A Simple Homoleptic Gallium(I) Olefin Complex: Mimicking Transition-Metal Chemistry at a Main-Group Metal?
Abstract
The earth-metal olefin complex [GaI(COD)2]+[Al(ORF)4]− (COD=1,5-cyclooctadiene; RF=C(CF3)3) constitutes the first homoleptic olefin complex of any main-group metal accessible as a bulk compound. It is straight forward to prepare in good yield and constitutes an olefin complex of a main-group metal that—similar to many transition-metals—may adopt the +1 and +3 oxidation states opening potential applications. Crystallographic-, vibrational- and computational investigations give an insight to the atypical bonding between an olefin and a main-group metal. They are compared to classical transition-metal relatives.
Transition metal olefin complexes are widely known and used as (pre-)catalysts in a variety of reactions. Since their first discovery in the 1820ies by Zeise,1 they developed into a mature class of compounds with a manifold of applications. Especially the properties of chelating 1,5-cyclooctadiene (COD) to stabilize low valent transition-metal complexes like [M(COD)2]0,+ (M=Ni, Pd, Pt)2, 3 or [ClM(COD)]2/[M(COD)2]+ (M=Rh, Ir)4, 5 sufficiently to be isolated, furnished a large interest into follow-up chemistry. Since the COD-ligands are easily displaced by other ligands such as phosphines, or alternatively may be hydrogenated in the course of the reaction and thus are removed from the metal atom leaving free and reactive coordination sites, they rapidly developed into very useful (pre-)catalysts for a variety of reactions.6 For example, Crabtree's famous catalyst [Ir(COD)(py)(PCy3)]+[PF6]− 4 or the Rhodium analogues7 activate H2 and hydrogenate, but also isomerize and hydroborate alkenes.
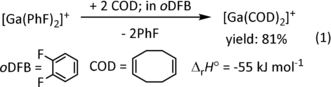
By contrast to transition-metal olefin complexes, homoleptic olefin complexes of a main-group metal remain unknown as isolable bulk compounds. Only a few examples are known under the special conditions of matrix isolation spectroscopy, that is, Li(C2H4)n8 or M(C2H4)n (M=Al,9 Ga,10, 11 In;12 n=1–3) as well as inside a mass spectrometer, that is, [M(C2H4)n]+ (M=Na13 or Al14). Turning to group 13, only a few bulk compounds are related; for example, the Al atom in AlCp3, but not GaCp3, is η2-coordinated by at least one charged [C5H5]−=Cp− ligand showing a related behaviour to olefins.15 Somewhat related are the classical Ga-cyclophane and -arene complexes.16 The first true group 13 olefin complex, the cyclohexene complex [Al(C6F5)3⋅(C6H10)], was published by Stephan et al.16c Very recently, Crimmin reported systems that could be described as AlI olefin complexes or AlIII metallacyclopropanes,17 Aldridge added versions, which include such situations as intermediates of AlI reactions with arenes18 and Inoue reactions of a dialumene with olefins and acetylenes giving the respective dialumina-cyclobutanes.19 Still, the simple access to an isolable bulk homoleptic olefin complex of any main-group metal—most favourably to an entry with switchable redox states that supports ligand substitution by steering ligands—is missing. The delineated profile suggests that possibly Ga or In, with their propensity to occur in the +I and +III oxidation states as well as their known capacity to bind phosphines,20, 21 appeared an interesting target for synthesis. In addition, the use of Ga and In in catalysis is increasing steadily.22 Moreover, we showed that the polymerisation of isobutene (IB) induced by [GaI(arene)2]+ complexes most likely proceeds by formation of a [GaI(IB)x]+ complex (x=3 or 4) that dismutates in the course of the reaction to a cyclo-galla(III) cation.23 Thus, we were interested to prepare a prototype Ga+-olefin complex. Starting point was our facile access to arene complexes of Ga+ salts with the non-reactive weakly coordinating anion (WCA) [Al(ORF)4]− (RF=C(CF3)3) that led to interesting coordination chemistry with a variety of σ-donors including phosphines,20, 21 carbenes,24 pyridines,25 and also crown ethers.26 And although η6-arene-coordination of gallium(I) is known since 1957,27 the classical η2-olefin-coordination was hitherto only matrix isolated10, 11 and calculated28 to occur, but never observed in an isolated compound. Here we report on the prototype complex salt [Ga(COD)2]+[Al(ORF)4]− that contains four η2-bound double bonds in two COD ligands.
