Stereochemical Determination of Tuscolid/Tuscorons and Total Synthesis of Tuscoron D and E: Insights into the Tuscolid/Tuscoron Rearrangement
Abstract
The stereochemistry of the structurally unique myxobacterial polyketides tuscolid/tuscorons was determined by a combination of high-field NMR studies, molecular modeling, and chemical derivatization and confirmed by a modular total synthesis of tuscorons D and E. Together with the discovery of three novel tuscorons, this study provides detailed insight into the chemically unprecedented tuscolid/tuscoron rearrangement cascade.
Tuscolid (1) and tuscorons A and B (2 and 3; Figure 1)1 present structurally unique and stereochemically elaborate polyketides from Sorangium cellulosum, which hold a special place among myxobacterial natural products2 and polyketides3, 4 as they originate from one joint biosynthesis. While tuscolid is characterized by a 22-membered macrolactone ring with an uncommon chiral furan-3-one bearing a bridgehead double bond,5 the tuscorons are linear compounds with a related furan-3-one and polypropionate; however, the appending diene is connected to a different dihydropyran by an sp2–sp3 fusion. While an interconversion has been tentatively proposed,1 proof or details have remained elusive,1 and further studies have been hampered by the lack of full stereochemical knowledge and a missing synthetic approach to resolve the supply issue of these scarce metabolites. Herein, we report the full stereochemical assignment of the tuscolid/tuscorons, the discovery of three novel tuscorons (4–6), and the total synthesis of tuscorons D and E, which unequivocally confirms our stereochemical proposal. In combination, these results provide detailed molecular insight into the unprecedented tuscolid/tuscoron rearrangement cascade.
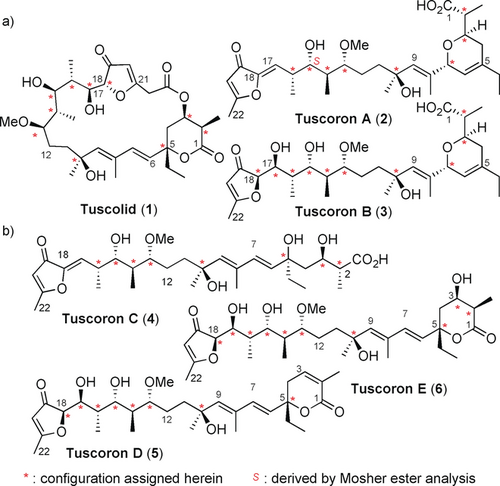
The tuscolid/tuscorons, biosynthetically interconverting polyketides from Sorangium cellulosum. a) Known representatives and b) novel intermediates of the tuscolid/tuscorons rearrangement reported herein.
The Sorangium cellulosum strain So ce1401 was identified as an efficient tuscolid/tuscoron-producing myxobacterium, leading to the discovery of the three novel tuscorons C, D, and E (4–6; Figure 2). The planar structures of 4 and 5 were assigned by extensive 1D and 2D NMR analyses. In detail, characteristic 1H,1H COSY correlations allowed for identification of the C-12 to C-17 and C-12 to C-18 subunits of 4 and 5, which was confirmed by C,H coupling data. HMQC and HMBC correlations, together with the double bond equivalents from HRMS analyses, defined the C-7 to C-12 and the C-18 to C-22 subunits of 4 and 5. The corresponding data for the C-1 to C-9 fragment of 5 suggested a cyclic lactone resembling the analogous subunit of tuscolid, but lacking one H2O equivalent. For tuscolid C, HMBC correlations from H-4 and H-6 to C-1 were missing, which together with its molecular formula led to the assignment of an open-chain analogue. In combination, these data led to the assignment of the planar structures of tuscorons C and D, which were further corroborated by close spectral similarities with the respective data for tuscorons A and B. For tuscoron E (6), 2D C,H coupling and 13C NMR data could not be obtained with sufficient quality because of the very low amounts available, and its structure could only be derived by comparison with the tuscolid/tuscoron data. In detail, the resonances for the C-18 to C-22 subunit were very similar to those of tuscolid and tuscoron B, while the C-1 to C-5 data resembled those of tuscolid and tuscoron C. As the remaining signals corresponded to those observed for all tuscolid/tuscorones, the planar structure of tuscoron E was tentatively assigned.
