Bioactive Luminescent Silver Clusters Confined in Zeolites Enable Quick and Wash-Free Biosensing
Abstract
The simultaneous capture and detection of biomolecules is crucial for revolutionizing bioanalytical platforms in terms of portability, response time and cost-efficiency. Herein, we demonstrate how the sensitivity to external stimuli and changes in the local electronic environment of silver clusters lead to an advantageous biosensing platform based on the fluorometric response of bioactive luminescent silver clusters (BioLuSiC) confined in faujasite X zeolites functionalized with antibodies. The photoluminescence response of BioLuSiC was enhanced upon immunocomplex formation, empowering a wash-free and quick biodetection system offering optimal results from 5 min. Proteins and pathogens (immunoglobulin G and Escherichia coli) were targeted to demonstrate the biosensing performance of BioLuSiC, and a human serum titration assay was also established. BioLuSiC will pave the way for innovative bioanalytical platforms, including real-time monitoring systems, point-of-care devices and bioimaging techniques.
Biosensing platforms based on the optoelectronic responses of metal nanoclusters are a flourishing field. Oligonucleotides and proteins have been utilized as biocompatible templates confining metal clusters to establish (bio)sensing systems targeting heavy metal ions (e.g., Hg2+, Cu2+, Ag+, Pb2+, Fe3+)1-4 and some biomolecules such as cysteamine, hemin, dopamine, adenosine triphosphate (ATP), microRNA or DNA5-9 using diverse detection mechanisms, including cluster formation upon analyte detection,1 fluorescence quenching by hindering cluster formation upon analyte detection,10 or in some cases a photoluminescent red-shift/quenching due to an energy transfer phenomenon.2, 11 The resulting analytical systems are generally advantageous in terms of limit of detection (i.e. in the nM range)2, 12 and response time (10 to 40 min)3, 6 when compared to conventional biodetection techniques such as enzyme-linked immunosorbent assay (ELISA), which generally require several steps/reagents to capture and detect the analyte, respectively.13, 14 Nevertheless, as observed in the summary of the state-of-the-art in Table S1–S2 in the Supporting Information, luminescent clusters have not been explored in wash-free biosensing or a biosensing system that performs capture and detection of the analyte in a single step, thus offering a homogeneous assay.15-17 In this context, photoluminescent silver clusters confined in zeolites through a ship-in-a-bottle approach followed by an activation procedure, have been poorly employed in (bio)sensing platforms.18, 10
Several reports indicate that the emission of metal clusters of around 6 to 10 Å19 confined in zeolites is mostly controlled by the electronic environment determined by neighboring species surrounding the metal cluster conferring them a high versatility and modulation in their emissive response.20, 21 Therefore, by employing molecules of different nature, the electronic environment surrounding the cluster could suffer changes, producing a response in the emission of the entire system, which can be observed as a wavelength shift or as an increase/decrease in the emission intensity, depending on the affinity of these molecules to the metal cluster.21 Hence, as illustrated in Scheme 1, we hypothesized that the sensitivity of these clusters to external stimuli and changes in the local environment could potentially allow for the fluorometric detection of an analyte.
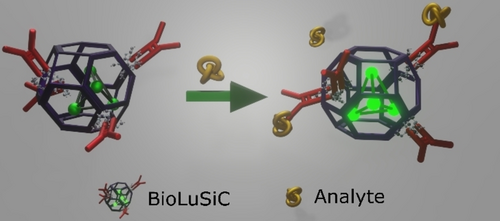
The biosensing mechanism of BioLuSiC. Photoluminescent silver clusters confined into antibody-functionalized zeolites offer simultaneous capture and detection of biomolecules because immunocomplex formation triggers the fluorometric enhancement of the composite material.
Herein, faujasite X containing luminescent silver clusters (FAUX-Ag6) was biofunctionalized with antibodies to develop bioactive luminescent silver clusters (BioLuSiC), a wash-free and quick biosensing platform that captures and detects the analyte in a single-step. Emissive properties of silver clusters in FAUX have displayed higher stability as compared to other sodalite-containing counterparts.22 Experimental details are provided in the SI. Different surface chemistries were investigated to evaluate the bio-functionalization of BioLuSiC in terms of biosensing performance (dynamic range, response time, precision, limit of detection, sensitivity and specificity). Particularly, four amines of different lengths and branches were employed, including diethylenetriamine (DETA), tetraethylenepentamine (TEPA) and two branched polyethyleneimines with different molecular weights, including (Mn) ≈10,000 (PEI); and Mn ≈60,000 in water solution (PEI-H2O). The morphology, surface chemistry and optical properties of the explored materials were determined using scanning electron microscopy (SEM), Fourier transform infrared (FTIR) spectroscopy and UV/Vis spectroscopy, respectively. Subsequently, we demonstrated the innovative biosensing performance of BioLuSiC targeting Immunoglobulin G (IgG) antibodies and Escherichia coli. To prove that BioLuSiC can operate with biological matrices, a serum titration assay was developed, as well.
