1,4-Aminoarylation of Butadienes via Photoinduced Palladium Catalysis
Abstract
A visible-light-induced, three-component palladium-catalyzed 1,4-aminoarylation of butadienes with readily available aryl halides and aliphatic amines has been developed, affording allylamines with excellent E-selectivity. The reaction exhibits exceptional control over chemo-, regio-, and stereoselectivity, a broad substrate scope, and high functional group compatibility, as demonstrated by the late-stage functionalization of bioactive molecules. Mechanistic investigations are consistent with a photoinduced radical Pd(0)-Pd(I)-Pd(II)-Pd(0) Heck-Tsuji–Trost allylation cascade.
Palladium-catalyzed Heck couplings, Buchwald–Hartwig aminations, and hydroamination reactions with readily available aryl halides, alkenes, and amines have revolutionized both academic and industrial chemistry by enabling the construction of C−C and C−N bonds (Figure 1A).1 Despite, or maybe even due to, these robust reactions, a three-component coupling of aryl halides, butadienes, and amines for selective aminoarylation to afford aryl-substituted allylamines has remained elusive thus far.2 Herein, we report a highly chemo-, regio-, and stereoselective 1,4-aminoarylation addition to butadienes with aryl halides and amines under photoinduced palladium catalysis. The transformation is based on the photoexcitation of a low-valent palladium catalyst for oxidative addition,3 interrupted by radical addition to a diene, which results in a single electron radical Heck-Tsuji Trost allylation cascade pathway. The allylamines are produced selectively with E configuration, in a single step, in contrast to traditional multistep processes for their syntheses, which we illustrated at the example of the synthesis of clofilium, an antiarrhythmic agent. The mild reaction conditions tolerate a wide range of functional groups and bioactive molecules that are typically not compatible with traditional palladium catalysis.
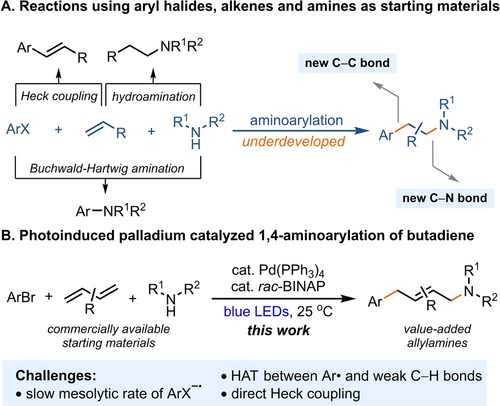
Synthesis of allylamines through aminoarylation of butadienes with widely available aryl halides and amines.
Allylamines are not only commonly found in pharmaceuticals and natural products but also utilized as versatile building blocks in organic synthesis.4 The amino-functionalization of butadienes offers a direct route to synthesize allylamines, eliminating the requirement of pre-functionalized olefins and reducing the number of synthetic steps.2 Transition metal-catalyzed three-component bifunctionalization of butadienes are currently possible for carbosilylation, carboborylation, and dicarbonation,2a, 5 yet, the aminoarylation of butadienes with high selectivity remains a challenging task. Heck reported a palladium-catalyzed aminoarylation of butadienes, but it required excess of amines (2.5 to 30 equiv) and still yielded significant Heck coupling byproducts (20–60 %), indicating that the rate of β-H elimination from allyl palladium complexes is comparable to the rate of substitution with amines.6 As a consequence, the scope of aminoarylation of butadienes is currently limited to two-component systems, wherein two out of the three reaction partners are tethered.7 Radical-mediated three-component amino-functionalizations have also been developed, leveraging the efficiency of butadienes as effective radical trapping agents.2b, 2e Although diamination, aminooxylation, amidocyanation, and aminoalkylation of butadienes have been successfully achieved,8 the radical-mediated aminoarylation of butadienes with high selectivity has not.9 Overall, a general and practical platform for the aminoarylation of butadienes to produce allylamines is currently lacking, both in terms of traditional two-electron transition metal catalysis as well as radical-based approaches alike.
