Disproportionation of Co2+ in the Topochemically Reduced Oxide LaSrCoRuO5
Abstract
Complex transition-metal oxides exhibit a wide variety of chemical and physical properties which are a strong function the local electronic states of the transition-metal centres, as determined by a combination of metal oxidation state and local coordination environment. Topochemical reduction of the double perovskite oxide, LaSrCoRuO6, using Zr, yields LaSrCoRuO5. This reduced phase contains an ordered array of apex-linked square-based pyramidal Ru3+O5, square-planar Co1+O4 and octahedral Co3+O6 units, consistent with the coordination-geometry driven disproportionation of Co2+. Coordination-geometry driven disproportionation of d7 transition-metal cations (e.g. Rh2+, Pd3+, Pt3+) is common in complex oxides containing 4d and 5d metals. However, the weak ligand field experienced by a 3d transition-metal such as cobalt leads to the expectation that d7+ Co2+ should be stable to disproportionation in oxide environments, so the presence of Co1+O4 and Co3+O6 units in LaSrCoRuO5 is surprising. Low-temperature measurements indicate LaSrCoRuO5 adopts a ferromagnetically ordered state below 120 K due to couplings between S=1/2 Ru3+ and S=1 Co1+.
Complex metal oxides have been the subject of extensive study due to the wide variety properties they exhibit. These range from electronic and magnetic behaviors such as ferroelectricity, superconductivity and magnetoresistance to an extensive array of catalytic and electrochemical phenomena. As the chemical and physical behaviors exhibited by metal oxides tend to depend strongly on the electric configurations of the metal cations they contain (defined by a combination of oxidation states and coordination environments), there has been an enduring interest in establishing composition-structure-property relations in extended oxide systems to explore these features. These studies have revealed that a number of elements exhibit ‘disfavored’ oxidation states in oxide environments, i.e. oxidation states that appear to be thermodynamically accessible (sufficient lattice energy to overcome the required ionization energy) but are unstable with respect to disproportionation, when the metal is located in an extended oxide framework.
The instability of some of these disfavored states, such as the disproportionation of Pb3+ and Bi4+ in Pb2O3 and BiO2 respectively,1, 2 can be accounted for by universal chemical concepts—in this instance the global instability of ns1 electron configurations in main group metals leads to Pb2O3 and BiO2 being better described as PbIIPbIVO3 and BiIIIBiVO4 respectively.
However, similar disproportionations are observed in transition-metal systems, where the instability of the metal oxidation state cannot be easily attributed to a global instability of a particular electron count but appears to arise from the favorability of particular combinations of d-electron count and local coordination environment. For example, AgO is better described as AgIAgIIIO2,3 with the disproportionation of AgII attributed to the favorability of locating d10 AgI in a linear coordination and d8 AgIII in square-planar coordination sites within the oxide framework. Likewise, analogous disproportionations of d7 cations, such as PdIII or PtIII are observed, driven by the favorability of locating d6 PdIV/PtIV cations in octahedral environments and d8 PdII/PtII cations in square-planar coordinations, in phases such as K2PdII3PdIVO6 and CdPtIIPtIV2O6.4, 5
Recently we observed the disproportionation of d7 RhII during the topochemical reduction of the Ruddlesden-Popper LaSrM0.5Rh0.5O4 (M=Co, Ni) and perovskite LaM0.5Rh0.5O3 oxides, with the reduced phases (LaSrM0.5Rh0.5O3.25 and LaM0.5Rh0.5O2.25 respectively) hosting d8 RhI in square-planar coordination sites, and d6 RhIII in 5-coordinate, square-based pyramidal sites.6, 7 Here we describe the first observation of the disproportionation of d7 CoII in an extended oxide, which occurs during the topochemical reduction of the double perovskite oxide LaSrCoRuO6 to LaSrCoRuO5.
