Stable Isotope Phosphate Labelling of Diverse Metabolites is Enabled by a Family of 18O-Phosphoramidites**
A previous version of this manuscript has been deposited on a preprint server (https://doi.org/10.33774/chemrxiv-2021-0r9g7).
Abstract
Stable isotope labelling is state-of-the-art in quantitative mass spectrometry, yet often accessing the required standards is cumbersome and very expensive. Here, a unifying synthetic concept for 18O-labelled phosphates is presented, based on a family of modified 18O2-phosphoramidite reagents. This toolbox offers access to major classes of biologically highly relevant phosphorylated metabolites as their isotopologues including nucleotides, inositol phosphates, -pyrophosphates, and inorganic polyphosphates. 18O-enrichment ratios >95 % and good yields are obtained consistently in gram-scale reactions, while enabling late-stage labelling. We demonstrate the utility of the 18O-labelled inositol phosphates and pyrophosphates by assignment of these metabolites from different biological matrices. We demonstrate that phosphate neutral loss is negligible in an analytical setup employing capillary electrophoresis electrospray ionisation triple quadrupole mass spectrometry.
Introduction
Isotopologues are molecular entities that differ only in isotopic composition.1 Their chemical properties are almost identical, yet they can be readily distinguished by several analytical methods. This ambivalence has been the basis for many applications in chemistry, biology and medicine for decades.2-7 The synthesis of isotopologues is called isotope labelling and can be categorised into stable isotope labelling (SIL) and radiolabelling.8
The phosphate group is among the most important functional groups in organisms, playing a pivotal role in energy transfer, enzyme activation and genetic information storage.9 For more than 50 years, isotope labelling of phosphate groups has contributed to our understanding of phosphate reactivity and function. SIL in the context of phosphates focuses on oxygen isotopes, since the P-isotopes 32P and 33P are beta-emitting radionuclides (t1/2, 32P=14 d, t1/2, 33P=25 d).10 Potentially, both 17O and 18O are suitable isotopes for phosphate SIL. The higher natural abundance of 18O and its mass-shift of M+2 render this isotope more useful for mass-spectrometry-based applications, while important applications for the NMR-active 17O nucleus exist.11
Since the 1970s 18O-phosphates have been used as probes to clarify questions in chemistry and biology (Figure 1). 18O-phosphates were indispensable, for example, in the elucidation of structure, reactivity, and reaction mechanisms involving phosphate esters and anhydrides. Already in 1977, Gorenstein et al. elucidated metaphosphate-involving mechanisms in phosphate ester hydrolysis reactions by exploiting 18O-kinetic isotope effects.12 In 1988, Takeuchi et al. used 18O-ATP to determine Mg2+ coordination sites by Raman spectroscopy.13 In 1990, Cleland described how 18O-labelled phosphate esters could be used to study phosphate transesterification mechanisms. He emphasised that “the use of [secondary] 18O […] isotope effects has proved a powerful tool for studying transition state structure in phosphoryl transfer reactions.”14 Later, similar approaches to clarify the catalytic mechanism of RNase A were developed.15 Additionally, Lee et al. investigated 18O-kinetic leaving group effects in 2011 to gain mechanistic evidence for an SNi-type reaction of a trehalose-6-phosphate synthase.16 Another application of 18O-phosphate esters was presented by Hamasaki et al. in 2013 by synthesising 18O-labelled RNA as an alternative to fluorescently labelled RNA. The 18O-RNA was then visualised by isotope microscopy in human cells.17
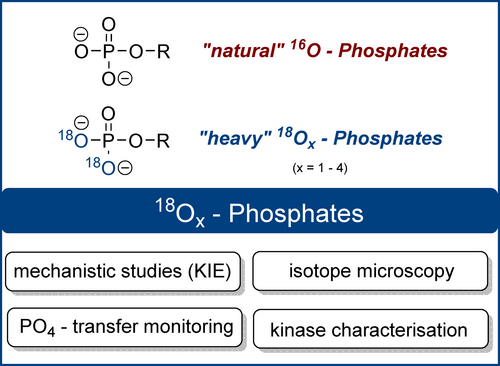
Structures of natural 16O4-phosphates and labelled 18Ox-phosphates. 18O2-phosphate is shown as an example (KIE: kinetic isotope effect).