Intrigued by the photoinduced palladium-catalyzed aminoalkylation of butadienes reported by Glorius, Gevorgyan, and others,8a-8f we presumed that aminoarylation of butadienes could potentially be achieved using aryl halides as aryl radical precursors. Although both alkyl- and aryl halides possess highly negative reduction potentials, generation of aryl radicals from electron-poor aryl bromides and -chlorides via single electron transfer (SET) is substantially more challenging, especially when the rate of mesolytic bond cleavage of aryl halide radical anions (ArX−⋅) is slow (Scheme 1).10 The slower rate of electron-poor ArX−⋅ mesolytic cleavage arises from the resonance stabilization of electron delocalization within the aromatic ring. An additional challenge is therefore posed by the unproductive back electron transfer (BET) between ArX−⋅ and the photocatalyst. Introducing an electron-donating group on the arene moiety results in destabilization of the ArX−⋅, which increases the rate of mesolytic cleavage; however, it also leads to a more negative reduction potential for aryl halides, making single electron reduction more difficult.10 In contrast, for the generation of alkyl radicals from alkyl halides, both theoretical and experimental studies suggest that a concerted dissociative electron transfer process is preferred over a two-step process with radical anion intermediates.11 Such considerations make the aminoarylation of butadiene reported here conceptually distinct from for example the aminoalkylation of butadienes as reported by Glorius, Gevorgyan, and others, and represent the advance of this work reported here.8a-8f Moreover, ambiphilic aryl radicals are more prone to abstract hydrogen atoms from both electron-rich allylic positions and α-C−H bonds of amines, when compared to nucleophilic alkyl radicals, due to polarity match and stronger C(sp2)−H bond energies.12 Lastly, the preference for Heck coupling is higher for aryl halides than it is for alkyl halides due to the formation of thermodynamically more stable conjugated arylbutadienes. Owing to these factors, the utilization of aryl halides as synthetically useful aryl radical precursors for alkene functionalization is rare compared to alkyl halides as alkyl radical precusors,13, 14 and their application in the functionalization of butadiene remains unreported. As part of our ongoing research in the field of arylfunctionalization of alkenes,15 we sought to develop a practical and broadly applicable method for the aminoarylation of butadienes utilizing readily accessible aryl halides and amines (Figure 1B).
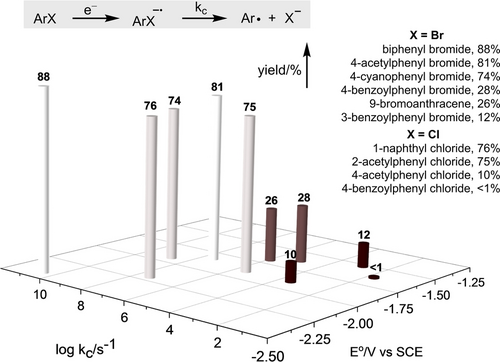
Correlation between the yields and the reduction potential (E°) of aryl halides and dissociation rate constant (kc) of the corresponding radical anions.
Optimization of reaction conditions revealed that the combination of Pd(PPh3)4 and rac-BINAP as catalyst under blue LEDs irradiation was effective in the 1,4-aminoarylation addition of butadiene, resulting in the formation of allylamine (E)-1 in 82 % yield (Figure 2). The hydrodebromination byproduct of aryl bromide was successfully suppressed to less than 5 % by using 5.0 equivalent butadiene. The reaction exhibits high regio- and stereoselectivity, as evidenced by the absence of 1,2-addition isomer and formation of less than 2 % of the Z-configured stereoisomer. The photocatalytic conditions also result in high chemoselectivity by effectively circumventing the formation of byproducts related to otherwise common two-component coupling reactions. For example, the formation of Heck coupling byproduct, frequently observed under classic thermal conditions (100–130 °C),6 was effectively suppressed, likely owing to the milder visible light-induced conditions, enabling the nucleophilic interception of the allyl Pd(II) complex.
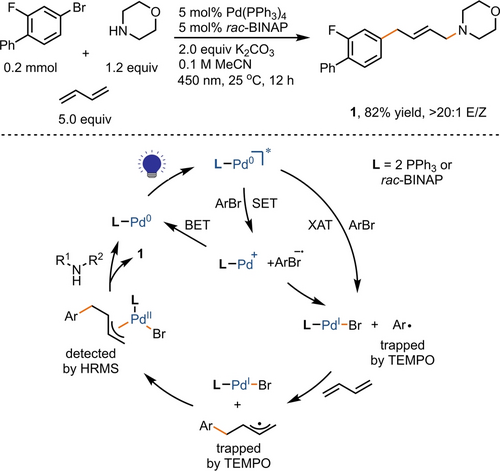
Optimized reaction conditions and proposed mechanism. XAT=halogen atom transfer; SET=single electron transfer; BET=back electron transfer.