Previous work revealed that rapidly quenching the double perovskite oxide LaSrNiRuO6 through a R-3 to P21/n phase transition (T≈400 °C)8 increased the reactivity of this oxide phase with CaH2, allowing the preparation of the infinite layer phase, LaSrNiRuO4, by topochemical anion deintercalation.9, 10 The corresponding cobalt phase, LaSrCoRuO6,11, 12 exhibits an analogous phase transition at T≈450 °C. Rapidly quenching LaSrCoRuO6 through its R-3 to P21/n phase transition also enhances its reactivity enabling the preparation of the infinite layer phase LaSrCoRuO4 via reaction with binary metal hydrides, as will be described in detail elsewhere. However, in contrast to the LaSrNiRuO6-x system, quenched samples of LaSrCoRuO6 can be reduced to a phase of intermediate oxygen content (shown to be LaSrCoRuO5 by oxidative thermogravimetric analysis) by reaction with a Zr getter at 450 °C.
Synchrotron X-ray powder diffraction (SXRD) data collected from LaSrCoRuO5 could be indexed using a body-centered monoclinic unit cell (a=5.40 Å, b=5.41 Å, c=8.16 Å, γ=90.5 °) consistent with the retention of the perovskite framework from the LaSrCoRuO6 parent phase. However, close inspection revealed a series of weak additional reflections in the SXRD data that could not be indexed by this cell. Electron diffraction (ED) data collected from LaSrCoRuO5, shown in Figure 1, are consistent with a 2×2×1 cell expansion compared to the LaSrCoRuO6 parent phase (2√2×2√2×2 compared to a simple ABO3 perovskite unit cell).13, 14 This expanded cell accounts for all the additional weak peaks observed in the SXRD data and can also index neutron powder diffraction (NPD) data collected at room temperature from LaSrCoRuO5.
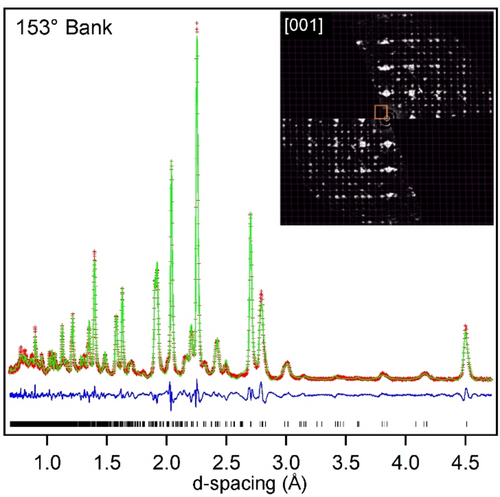
Observed, calculated and difference plots from structural refinement of LaSrCoRuO5 against NPD data. Inset shows ED pattern demonstrating 2√2×2√2×2 cell expansion.
Considering the A2BB'O5 composition and the 2√2×2√2×2 cell expansion of the phase, a number of anion-vacancy ordered and B-site cation ordered perovskite structural models were considered for LaSrCoRuO5. It was observed that a good fit to the SXRD and NPD data could be achieved using a model based on the anion-vacancy ordered structure of LaNi0.9Co0.1O2.5 which consists of a network of apex-linked 6-coordinate octahedral, 5-coordinate square-based pyramidal and 4-coordinate square planar BOx units.15 The model was modified to take account of the rock salt ordering of the Co and Ru cations, so that the Ru centers were exclusively located within 5-coordinate sites, while the Co centers occupied both 6- and 4-coordinate sites within a monoclinic unit cell (a=10.8128(2) Å, b=10.8231(2) Å, c=8.1626(1) Å, γ=90.55(1) °) with P1121 space group symmetry, as shown in Figure 2. The model was refined against the NPD data to achieve a good fit (wRp=6.33 %) as shown in Figure 1 and described in detail in the Supporting Information.16
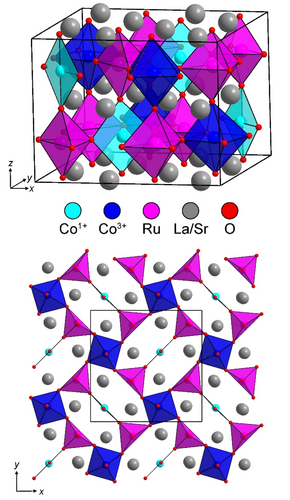
Crystal structure of LaSrCoRuO5 (top) and a projection of the transition metal coordination polyhedra at z≈0.75 (bottom).