Phosphate groups, usually from P-anhydrides (e.g. ATP), can be transferred to nucleophiles in vivo. Detailed studies into phosphate transfer events are essential for our understanding of complex cellular processes.18 In this context, 18O-phosphates proved particularly powerful. In 1976, Midlefort and Rose used an 18O-isotope scrambling method to clarify transient dephosphorylation reactions of ATP catalysed by glutamine synthetase.19 Phelan et al. characterised adenylation efficiency of non-ribosomal peptide synthetases by measuring pyrophosphate exchange of 18O4-ATP.20 Scian et al. investigated the mechanism of 18O4-ATP hydrolysis catalysed by human P-glycoprotein.21 Boyer elucidated ATPase's mechanism by analysing 18O-exchange between 18O4-ATP, Pi and water under uncoupling conditions. In his Nobel lecture he stated: “The use of the 18O-exchange measurements to study the process provided a crucial insight.”22
γ-18Ox-ATP phosphorylation analysis also became a key tool in kinase characterisation, because the 18O-approach is non-radioactive and does not require fluorescent substrates.
Zhou et al. used 18O4-ATP and MS/MS to identify the phosphorylation sites within substrates of a human tyrosine/serine kinase.23 Analogously, Sulbaran et al. characterised kinase phosphorylation sites on myosin regulatory light chains.24 Fu et al. reported a kinase assay based on 18O4-ATP, to determine effectiveness and specificity of kinase inhibitors.25 Molden et al. demonstrated the application of γ-18O4-ATP in nucleo to follow protein phosphorylation rates.26 Furthermore, several proteomics-based assays with 18Ox-ATP (x=2–4) were developed, identifying substrates of specific kinases of interest.27-31
These various applications underline the tremendous potential of 18O-labelled phosphates to serve as tool compounds for addressing chemical and biological questions. Notably, most methods rely on synthetically well-studied 18Ox-ATP as a probe. Other 18O-labelled phosphates or phosphoanhydrides are scarce. The high potential of 18O-labelled phosphates beyond 18O-ATP is insufficiently exploited in large part because of a “dearth of synthetic methods”.32
The most common strategies are based on hydrolysis of P−Cl bonds (Scheme 1 A). Especially 18O4-Pi (2) is an important building block. Formed by hydrolysis of PCl5 (1) in H218O, 18O4Pi (2) may undergo SN-reaction with activated PV-electrophiles (3), leading to 18O4-containing phosphoanhydrides (4).13, 33, 34 Furthermore, hydrolysis of phosphorodichloridates in H218O is suitable to access 18O2-monophosphates.35 18O-labelled RNA was obtained by Hamasaki et al. by using H218O as solvent in the oxidation step of solid-phase synthesis.17 Also, the Stec reaction is suitable to incorporate 18O from benzaldehyde into phosphoramidates.36 In addition, enzymatic approaches were described to access γ-18O4-ATP and γ-18O4-GTP from 18O4-Pi (2).37 Our group presented a method for γ-18O2-NTP (10) synthesis based on an 18O2-dibenzyl-P-amidite 8.38 However, during H2-reduction, some nucleobases (C, G) were also reduced and substantial amounts of P-anhydrides (>20 %) were hydrolysed. Therefore, even though there are several methods available for the synthesis of 18Ox-phosphates, significant improvements regarding substrate scope, yields, scalability, 18O-enrichment ratios, and reduction of H218O consumption are required to unleash the true potential of 18O-phosphate labelling.
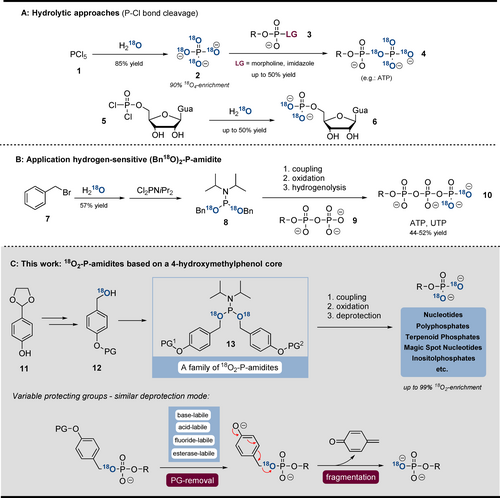
A) hydrolytic approaches towards 18O4-phosphates. B) Application of (Bn18O)2-P-amidite towards labelled NTPs. C) New synthetic concept towards 18O2-phosphates. The general deprotection mode of all 18O-P-amidites developed is shown.
