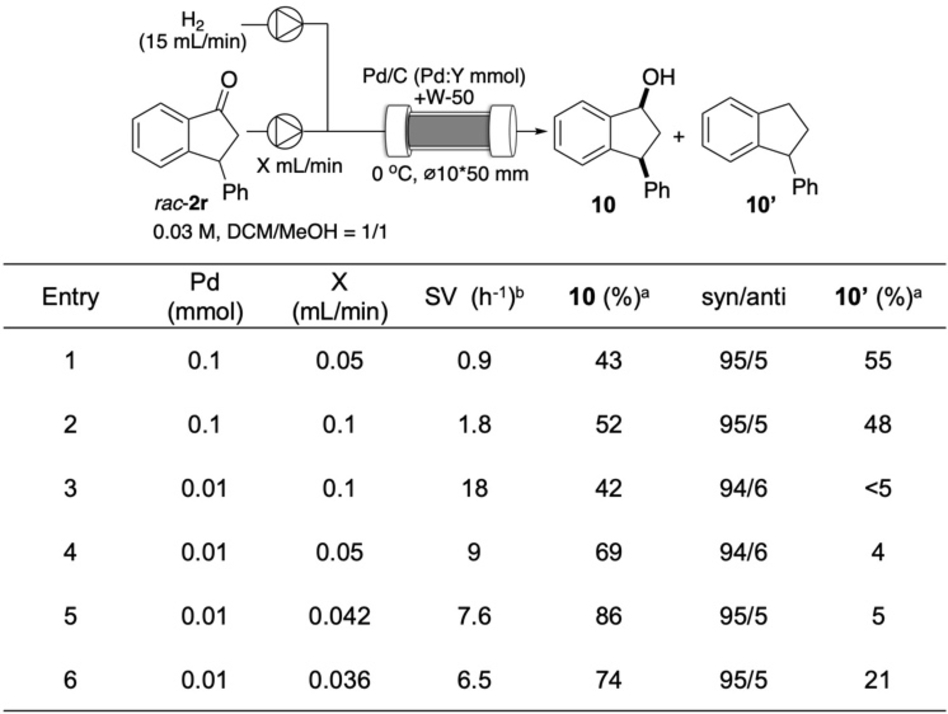
- [a] Yield was determined by 1H NMR analysis using 1,2,4,5-tetramethylbenzene as an internal standard. [b] SV=[rac-2 r(mmol)][Pd(mmol)]−1 h−1.
Through these experiments, precise control of the SV value was found to be crucial to achieving high alcohol/alkane selectivity, and high yield and diastereoselectivity were realized. Under the optimized reaction conditions, the TOF value under flow conditions was seven times higher than that of batch conditions, which can be explained by the efficient contact of H2 gas with heterogeneous catalysts.46
Based on the optimized flow conditions for the selective hydrogenation of ketone, a sequential reaction of hydroacylation/hydrogenation was performed (Scheme 2(a)). A stream of a solution containing optically active ketone 2 r after flow hydroacylation was merged with streams of MeOH and H2 gas and pumped into a column reactor packed with the prepared Pd/C catalyst, and optically active alcohol 10 was obtained in good yield and stereoselectivity without loss of enantioselectivity. This hydroacylation/hydrogenation sequential-flow reaction could be applicable to the synthesis of a synthetic intermediate of a biologically active molecule. Using 3,4-disubstituted starting material 1 af, the first enantioselective hydroacylation took place in excellent yield with high enantioselectivity to afford the intermediate 2 af. However, the second hydrogenation with supported Pd catalysts was sluggish due to the reductive cleavage of the C−Cl bond. The problem of the dechlorination was overcome by changing the catalyst to Pt/C. The target product 11 was obtained in good yield with high stereoselectivity, it is a synthetic intermediate of a nonselective monoamine transporter inhibitor indatraline.47
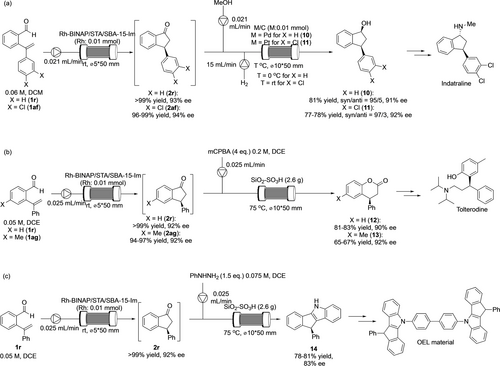
Sequential flow catalyses for the syntheses of fine chemicals.