The crystal structure of LaSrCoRuO5 shown in Figure 2 reveals that the cobalt cations occupy two distinct sites within the oxide framework. A 4-coordinate planar site and a 6-coordniate octahedral site. The location of the cobalt cations in two distinct sites is reminiscent of the local-coordination-driven disproportionation of transition metals with d7 electron counts observed for Pd3+ and Pt3+ and more recently Rh2+, and suggests the disproportionation of Co2+ into Co1+ (square-planar) and Co3+ (octahedral). Analysis of the local coordination environments of the cobalt centers is hampered by the lack of reported Co1+O4 units for comparison. However, the observed bond lengths of the CoO4 units in LaSrCoRuO5 (Co−O=2.032(11) Å×2; 2.119(9) Å×2) are significantly longer than those in the Co2+O4 units reported in Sr3Co2O4Cl2 (Co−O=2.007(1) Å×4)17 or Sr2CoO2Cu2S2 (Co−O=1.995(1) Å×4)18 consistent with assignment of Co1+O4 for the units present in LaSrCoRuO5. Bond valance sums (BVS)19 calculated using parameters for Co2+ yield values of LaSrCoRuO5 : Co+1.42, Sr3Co2O4Cl2 : Co+1.70 and Sr2CoO2Cu2S2 : Co+1.76. The CoO6 units in LaSrCoRuO5 have a rather irregular shape but exhibit an average bond length of <Co−O=2.004 Å> (BVS=Co+2.69) compared to <Co−O=2.033 Å> (BVS=Co+2.38) the CoIIO6 units in the LaSrCoRuO6 parent phase.12 Thus, it can be seen that the BVS values of the square-planar (BVS=Co+1.42) and octahedral sites (BVS=Co+2.69) in LaSrCoRuO5 differ by 1.27 units. This difference is significantly larger than the difference between the octahedral and tetrahedral sites in the Co2+ brownmillerite phase La2Co2O5 (CoO6 BVS=Co+2.23; CoO4 BVS=Co+2.07, Δ=0.16)20 or the difference between octahedral and square-planar sites in the Ni2+ phase La2Ni2O5 (NiO6 BVS=2.08; NiO4 BVS=2.11, Δ=0.03)21 and provides strong support for the disproportionation of Co2+ in LaSrCoRuO5.
In an attempt to further confirm the disproportionation of Co2+, cobalt EELS data collected from LaSrCoRuO5. These data show a single set of Co L2 and L3 peaks (Figure S14 in the Supporting Information) and thus represent the superposition of signals from both the square-planar and octahedral cobalt sites. In the absence of a Co1+ oxide standard we are unable to know if a Co1+/Co3+ oxidation state combination would be expected to lead to a resolvable splitting of the L2 and L3 peaks. It should be noted that splitting of Co2+/Co3+ signals is not resolvable for Co3O4.22 The L3/L2 intensity ratio (4.83) and L3-L2 energy difference (15.06 eV) from the data are broadly consistent with Co2+.
Magnetization data collected from LaSrCoRuO5 indicate that, in common with many other topochemically reduced phases containing cobalt, samples of LaSrCoRuO5 contain small quantities of ferromagnetic, elemental cobalt not detectable by diffraction. The magnetization of LaSrCoRuO5 was therefore measured using the ‘ferromagnetic subtraction’ method described in the Supporting Information. A plot of the magnetic susceptibility of LaSrCoRuO5 against temperature (Figure 3a) can be fit by the Curie–Weiss law in the range 140<T/K<300. However, the extracted Curie constant (C=3.76 cm3 K mol−1; θ=+82.7 K) is much larger than can be accounted for by a combination of Co1+, Co3+ and Ru3+ cations (even with the cobalt centers in high-spin states), suggesting strong magnetic interactions are present between the metal centers in this temperature range.