Herein, we describe a unifying and modular concept for the synthesis of 18O2-phosphate products, addressing the limitations specified above. The concept involves a family of 18O2-phosphoramidites (13), equipped with various protecting groups (removable by piperidine, DBU, H+, F−, or esterases (Scheme 1 C)). After triggering deprotection, the fragmentation proceeds analogously via self-immolation of a divergently modified 18O-4-(hydroxymethyl)phenol adapter (Scheme 1 C). The broad applicability of the P-amidite family is demonstrated by the synthesis of various important naturally occurring phosphates: nucleotides, inorganic polyphosphates (polyphosphates thereafter), terpenoid phosphates, magic spot nucleotides, inositol phosphates, and DNA. The synthesis relies on telescoping sequences of phosphitylation, oxidation and deprotection39 and usually results in high yields and complete 18O incorporation while enabling gram-scale synthesis of labelled products. We demonstrate the utility of phosphate-labelled inositol phosphates in capillary electrophoresis mass-spectrometry-based (CE-ESI-QqQ) analytics. Our CE-ESI-QqQ setup enables accurate assignment of analytes without neutral loss from diverse biological matrices.
Results and Discussion
Synthesis of Functionalised 18O-4-(Hydroxymethyl)phenols
The key intermediate on the way to 18O-P-amidites is 18O-4-(hydroxymethyl)phenol (16, Scheme 2). Its synthesis commenced from 4-hydroxybenzaldehyde (14) by acetalisation in 50 % yield on multi-gram scale.40 To ensure the required purity (>99 %) of acetal 11, we developed a crystallisation procedure from cyclohexane/ethylacetate. Other acetalisation conditions were unsuccessful, as p-donor substituted benzacetals are highly prone to hydrolysis.41
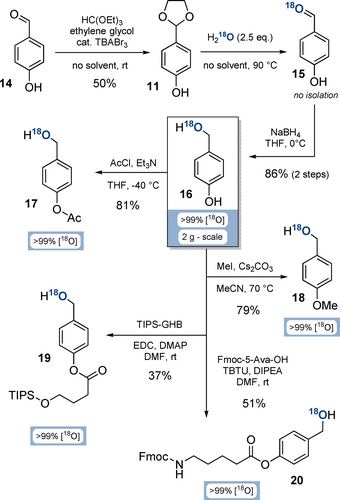
Syntheses of functionalised 18O-(hydroxymethyl)phenols from 4-hydroxybenzaldehyde (16). Abbreviations: TBABr3: tetrabutylammonium tribromide; THF: tetrahydrofurane; TIPS-GHB: 4-((triisopropylsilyl)oxy)butanoate; EDC: 1-ethyl-3-(3-dimethylaminopropyl)carbodiimide; DMAP: 4-dimethylaminopyridine; DMF: dimethylformamide; Fmoc-5-Ava-OH: 5-(Fmoc-amino)valeric acid; TBTU: 2-(1H-benzotriazole-1-yl)-1,1,3,3-tetramethylaminium tetrafluoroborate; DIPEA: diisopropylethylamine.
The high tendency of acetal 11 to hydrolyse was subsequently exploited by melting a mixture of 11 and H218O (2.5 equiv, >99 % isotopic purity). 18O-Labelled aldehyde 15 was unstable towards humidity-induced H216O back-exchange, resulting in loss of labelling efficiency. To overcome this issue, 15 was directly reduced with dry NaBH4 in triethylene glycol dimethyl ether to access key building block 18O-(hydroxymethyl)phenol 16 in 86 % yield over 2 steps, and with an isotopic purity of >99 % on a multi gram-scale.
Subsequently, the phenol group of 18O-diol 16 was functionalised divergently to access various P-amidite precursors under conservation of the 18O isotopic purity (Scheme 2): Acetylation towards 17 with AcCl and Et3N was performed in 81 % yield on gram-scale. The methylated derivative 18 was accessed in 79 % yield on gram-scale by using MeI and Cs2CO3. Silyl-protected derivative 19 was obtained in 37 % yield by esterification of 4-TIPSO-butanoate with EDC and DMAP. Finally, 18O-BigFM-alcohol (20) was synthesised from 5-Fmoc-aminovaleric acid, TBTU and DIPEA in 51 % yield.