After the establishment of the hydroacylation/selective hydrogenation sequence, other types of sequential-flow transformations were investigated. We focused on acid-catalyzed Baeyer–Villiger oxidation affording optically active dihydrocoumarins.48 While continuous-flow Beayer-Villiger oxidation using a supported enzyme catalyst was reported recently,49 we found that commercially available SiO2-supported sulfonic acid (SiO2−SO3H) was an efficient catalyst for this transformation when using rac-2 r as a substrate (see Table S5 in SI). Although the solvent for the hydroacylation had to be changed to 1,2-dichloroethane (DCE) because the second Baeyer–Villiger oxidation requires a high reaction temperature, the yield and the enantioselectivity were unaffected by this solvent change. The sequential-flow reaction was performed by pumping the combined stream of the solution of the hydroacylation and mCPBA into a column reactor packed with SiO2−SO3H. The target dihyrocoumarin 12 could be synthesized in good yield with high enantioselectivity by this hydroacylation/Baeyer–Villiger oxidation sequence (Scheme 2(b)). We also investigated the continuous-flow synthesis of a key intermediate of an API. 4-Me-substituted starting material 1 ag underwent enantioselective hydroacylation to afford 2 ag in excellent yield with high enantioselectivity. The second Baeyer–Villiger oxidation proceeded without loss of optical purity and the sequential-flow synthesis of dihydrocoumarin 13 was achieved in moderate yield presumably due to the shorter residence time. Compound 13 is a known synthetic intermediate of tolterodine, a medication used to treat frequent urination, urinary incontinence, or urinary urgency.50
We also found that the same solid acid was effective for Fischer indole synthesis. Although the reaction required a stoichiometric amount of solid acid, the target indole 14 was obtained in good yield by the reaction with phenylhydrazine under heating conditions (see Table S6 in SI). Finally, sequential hydroacylation/Fischer indole synthesis was performed using 1 r as a starting material. After the two-step flow reaction, 14 was obtained in good yield but with slightly decreased optical purity presumably due to the highly conjugated nature of the product. Interestingly, the product can be used as a synthetic intermediate of optically active organic electroluminescence materials (Scheme 2(c)).51, 52
The demonstrated sequential transformations do not require any isolation of intermediates and work-up such as quenching or solvent switch, and continuous-flow syntheses of intermediates of optically active fine chemicals were realized.
Conclusion
In summary, we have developed heterogeneous chiral Rh catalysts for continuous-flow enantioselective hydroacylations. It was revealed that the structure of supports is crucial to achieving high activity and robustness of the catalyst. Under the optimized reaction conditions, excellent yields and enantioselectivities were realized. White the currently inevitable catalyst deactivation was observed after 6 h, the TON was >300 and the TOF was 60 h−1 without leaching of Rh species. The catalyst not only demonstrated a wide substrate scope but also possessed versatility in the chiral ligand structure. By tuning the chiral ligands, more challenging reactions such as a desymmetrization of alkenes, an isomerization/hydroacylation sequence, and an intermolecular reaction were realized. Moreover, the continuous-flow enantioselective hydroacylation could be applied in sequential transformations combined with hydrogenation or solid acid-catalyzed/mediated transformations, and the continuous synthesis of optically active useful molecules was demonstrated. Further studies to improve the catalyst lifetime and on the immobilization method for challenging continuous-flow catalysis are now ongoing in our group.
Supporting Information
Catalyst preparation and characterization including ICP, N2 ads./des. isotherm, solid-state NMR, STEM, EDS mapping, detailed experimental procedures, detailed optimization data, characterization of products, copies of product NMR spectra and HPLC charts (PDF)
Acknowledgments
This work was supported in part a The Grant-in-Aid for Transformative Research Areas (A) JP21A204/22H05345 Digitalization-driven Transformative Organic Synthesis (Digi-TOS) from the Ministry of Education, Culture, Sports, Science & Technology, Japan, a Grant-in-Aid for Scientific Research from the Japan Society for the Promotion of Science, the Japan Agency for Medical Research and Development (AMED), and a Grant-in-Aid for Scientific Research from the New Energy and Industrial Technology Development Organization (NEDO) project (Grand No. JPNP 19004), Japan. We thank Dr. Tei Maki (The University of Tokyo) for STEM and EDS analyses.
Conflict of interest
The authors declare no conflict of interest.
Open Research
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.