(FAUX-Ag6) composites were prepared by cation exchange following previously reported procedures,22 displaying a particle size centered at 1.4±0.4 μm, which was determined via SEM images analysis, see Figure 1A–B. Subsequently, to facilitate biofunctionalization through specific antibodies and to build a selective biosensing probe, FAUX-Ag6 surface was modified with amines. According to X-ray diffraction patterns, no significant changes in the crystalline structure of FAUX-Ag6 were observed after modification with the proposed amines, see Figure S1 in the SI. FTIR was employed to demonstrate the resultant amino modification. As shown in Figure 1C, in all samples, FTIR spectroscopy reveals peaks of medium intensity at 560, 671, 739 cm−1 and an intense peak at 957 cm−1, which are typical of O−Si−O and Al−O zeolite bonds.23 The low intensity peak at 1639 cm−1 and the broadband at 3357 cm−1 in the FAUX-Ag6 sample before amino modification have been related to characteristic vibrations of O−H bonds of water molecules internalized within the zeolite structure and to hydroxyl groups present onto the surface of the zeolite.24 The samples modified with PEI, PEI-H2O and TEPA display two peaks of low intensity at 2850 and 2920 cm−1, corresponding to symmetric and antisymmetric stretching of C−H bonds of the amine structures. A broad peak at 3266 cm−1 associated to N−H bonds, was observed in the four amino modified samples and two peaks of medium intensity at 1147 and 1208 cm−1 associated to stretching vibrations of the C−N bond for tertiary amines24, 25 were observed in the samples modified with PEI and PEI-H2O, thereby corroborating the amino-modification process.
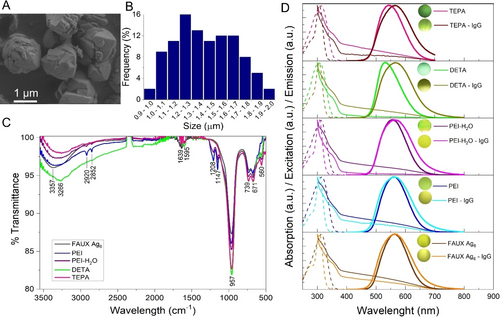
Characterization of BioLuSiC. A. SEM image of pristine FAUX-Ag6. B. Histogram displaying the resulting particle size distribution (n=100). C. FTIR spectra of the explored materials: pristine FAUX-Ag6, PEI, PEI-H2O, DETA and TEPA. D. Absorption (solid lines), excitation (dashed lines) and emission (bold lines) spectra of the explored composites: pristine FAUX-Ag6, PEI, PEI-H2O, DETA and TEPA before and after bioconjugation with IgG antibodies. Excitation wavelength 310 nm.
As observed in Figure 1D, and Table S4 in the SI, UV/Vis spectroscopy revealed that the maximum emission wavelength of pristine FAUX-Ag6 composites in water displayed a blue shift from 562 nm to 560 nm and 557 nm after modification with PEI and PEI-H2O, respectively, whereas a blue shift from 562 nm to 532 nm and 541 nm was exhibited after modification with TEPA and DETA, respectively. Such changes in the emissive properties of silver clusters can be explained by a modification of the resulting electronic environment induced by free electrons from the terminal nitrogen of the amino groups, as predicted by Bun Chan,21 where the smallest linear amine (DETA) probably permeated within the pores of FAUX-Ag6, thereby strongly modifying the electronic states of the silver clusters, whereas TEPA, which is a slightly larger linear amine, could have a less observable effect in the same context. Besides, probably due to their branched structure, the two PEIs mainly remained onto the surface of the zeolite modulating the electronic environment of the silver clusters to a lesser extent.25, 26 Besides, a red shift to approximately 565 nm after antibody incorporation and suspension in phosphate buffer saline (PBS) was observed for both pristine FAUX-Ag6 and amine-functionalized composites. This variation could be related to a change in the ions concentration in the solvent (water/PBS), as observed in the experimental evidence depicted in Figure S2 in the SI.