Synthesis of 18O-P-Amidites
In the next steps, the functionalised 18O-alcohols were converted into the corresponding 18O-P-amidites (Scheme 3). Symmetric 18O2-P-amidites (22) could be accessed by subjecting functionalised 18O-alcohols 17–20 to SN-conditions with iPr2N-PCl2 (21) and Hünigs base (Scheme 3 A). 18O-P-diamidites (24) were synthesised analogously from (iPr2N)2P-Cl (23) and were then transformed with ETT and 18O-alcohols to obtain unsymmetric 18O2-P-amidites (26). Application of these methods to 18O-alcohols 17–20 provided access to a family of 18O-P-amidites (Scheme 3 B), equipped with orthogonal protecting groups. The isotopic enrichment was preserved during all transformations, and the P-amidites were isolated in 18O/16O-ratios of >99 %. 18O2-AB-P-Amidite 27 was synthesised from 18O-AB-alcohol 17 in 69 % yield on gram-scale. Its cleavage can be triggered with amine nucleophiles (e.g. pyrrolidine) or esterases.42 18O2-PMB-P-Amidite 28 was obtained from 18O-PMB-alcohol 18 in 72 % yield on gram-scale. PMB is labile towards acids, such as trifluoroacetic acid.43 18O-AB-P-Diamidite 29 was accessed from 18O-AB-alcohol 17 in 42 % yield and was transformed further with 18O-PMB alcohol 18 to unsymmetrically modified 18O2-AB-PMB-P-amidite 30.
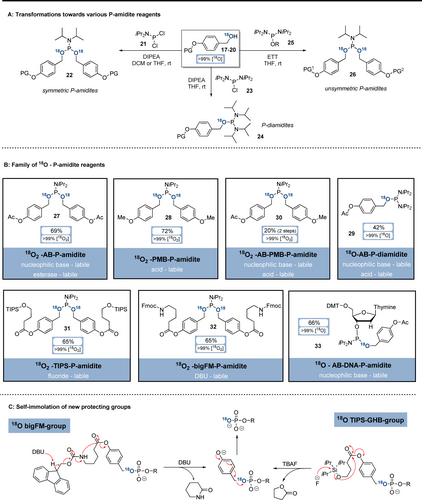
Synthesis of 18O-P-amidites (A,B). Self-immolation of novel phosphate protecting groups after the trigger (C). Abbreviations: AB: para-acetoxybenzyl;PMB: para-methoxybenzyl, DCM: dichloromethane; ETT: 5-ethylthio-1H-tetrazole.
In addition, the novel P-amidites 31 and 32 based on the 4-(hydroxymethyl)phenol core were developed to enable further orthogonal deprotection conditions. TIPS-containing 18O-alcohol 19 was transformed to silyl-protected 18O2-P-amidite 31 in 65 % yield. Its deprotection mechanism (Scheme 3 C) includes TBAF-induced TIPS-removal triggering 5-ring lactonisation and fragmentation towards unprotected 18O-phosphates. 18O2-BigFM-P-amidite 32 was synthesised from 18O-BigFM-alcohol 20 in 65 % yield. The deprotection mechanism of 32 (Scheme 3 C) is based on a DBU-induced self-immolation cascade of Fmoc-elimination, 6-ring lactamisation and fragmentation, liberating 18O-phosphates.
Finally, the DNA precursor 18O-AB-DMT-thymine-P-amidite 33 was accessed in 66 % yield from 18O-AB-alcohol 17 and the corresponding P-diamidite. P-amidite 33 is fully compatible with automated solid-phase DNA-synthesis, as the AB-group is removed under comparable conditions required for β-CE-group cleavage. The whole family of 18O-P-amidite reagents can be stored for months at −20 °C without substantial decomposition and deterioration of isotopic purity.
Synthesis of 18O-Phosphorylated Products
18O2-P-amidites 27–33 were then applied in the syntheses of diverse 18O-labelled phosphates mostly according to methods developed in our group in recent years.39, 44 The general 18O2-phosphorylation reactions (Figure 2, top) were based on ETT-promoted phosphitylations of alcohols and phosphates by 18O2-P-amidites followed by oxidation and deprotection. In contrast, 18O-P-diamidites were used for phosphate dimerisation reactions. In many cases, the chemoselective nature of P-amidite phosphitylation was exploited, so unprotected nucleotides were suitable starting materials. Detailed synthesis schemes are provided in the SI.