The observed small Hammett ρ value of 0.5 in our study is in agreement with the aryl radical being generated through halogen atom transfer (XAT), with relatively little polar contribution to the bond breaking step from the arene π system. Nevertheless, we found positive correlations between yield and rate constant kc and reduction potentials as shown in Scheme 1, which is consistent with an SET/mesolytic cleavage pathway for aryl radical generation. The reported reduction potential of excited Pd(PPh3)4 (−2.51 V vs Fc+/Fc) further supports the plausibility of an SET pathway.16 These findings cannot distinguish between XAT and SET pathways. The former may be favored when aryl halides have higher kc values and more negative reduction potentials, while the latter becomes more favorable when aryl halides are more easily reduced or when the corresponding radical anion can be stabilized. Further supported by a UV/Vis study, HRMS, and TEMPO trapping experiments (see Supporting Information, Figure S1–2), the proposed mechanism for the reaction is shown in Figure 2. Following the generation of the aryl radical through either the XAT or SET/homolytic cleavage pathway in the presence of excited Pd(0), the allyl Pd(II) species is formed via aryl radical addition and oxidative ligation, followed by Tsuji–Trost allylation, resulting in the allylamines product.8a-8f
While the rate of mesolytic cleavage (kc) of the aryl halide radical anion has been well-established in the field of physical organic chemistry for decades,10, 17 its application and discussion in the context of organic reactions is rare. As shown in Scheme 1, high yields (>74 %) are achieved for aryl bromides and -chlorides with kc above 105 s−1, while inefficient product formation is likely caused by BET between the ArX−⋅ and the Pd(I) species (Figure 2), if kc is smaller. Moreover, in contrast to traditional transition metal catalysis, which shows slower rates for electron-rich aryl halides with more negative reduction potentials,17 the substrates for the reaction presented here exhibit high reactivity, regardless of whether aryl bromides or -chlorides are used. 4-Bromoanisole, despite its extremely negative reduction potential (−2.72 V vs SCE), can also participate (see Supporting Information, Table S2), which is rare for reactions that use aryl halides as aryl radical precursor,18 and can be attributed to a direct XAT pathway or strong reducing ability of excited Pd(0).16
Further evaluation of the substrate scope using commercially available (hetero)aryl bromides, amines, and butadienes is summarized in Scheme 2. The allylamines are typically obtained in moderate to high yields (43–89 %), and their purification is straightforward owing to the high chemo-, regio-, and stereoselectivity of the reaction when butadiene is employed. Electron-rich, -neutral, and -poor groups on the aryl halides all display excellent compatibility, supporting the notion that the SOMO of aryl radical is situated within the aromatic ring, thereby minimizing the effect of electron-donating or electron-withdrawing substituents on reactivity.12, 19 For most substrates with low kc values shown in Scheme 1, extended reaction times up to 48 hours can lead to improved yields (see Supporting Information, Table S2).The methodology exhibits tolerance towards a diverse array of functional groups, commonly problematic in traditional palladium catalysis, encompassing protic groups (6, 7, 15, 21, 22), strong electrophiles (8–10), strong nucleophile (4), groups with potential for poisoning the transition metal (5, 19), and sterically encumbered 2,6-disubstituted aryls (22)1. Substrates with weak C−H bonds (22, 31, 36), usually problematic in aryl radical chemistry owing to competing hydrogen atom transfer (HAT), show good tolerance here, possibly attributed to the fast addition rate of aryl radicals to 1,3-butadiene.20 A range of medicinally crucial heteroarenes are well-tolerated. Aryl chlorides (11, 13) are compatible but less reactive than aryl bromides in general, whereas aryl triflates are not compatible; thus, selective conversion of aryl bromides in the presence of chlorides and triflates is achievable (9, 16). Aryl iodides, being preferred substrates as aryl radical precursors over aryl bromides owing to their less negative reduction potentials in other reactions,16 result in lower conversion and yields, despite possessing generally faster mesolytic cleavage rates (>105 s−1) or weaker C(sp2)−I bonds, likely attributed to rapid oxidative addition with Pd(0) before excitation can take place. This hypothesis is further supported by the fact that electron-rich aryl iodides provide higher conversions and yields compared to electron-deficient aryl iodides (see Supporting Information, Table S3). With an extended reaction time, aryl iodides can also produce the corresponding allylamine products in 43–58 % yields (2, 3).