Given their sensitivity to external stimuli and changes in the local environment, we hypothesized that the photoluminescence of BioLuSiC could be modulated by immunocomplex formation. To prove it, we incubated different configurations of IgG-conjugated BioLuSiC (pristine FAUX and amino-modified) with different concentrations of IgG-FITC (from 0.3125 to 10 ng/mL), the target of this model biosensing system, which was an anti-human Immunoglobulin antibody labeled with fluorescein isothiocyanate (FITC) and blank samples. The excitation and emission wavelengths of BioLuSiC were centered at 310 and 585 nm, respectively; therefore, in this series of experiments the detected fluorescence emission was not derived from FITC because there was no significant spectral overlap of the excitation of BioLuSiC and FITC. In fact, FITC was employed as a reporter to confirm immunocomplex formation. Figure S3 (see SI) provides detailed experimental evidence of immunocomplex formation via BioLuSiC. We observed that BioLuSiC was able to report the presence of the analyte (IgG-FITC antibodies) by increasing the intensity of the resulting photoluminescence without changing the wavelength emission, see Figures S3–S7 and Tables S6–S8. This intensity modulation could be ascribed to an indirect modification of the electronic environment via an antenna effect,27 where the luminescent donor system is depicted by silver clusters, while the non-luminescent acceptor is represented by surface zeolite molecules that undergo specific modifications during antibody-analyte interactions, inducing an energy transfer from the non-luminescent immunocomplex on the surface of the zeolite to the luminescent silver clusters, modifying the overall emission of the system. Hence, BioLuSiC was proven useful as a biosensing probe, leading to a wash-free and quick biosensing platform.
To optimize the analytical performance of IgG-decorated BioLuSiC for IgG-FITC detection, a real-time monitoring process was established every 5 min for 90 min, and the aforementioned multiple configurations of BioLuSiC were assessed in terms of surface functionalization (depending on the amino modification or its absence), concentration of the antibodies functionalizing BioLuSiC (from 50 to 200 μg/mL) and concentration of BioLuSiC (from 58 to 233 μg/mL) in a constant volume (50 μL). To this end, considering a nonlinear mathematical model to characterize the analytical behavior of this biosensing system, we employed the limit of detection (calculated through the interpolation of the blank plus three times its standard deviation in the corresponding calibration curve), the rate constant k of the corresponding calibration curve, the coefficient of determination (R2) and the coefficient of variation (%CV) as quantitative indicators of the resulting analytical sensitivity, quality of the curve fitting and analytical precision, see Figure S5–S55 and Table S6–S56 in the SI. As observed in Table S49 in the SI, given the resulting indicators, DETA modification yielded the optimal BioLuSiC configuration with IgG conjugation occurring at 200 μg/mL, and a final concentration of [BioLuSiC] at ≈117 μg/mL, obtaining a relatively stable k value from 20 to 40 min, which suggests that the biosensing system remains stable during this interval. The emission stability troughout time of BioLuSiC functionalized with DETA is much better than the emission stability of BioLuSiC non-funtionalized with amines, which could probably be due to a buffer effect caused by DETA, which is the amine that offers closer proximity to the silver clusters when compared with the other amines. Since the emission of the clusters depends on the electronic environment of their near vecinity, the proximity of DETA to the silver clusters could render a more stable system when compared with the non-functionalized system. At 20 min, BioLuSiC targeting IgG-FITC exhibited a limit of detection of 250.2035 pg/mL, the minimum value for the rate constant k (0.00112 a.u.) and a coefficient of determination R2 of 0.9987, whereas the CV accounted from 2.33 to 4.41 %, which is less than 15 % and thus acceptable for immunoassays.28 This optimal analytical performance is displayed in Figure 2A–C. It is worth mentioning that the achieved limit of detection is comparable to that offered by a conventional ELISA.29
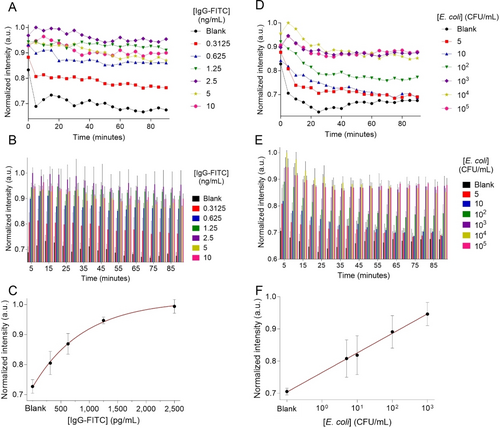
BioLuSiC as a biosensing platform. A–C. IgG-FITC antibodies detection. D–F. Escherichia coli detection. Surface configuration: DETA. A,D. Kinetic analysis during 90 min of biosensing reaction. B,E. Bar charts depicting kinetic behavior and respective standard deviation. C. Calibration curve at 20 min of reaction. F. Calibration curve at 5 min of reaction. The excitation wavelength was 310 nm, and the detection wavelength was 585 nm. BioLuSiC complexed with antibodies (Ab) was prepared at a final concentration of [BioLuSiC]≈117 μg/mL and [Antibody]=200 μg/mL. The error bars represent the standard deviation of three parallel experiments.