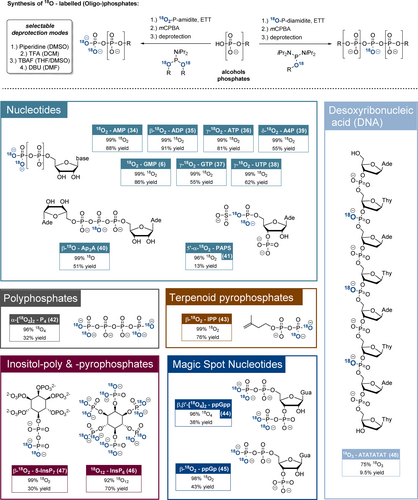
Top: Main synthetic concept towards the 18Ox-labelled natural products. DNA solid-phase synthesis is not shown. Bottom: Overview of 18O-labelled phosphate products, including yields and 18Ox-enrichment rates. The yields usually refer to the 18O2-phosphate introduction procedure (coupling, oxidation, global deprotection) only, except in the cases of PAPS (41), ppGp (45) and DNA (see SI for detailed information). In the case of PAPS (41) the product represents an isotopomeric mixture, where only one isotopomer is shown. Abbreviations: DMSO: dimethylsulfoxide; mCPBA: meta-chloroperbenzoic acid; DBU: diazabicycloundecene; AMP: adenosine-5’-monophosphate; GMP: guanosine-5’-monophosphate; ADP: adenosine-5’-diphosphate; ATP: adenosine-5’-triphosphate; GTP: guanosine-5’-triphosphate; UTP: uridine-5’-triphosphate; A4P: adenosine-5’-tetraphosphate; Ap3A: diadenosine triphosphate. IPP: isoprenylpyrophosphate; ppGpp: guanosine-3’,5’-bisdiphosphate; ppGp: guanosine-3’-phosphate-5’-diphosphate; PAPS: adenosine-3’-phosphate-5’-phosphosulfate.
We initially evaluated the reagents regarding access to 18O-labelled nucleotides (Figure 2, bottom). 18O2-AMP (34) and 18O2-GMP (6) were synthesised from the corresponding 2′,3′-isopropylidene nucleosides using 18O2-PMB-P-amidite 28 in yields of 88 % and 86 % as well as excellent 18O2-enrichment ratios of 99 % after global deprotection. Nucleoside oligophosphates NPx were accessed from NPx−1 precursors by using 18O2-AB-P-amidite 27. Accordingly, β-18O2-ADP (35), γ-18O2-ATP (36), γ-18O2-GTP (37), γ-18O2-UTP (38) and δ-18O2-AP4 (39) were isolated in yields of 55–91 % and consistently with high 18O2/16O2 ratios of >99 %. Notably, 18O2-AMP and γ-18O2-ATP were synthesised on gram-scales, underlining the method's robustness and the significant advantage of introducing the stable isotope labels in the final steps. Chemoselective dimerisation of AMP using 18O-AB-diamidite 29 gave β-18O-Ap3A (40) in 51 % yield and with 99 % 18O-enrichment.
As additional targets, the first 18O-labelled polyphosphate representative 18O4-P4 (42) was accessed from pyrophosphate in 33 % yield using a bisphosphorylation procedure with 18O2-AB-P-amidite 27 and with a >95 % 18O4-enrichment. The terpenoid phosphate β-18O2-isoprenylpyrophosphate (43) was synthesised from isoprenylphosphate and TIPS-protected 18O2-P-amidite 31 followed by deprotection in 78 % yield and 99 % 18O2-enrichment.
18O4-ppGpp as a representative of the important magic spot nucleotides was synthesised from guanosine-3,5-bisphosphate (pGp) using 18O2-BigFM-P-amidite (32) in a chemoselective bisphosphorylation procedure.45 Other 18O2-P-amidites failed in the construction of magic spot nucleotides as the guanosine-2’,3’-cyclophosphate-5’-pyrosphosphate (ppGcp) byproduct was formed quantitatively under deprotection conditions. Only when applying 18O2-BigFM-P-amidite, DBU-induced deprotection led to a 1:1 mixture of 18O4-ppGpp (44) and 18O2-ppGcp. The products were separated and 18O2-ppGcp was transformed into 18O2-ppGp (45) by RNase T2. Consequently, 18O4-ppGpp (44) and 18O2-ppGp (45) originated from the same reaction mixture in yields of 38 % and 43 %. 18O4- and 18O2-enrichment ratios were 96 % and 98 %, respectively.