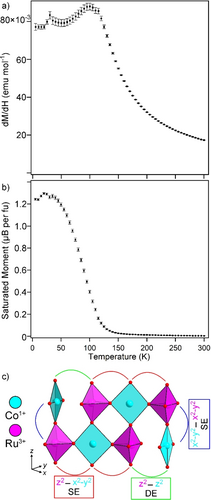
a) Paramagnetic susceptibility and b) saturated ferromagnetic moment of LaSrCoRuO5 measured via the ‘ferrosubtraction’ method and plotted as a function of temperature, c) The direct exchange and super exchange pathways in LaSrCoRuO5.
On cooling below 120 K there is a large increase in the saturated ferromagnetic moment of the samples (Figure 3b), increasing from 0.03 μB per fu at 150 K (a value that is attributed to the presence of an elemental Co impurity) to ≈1.25 μB per fu at 2 K, indicative of a ferromagnetic state, however NPD data collected at 5 K show no additional features indicative of magnetic order, as described in the Supporting Information.
The bond lengths of the square-planar and octahedral cobalt sites in LaSrCoRuO5 are consistent with a high spin, S=1 Co1+ center, and a low spin, S=0 Co3+ respectively. Thus, the most significant magnetic couplings in the system will be between the square-planar Co1+ centers, which have a (dxz/yz)4(dxy)2(dz2)1(dx2−y2)1 electronic configuration, and the Ru3+ centers located in square-based pyramidal sites which have a (dxz/yz)4(dxy)1(dz2)0(dx2−y2)0 electronic configuration.
As shown in Figure 3c, the Co1+ and Ru3+ centers are magnetically coupled by either (Ru4dx2−y2)−O2p−(Co3dx2−y2) or (Ru4dz2)−O2p-(Co3dx2−y2) σ-type super exchange or (Ru4dz2)−(Co3dz2) direct exchange. Given that the Ru 4dx2−y2 and 4dz2 orbitals are empty and the corresponding Co3d orbitals are half filled, all of these interactions will be ferromagnetic,23 consistent with the low-temperature magnetization data.
The disproportionation of Co2+ evident in LaSrCoRuO5 is surprising. As noted above, other transition metal cations with d7 electron counts (e.g., Pd3+, Pt3+, Rh2+) are observed to disproportionate in oxide environments, driven by the presence of ‘preferred’ coordination sites. However, to date, this behavior has been restricted to 4d and 5d transition metals where the stronger ligand fields (compared to 3d metals) provide a larger energetic stabilization for the d6 octahedral and d8 square-planar electron-count/coordination combinations. It is therefore unexpected to see Co2+, a common oxidation state with a modest ligand field in oxides, undergo a coordination-site driven disproportionation.
There are limited examples of 3d transition metal cations, such as Fe4+ and Ni3+ disproportionating in extended oxides. However, in these cases the disproportionation of the metal center (e.g. Fe4+ in CaFeO3 or BaFeO3; Ni3+ in TlNiO3)24-26 is driven by a metal-insulator phase transition driven by the presence of a single electron in the σ-band of these oxides phases, rather than coordination site preference.
The unique observation of coordination-site driven disproportionation of Co2+ in LaSrCoRuO5 suggests that the topochemical reaction which forms LaSrCoRuO5 may act to ‘select’ this phase, as the disproportionated structure is a local energy minimum in composition-structure space in the reaction path between LaSrCoRuO6 and LaSrCoRuO4. Indeed, the same argument can be applied to the topochemical reactions which form the RhI/RhIII disproportionated phases reported previously.6, 7 In combination these observations suggest further coordination-site driven disproportionated oxide phases could be accessible by this type of low-temperature reaction, presenting an opportunity to prepare a range of transition metal oxides with a rich variety of novel metal oxidation-state/coordination geometry-combinations.
Acknowledgments
Experiments at the Diamond Light Source were performed as part of the Block Allocation Group award “Oxford Solid State Chemistry BAG to probe composition-structure–property relationships in solids” (CY25166). Experiments at the ISIS pulsed neutron facility were supported by a beam time allocation from the STFC (doi.org/10.5286/ISIS.E.RB2220199). ZL and MAH thank the EPSRC (EP/T027991/1) for funding. We thank Daphne Vandemeulebroucke for assistance collecting the EELS data.
Conflict of interest
The authors declare no conflict of interest.
Open Research
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.