We also evaluated the analytical specificity of BioLuSiC modified with DETA by adding high concentrations of BSA (1 μg/mL) as an interfering molecule in the immunoreaction, see Figure S56–S57 and Tables S57–S58 in the SI. In this series of experiments, we observed that the conjugation occurring at 800 μg/mL of IgG offered the best specificity when compared with other conjugations occurring at lower concentrations of the antibody (i.e., 200 μg/mL of IgG). Hence, we concluded that the interference of non-target molecules can be minimized by increasing the abundance of bioactive sites in BioLuSiC. Besides, both the sensitivity and dynamic range of BioLuSiC can be modulated by varying the concentration of antibodies decorating the zeolite surface, thus expanding the dynamic range up to four times by increasing the concentration of antibodies from 200 to 800 μg/mL as observed in Figures S54–56 and Tables S55–56 in the SI.
To prove the highly transformative character of BioLuSiC, we prepared another bioprobe targeting pathogens, namely anti-Escherichia coli-conjugated BioLuSiC modified with DETA. Biofunctionalization was carried out with 200 μg/mL of anti E. coli antibody and different final concentrations of this bioprobe were evaluated (58–233 μg/mL). A linear mathematical model with logarithmic scale in the x-axis was used to fit the behavior of this biosensing system (a value of 0.001 was used to model the blank sample). As shown in Figure S58–S60 and Tables S59–S61 in the SI, anti-E. coli-functionalized BioLuSiC at ≈117 μg/mL offered the optimal analytical behavior according to the employed indicators, which is consistent with the previous optimization targeting IgG. The slope of the biosensing response of BioLuSiC targeting E. coli remained relatively constant from 5 to 20 min, see Table S60. Under optimal conditions, BioLuSiC allowed for the detection of E. coli at 5 min of immunoreaction, with a limit of detection of 4.8983 CFU/mL and R2 of 0.9977, see Table S59 in the SI. This also proves the high analytical sensitivity as well as the versatility of the BioLuSiC approach.
Finally, to prove that BioLuSiC is technically sound for the analysis of biological matrices, as a proof-of-concept, a human anti-IgG antibody titer serological assay was established by analyzing IgG in the sera of three volunteers. As observed in Figure 3 and S61, BioLuSiC modified with DETA was conjugated with 200 μg/mL of anti-IgG and human sera were diluted from 64,000 to 512,000 times. The biosensing system offered the optimal response at 5 min of immunoreaction, displaying a photoluminescent intensity that was clearly distinguished from that of the blank sample. An increase in the photoluminescence intensity of the samples diluted from 512,000 to 256,000 times was observed, respectively, and a subsequent saturation was unfolded. This series of experiments proved that BioLuSiC can be potentially useful for the analysis of human serum or other biofluids.
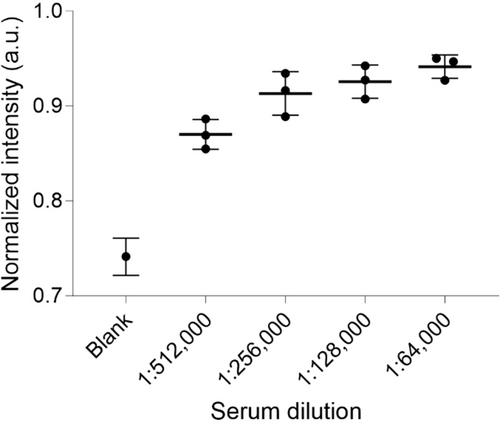
Human IgG antibody titer serological assay using BioLuSiC. Surface configuration: DETA. Evaluation at 5 min of interaction with human serum dilutions of three different volunteers. BioLuSiC complexed with antibodies (Ab) was prepared at a final concentration of [Ab]=200 μg/mL and [BioLuSiC]≈117 μg/mL. The box plots display the median and the extreme values of the respective distribution. Excitation wavelength 310 nm, detection wavelength 585 nm.
In summary, we developed a straightforward and low-cost biosensing system ($0.51 per assay, see Table S62 in SI), offering a conceptually innovative wash-free and quick optical biosensing system that only requires a single antibody to capture and detect the analyte. The BioLuSiC architecture/biosensing principle can be extended to other types of frameworks embedding silver clusters (i.e. LTA zeolite) and/or biorecognition elements (ex., aptamers). Although the overall concept was demonstrated using a microplate reader, we are confident that BioLuSic will pave the way for miniaturized bioanalytical platforms including real-time monitoring systems, point-of-care devices, bioimaging techniques and theranostic approaches.
Acknowledgments
The authors acknowledge the financial support from CONACYT, grant numbers 376135 and A1-S-44458. The authors are grateful to Wiley and Universidad Nacional Autónoma de México (UNAM) for signing an Open Access Agreement. The authors thank the volunteers who donated serum and Clariant for the kind donation of pristine zeolites. The authors gratefully thank Maria Christian Albor for SEM characterization conducted at CIO facilities.
Conflict of interest
The authors declare no conflict of interest.