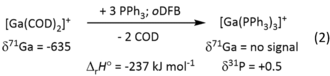
Formation of the complex [Ga(PPh3)3]+ followed from the disappearance of the 71Ga-NMR signal of the COD complex and the appearance of the known 31P-NMR signal of the phosphine complex.21 In addition, Equation (2) is in agreement with B3LYP/D3(BJ)/def2-TZVPP calculations1 and exothermic (exergonic) by −237 (−142) kJ mol−1.1
Molecular Structure of 1: The complex salt shown in Figure 1 contains one Ga+ ion coordinated distorted tetrahedrally by the four double bonds of two COD ligands and includes an almost non-interacting [Al(ORF)4]− anion. The shortest cation-anion H–F (Ga–F) contacts in 1 amount to 264.6 (306.5) pm. They are close to the sum of the van der Waals radii (H+F: 256 pm; Ga+F: 337 pm) and thus should influence the structure only little.29 One COD ligand is bound closer (CODc) and one is further away (CODf) to the Ga+ attractor: dGa-C=285.1(6) to 321.3(6) pm in CODc vs. 297.4(5) to 323.2(5) pm in CODf. All C2Ga-triangles include by 9 to 26 pm asymmetric Ga-C distances. These dGa-C values are in a similar range to the ones observed in several [Ga(arene)2–3]+ complexes with the like counterion [in pm]: 291–308 [Ga(PhF)3]+, 287–303 [Ga(PhF)2]+ or 286–302 [Ga(1,3,5-Me3C6H3)2]+. Yet, they are much longer than the sum of the radii rcov., C+Ga of 203 pm.
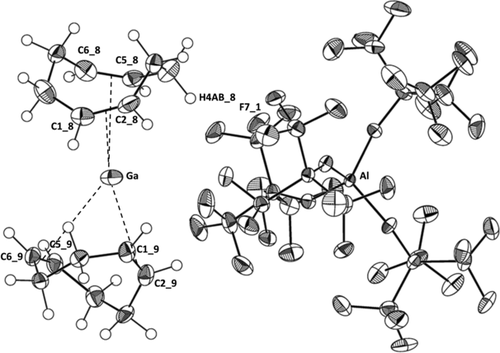
Molecular structure of 1. The carbon atoms of the double bonds (C1 9=C2 9; C5 9=C6 9) and the centroid metal distance are highlighted, as well as the shortest anion–cation contact (dH4AB 8–F7 1=265 ppm). Thermal ellipsoids are set at 50 % probability and hydrogen atoms are included as isotropic spheres of arbitrary radius. See Supporting Information for details and CCDC numbers.
In the tighter bound CODc ligand, dC=C are longer at 136.3(8) and 136.6(8) pm. The two double bonds in CODf are shorter at dC=C=134.0(8) and 132.5(8) pm and resemble more closely to dC=C=134 pm found for free COD by gas phase electron diffraction.30 The only structures that are reasonable to compare are “π-associated” dimers in [(CH3)2Ga-C≡C-Ph)]2 with a very asymmetric coordination of the gallium(III) atom to one triple bond of a second molecule (dGa-C=224.0(6) and 279.0(6) pm)31 or an intramolecular π-type coordination in [GaCl3{Me2Si(C5Me4)(N-t-Bu)}GaCl2]− with an average symmetric Ga-C distance to the GaIIICl2-unit of 239.9(4) pm,32 respectively. Both of the structures contain gallium in the oxidation state +3 and are by far stronger bound, since their multiple bond has anionic character. Therefore, and due to the much larger ionic radius of GaI vs. GaIII, all Ga-C distances in 1+ are by 18 to 99 pm longer than the distances in the cited GaIII compounds.
But how does the structure compare to transition-metal relatives? A mixture of experimental and DFT-calculated data on [M(COD)2]+ with M=Ga, Ag, Ni, Rh as well as free COD are compared in Table 1.
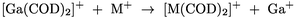 (3)
(3)Parameter |
Ga+[a] |
Ag+ |
Ni+ |
Rh+ |
COD |
|---|---|---|---|---|---|
d(C=C) [pm] |
132.5(8)–136.6(8) av. 134.7 |
134.2(4)–135.2(5) av. 134.5 |
135.0(4)–135.7(4) av. 135.3 |
136.1(4)–137.2(4) av. 136.8 |
134.0[d]
|
d(M-C) [pm] |
285.1(6)–323.3(6) av. 306.7 |
243.2(3)–255.6(3) av. 248.7 |
217.1(3)–228.5(3) av. 222.3 |
222.2(2)–227.3(2) av. 224.1 |
– |
θ [°] |
51.3 |
88.5 |
53.1 |
10 |
– |
ν(C=C) [cm−1][b] Δν(C=C) [cm−1][b] |
1635 (1636) −21 (−35) |
1607 (1589) −49 (−80) |
1570 (1548) −86 (−121) |
1491 (1499) −165 (−179) |
1656 (1669) – |
13C-NMR [ppm][c] |
131.2, 27.4 |
126.0, 28.0 |
paramagnetic33 |
127.7, 29.6 |
128.2, 27.9 |
ΔrH0 [kJ mol−1][e] |
0 |
−193 |
−419 |
−571 |
– |
- [a] This work. [b] Experimental values; italic values in parentheses are calculated (BP86-D3(BJ)/def-TZVP), Δν(C=C) is the difference to the ν(C=C) values of free COD. [c] sp2-CH, sp3-CH2 in COD. [d] From Ref. 30. [e] Isodesmic exchange enthalpies for Equation (3): [Ga(COD)2]+ + M+ → [M(COD)2]+ + Ga+ calculated at BP86-D3(BJ)/def2-TZVP.