Furthermore, several 18O-labelled inositolphosphates were synthesised. 18O12-InsP6 (46) with twelve 18O labels (M+24) was accessed directly from myo-inositol with 18O2-AB-P-amidite 27 in 70 % yield and with 92 % 18O12-enrichment. 18O2-5-InsP7 was synthesised according to literature precedent from AB10-(β-CE)2-InsP6 in 30 % yield and >99 % 18O2-enrichment with the labels on the β-phosphate. In this case, unsymmetric 18O2-AB-PMB-P-amidite (30) had to be used to ensure a >98 % purity of the product. Importantly, AB-protected and thus cell-permeable 18O-prometabolites46 were isolated as intermediate products, offering possible applications for in cellulo transphosphorylation experiments. The flexible 18O-(hydroxymethyl)phenol-based P-amidites compared favourably to the known (Bn18O)2-P-amidite, which was used for accessing 18O2-1-InsP7 (see SI).47 After hydrogenation, 18O2-1-InsP7 was isolated in a yield of 17 % and an 18O2-enrichment ratio of 92 %. Moreover, partial anhydride hydrolysis during deprotection resulted in the formation of 20 % InsP6, which is difficult to remove.
The 18O-labelled DNA sequence 18O3-5’-ATATATAT (48) was accessible by automated solid-phase synthesis using DMT-dT-P-(18OAB)-NiPr2 (33) and commercial DMT-dA(N-Bz)P-(β-CE)-NiPr2. AB- and β-CE-groups were deprotected using an aqueous mixture of ammonia and methylamine (AMA). The DNA sequence was isolated in 9.5 % yield and 75 % 18O3-enrichment. The 18O-DNA and its 16O3-sibling show clearly separated mass spectra, despite the comparably moderate enrichment. Probably the aqueous DNA-deprotection and oxidation steps impact the enrichment in comparison to other natural products.
The synthesis of another important cofactor,48 18O2-labelled adenosine-3’-phosphate-5’-phosphosulfate (41, PAPS) is shown in detail in Scheme 4. Our synthesis sequence relies on telescoping of the first five steps and ensures selective 18O-incorporation at the 5’-position: Adenosine (49) was selectively 5’-phosphitylated using 18O2-AB-P-amidite 27. Subsequently, cyclophosphate 52 was formed with (FmO)-P-diamidite (51) in a ring-forming phosphitylation reaction. After oxidation and basic deprotection, RNase T2 catalysis induced regioselective cyclophosphate hydrolysis towards adenosine-3’-5’-bisphosphate (54, pAp) in 44 % yield and with 96 % 18O2-enrichment after 5 steps without intermediate purification. pAp (54) was chemoselectively bis-sulfated by triethylamine-N-sulfonic acid (55) according to Horwitz et al.49 RNase T2 catalysis led to a net 3’-desulfation, delivering 18O2-PAPS (41) in 29 % yield from pAp and with 96 % 18O2-enrichment. This novel synthesis of 18O2-PAPS (41) further demonstrates the great utility of (hydroxymethyl)phenol-based 18O2-P-amidites for isotope incorporation into complex molecular frameworks.
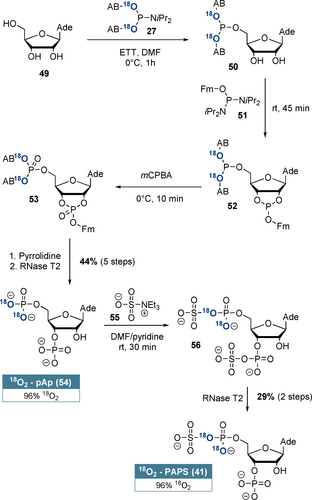
Synthesis of regioselectively labelled 18O2-PAPS (41) from adenosine (49). pAp was the only intermediate product that required purification.