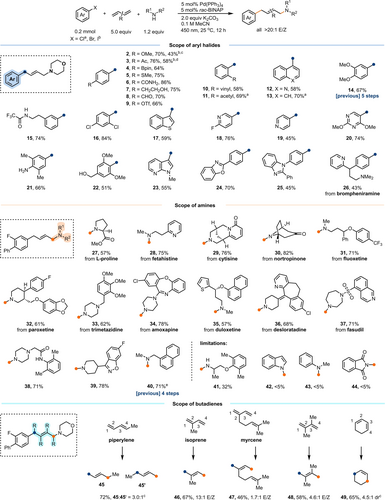
Substrate scope. [a] aryl chlorides were used; [b] aryl iodides were used; [c] 24 h; [d] 48 h; [e] bromobenzene was used.
Secondary aliphatic amines with strong nucleophilicity, including monocyclic, bicyclic, and acyclic amines, as well as five-, six-, and seven-membered N-heterocycles, are well-tolerated in the reaction. Primary aliphatic amines (41) with relatively weaker nucleophilicity display low reactivity, accompanied by the formation of Heck coupling byproducts, which can be attributed to the lower reaction rate with allyl Pd(II) species (see Supporting Information, Table S4).4a Amines with weak nucleophilicity (42–44), such as N-heteroaromatics, anilines, sulfonamides, imides, and carbamates, failed to produce the desired products in the reaction even with the use of strong bases for deprotonation. Thus, selective allylation of secondary aliphatic amines over amides and aniline is achievable (15, 21, 38). The robustness of the reaction conditions was further exemplified by successful reactions of functionalized pharmaceuticals containing amines (27–37).
The reactivity and selectivity of the aminoarylation reaction are significantly influenced by the substituents on the butadienes. The presence of a methyl substituent at the 1-position (piperylene, 45) reduces the reaction rate, leading to approximately 60 % conversion after 12 hours, likely owing to the slower rate of nucleophilic attack of the amine on the substituted allyl Pd(II) intermediate. On the other hand, substituents at the 2-position (46, 47) do not affect the reactivity but result in a mixture of Z/E isomers due to the presence of 1,3-allylic strain in the substituted allyl Pd(II) intermediate. Additionally, 2,3-disubstituted butadiene and 1,3-cyclohexadiene are also compatible, producing tetrasubstituted (48) and cyclic allylamines (49), respectively. Notably, the formation of 1,2-addition products was not observed in any case.
In contrast to conventional multistep synthesis, complex allylamines (14, 40, and 50) are obtained in a single step from commercially available aryl bromides, amines, and butadiene,21 highlighting the efficiency of the current approach (Scheme 3). Clofilium (51), an antiarrhythmic agent,22 can therefore be synthesized in fewer steps and higher yield than through currently accessible routes in just three-steps involving aminoarylation of butadiene, followed by hydrogenation and alkylation.
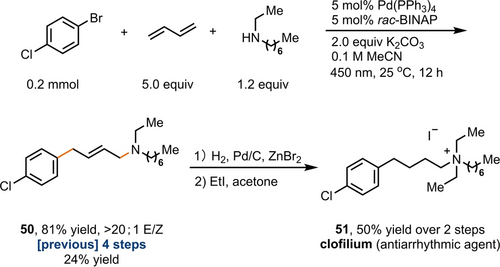
Synthesis of clofilium using 1,4-aminoarylation of butadiene as the key step.
In conclusion, a practical three-component aminoarylation of butadienes using readily available aryl halides and aliphatic amines is reported. The use of photoinduced palladium catalysis facilitates the sequential incorporation of aryl, 2-butenyl, and amino groups at room temperature, enabling the efficient synthesis of complex aryl-substituted allylamines that are typically obtained only through multiple steps. Future research will concentrate on exploring aminoarylation reactions with different types of alkenes beyond butadienes.
Acknowledgments
We thank the MPI für Kohlenforschung for funding. Y. C. acknowledges the Alexander von Humboldt Foundation for a Humboldt Research Fellowship. We thank F. Kohler and D. Kampen for mass spectrometry analysis, M. Leutzsch and C. Wirtz for NMR spectroscopy analysis. We thank Dr. Javier Mateos-Lopez and Dr. Le Li for helpful discussions. Open Access funding enabled and organized by Projekt DEAL.
Conflict of interests
The authors declare no conflict of interest.
Open Research
Data Availability Statement
The data that support the findings of this study are available in the supplementary material of this article.