With ΔrH0 [Eq. (3)]=−193 to 571 kJ mol−1, all transition-metal complexes are tighter bound than 1+. Solitary exchange with Na+, yielding the clearly electrostatic [Na(COD)2]+ complex, is unfavorable, but only by ΔrH0[Eq. (3)]=+75 kJ mol−1. Apparently, the bonding energetics in 1+ is closer to [Na(COD)2]+ than even the least favorable else investigated silver complex [Ag(COD)2]+ (ΔrH0[Eq. (3)]=−193 kJ mol−1). Therefore, the bonding situation in 1+ and related [M(COD)2]+ complexes including M=Na was investigated by AIM analyses (Table 2).
Parameter |
Rh(COD)2+[a] |
Ag(COD)2+[a] |
Ga(COD)2+[a] |
Na(COD)2+[a] |
Free COD[a] |
|---|---|---|---|---|---|
CPM-C type |
BCP |
TCP |
TCP |
TCP |
– |
ρ XCP, (M-db) |
0.51 |
0.27 |
0.14 |
0.08 |
|
ϵXCP, (M-db) |
1.60 |
1.27 |
0.47 |
0.31 |
|
ρ BCP, (C=C) |
2.22 |
2.33 |
2.36 |
2.36 |
2.39 |
ϵBCP, (C=C) |
0.26 |
0.30 |
0.32 |
0.34 |
0.36 |
- [a] This work, Na+ complex cation is hypothetical; optimized structures at B3LYP/D3(BJ)/def2-TZVPP.
Without π-back donation, the electron density at the bond critical points is rather T-shaped,35 in contrast to metalla-cyclopropanes36 with a more V-shaped electron density and more covalent bond character, for example, in the Rh complex in Table 2. A hypothetical [Na(COD)2]+ complex with clearly only electrostatic interaction to the ligands presents the other extreme. However, this compound shows the highest similarity in comparison to 1 with comparably low electron densities of ρ=0.08/0.14 e Å−3 residing on the TCPM-C and therefore suggests more of an electrostatic bond between ligand and metal.37 In addition, also the very long calculated Na-C distances in [Na(COD)2]+ (277 to 302 pm, av.: 288 pm) including their asymmetry (9–17 pm) are in a similar range to the Ga-C interactions. These findings are very much supported by the 13C-NMR shifts of the sp2-carbon atoms (see Table 1): Here the resonances of the transition-metal complexes shift to higher field, while the gallium complex shifts to lower field resonance. This observation indicates an inverse bonding situation in 1 with a very weak orbital-based ligand to metal bonding, but mainly electrostatic bonding interaction. This is also in agreement with the donor and acceptor orbital energies (cf. ESI, Table S10) and the calculated exchange energy according to Equation (3), which is close for M=Na and Ga, but by far inferior than for Ag.
In conclusion, we presented with [Ga(COD)2]+[Al(ORF)4]− the first representative of the novel class of homoleptic main-group metal-olefin complexes. The facile synthesis gives the title compound as a room temperature stable compound, IR and Raman spectroscopy find a slight redshift of the C=C vibration, which is much smaller than that of related transition-metal compounds. The AIM and frontier orbital energy analysis suggests similarity of 1 to the hypothetical [Na(COD)2]+ complex with no back-bonding to the ligands and would be in agreement with a predominantly electrostatic interaction between Ga+ and COD. Upon addition of phosphine, 1 reacts to give [Ga(PPh3)3][Al(ORF)4]20 and demonstrates its synthetic use.
Acknowledgements
This work was supported by the Albert-Ludwigs-Universität Freiburg and by the DFG. We would like to thank Fadime Bitgül and Dr. Harald Scherer from the magres facility of our faculty for the measurement of the NMR spectra, Dr. Daniel Kratzert and Jan Bohnenberger for crystallographic support and Dr. Thilo Ludwig for his effort concerning the x-ray powder diffraction. Open access funding enabled and organized by Projekt DEAL.
Conflict of interest
The authors declare no conflict of interest.
- 1 Contributions to entropy had to be calculated at the more economic BP86/D3(BJ)/def-TZVP level.