Determination of InsPs in Biological Samples by CE-MS
We have recently reported the use of 13C-labelled inositol phosphates and pyrophosphates as internal references for quantitative mass spectrometry.50 This approach requires the transformation of 13C-labelled glucose into myo-inositol with three consecutive enzymatic reactions.51 18O labelling could be an interesting alternative due to the lower cost of the 18O label and since it would not require the initial enzymatic conversion of glucose into inositol. However, these advantages would only be relevant if neutral phosphate loss resulting from in-source fragmentation could be avoided.52
To evaluate the utility of the new internal references ([18O12] InsP6 (46), [18O2] 5-InsP7 (47), [18O2] 1-InsP7), we used several biological extracts (mammalian cells, slime-mold, plant), which were spiked with the compounds. Next, we resolved the extracts by capillary electrophoresis and analysed them by on-line mass spectrometry. While an ESI-QToF system produced substantial neutral loss (ca. 10 %),50 this was not the case in an ESI-QqQ system, a common MS analyser for quantitative studies, where we found less than 0.3 % of neutral phosphate loss (Supplementary Figure 11).
Figure 3 shows electropherograms of several analysed samples spiked with heavy isotopologues. Clearly, the reference compounds co-migrate with their light analytes, enabling ready assignment of the different isomers, irrespective of the matrix. For example, we can easily assign the known distinct isomers present in mammalian HCT116 cells (1- and 5-InsP7) and would also be able to quantify them with the internal references. In slime mold and plants, an additional isomer could be detected which we have previously tentatively assigned as 4/6-InsP7.53 Once this new isomer will become accessible as its synthetic isotopologue following the procedures described herein, in-depth studies into its dynamic regulation will become possible.
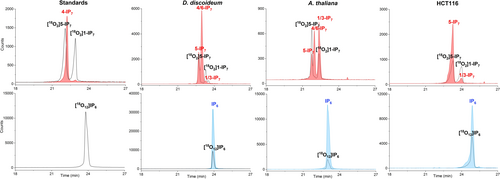
Electropherograms of InsP7 and InsP6 in standards and cell extracts (Dyctyostelium discoideum, Arabidopsis thaliana and mammalian HCT116 cells) spiked with the heavy isotopologues. The spiked-in [18O2] 5-InsP7, [18O2] 1-InsP7, [18O12] InsP6 concentrations are 4 μM, 4 μM and 20 μM, respectively. The detailed CE-ESI-QqQ method is described in the SI.
Conclusion
In summary, we introduce a unique 18O-P-amidite-based approach for the synthesis of various 18O-labelled phosphorylated products. The utility of the new family of reagents is demonstrated by the scalable synthesis of 18O-labelled nucleotides, such as for example, AMP, ADP, ATP, AP4, and PAPS, as well as the challenging magic spot nucleotides. Moreover, synthetically highly demanding 18O-inositolpyrophosphates are accessible. 18Ox-isotopic enrichment ratios of 92–99 % and good yields were obtained consistently, while the labels are introduced in the final steps of the synthesis, which can be a significant advantage. The heavy phosphate labels can be used as internal reference compounds for mass spectrometry applications, as demonstrated herein. Identical migration times allow a straightforward assignment of the isomeric identity of inositol phosphate analytes from different biological matrices, such as mammalian cells, slime mold, and plants. Importantly, our CE-ESI-QqQ setup shows less than 1 % phosphate neutral loss across the board of analytes, paving the way for their accurate quantitation. This novel toolbox will enable further innovative applications of 18O-labels in various fields of research involving nature's favourite modification: the phosphate group.
Acknowledgements
We thank Dr. Manfred Keller from MagRes of the University of Freiburg for a significant amount of time for NMR spectroscopy and Christoph Warth for HRMS measurements. This study was supported by the Deutsche Forschungsgemeinschaft (DFG) under Germany's excellence strategy (CIBSS, EXC-2189, Project ID 390939984). This project has received funding from the European Research Council (ERC) under the European Union's Horizon 2020 research and innovation program (grant agreement no. 864246, to H.J.J). We gratefully acknowledge financial support from the Studienstiftung des Deutschen Volkes, the Brigitte Schlieben-Lange Programm, and Cusanus-Werk. A.S. laboratory is supported by Medical Research Council UK grant MR/T028904/1. Open Access funding enabled and organized by Projekt DEAL.
Conflict of interest
The authors declare no conflict of interest.