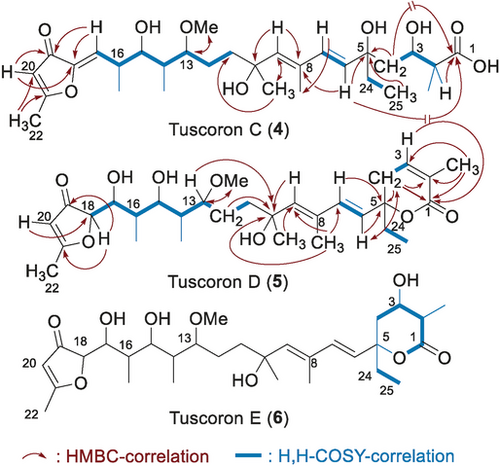
Planar structures of novel tuscorons C, D, and E.
The constitutions of tuscolid and tuscorons A and B had been elucidated by the Höfle group, and also the relative stereochemistry within the C-13 to C-17 moiety of 1 and the pyrans of 1 and 2 had been tentatively assigned.1 For full stereochemical determination, optimal NMR resolutions were obtained in CD3OD and CDCl3, allowing the determination of the relevant 3JH,H coupling constants. These data and ROESY correlations suggested the C-12 to C-21 subunits to be relatively rigid. For tuscolid and tuscoron D, large homonuclear coupling constants between H-14 and H-15 as well as between H-16 and H-17 indicated antiperiplanar relationships between these protons (Figure 3 a; data for 1 given). Small vicinal couplings between H-13 and H-14, H-15 and H-16, and H-17 and H-18 together with strong NOE correlations between H-11 and H-14, H-15 and H-16, H-17 and H18, and H-15 and OH-17 and C-12 CH2 protons and H-15 suggested this subunit to reside in conformation 7. Three further key NOESY correlations, OMe-13 to Me-14, H-14 to Me-16, and Me-14 to H-16, supported the relative assignment, which is in full agreement with a previous analysis of Höfle for 1. Following Murata's method,6 the assignment of a syn relationship between the adjacent oxygen substituents at C-17 and C-18 was based on small heteronuclear coupling constants observed from H-17 to C-18 and from H-18 to C-16 and C17, in combination with a small coupling constant from H-17 to H-18. This assignment was in agreement with a characteristic low-field shift of H-17 as compared to H-14 and H-15, caused by the carbonyl group residing on the same side, subsequently confirmed by modeling, and characteristic coupling constants of synthetic material (see below). Based on a common biogenesis and the close similarity of the spectral data, the stereochemistry of the C-12 to C-21 subunits of all tuscorons was assigned accordingly. In contrast to Höfle,1 detailed analysis of the 3JH,H coupling couplings observed for the C-1 to C-9 segment of 2 suggested the contribution of two or more rapidly interconverting conformations (Figure 3 b). Identical couplings from H-3 to H-4a and H-4b together with a medium value from H-6 to H-7 (3.4 Hz) supported considerable conformational flexibility. With strong NOE contacts between H-3 and H-9 and from H-2 to H-7, two interconverting conformers 8 a and 8 b were proposed, and a relative anti configuration between H-3 and H-7 was suggested, which was further supported by the absence of a significant NOE contact between these two protons, leading to a reassignment of this segment.1 The relative configuration between C-2 and C-3 in turn was based on a large coupling between H-2 and H-3 and a strong NOE contact between Me-2 and H-4a and H-4b. Finally, the relative configuration of the lactone fragments of 1 and 5 was deduced from strong NOE correlations between H-5 and H-4b, H-6 and H-7, and H-4a and Me-2 in combination with a small coupling from H-5 to H-4b and a large coupling from H-5 to H-4a (9, Figure 3 c, data for 1 given), leading to the assignment shown, which is in agreement with the Höfle proposal.1
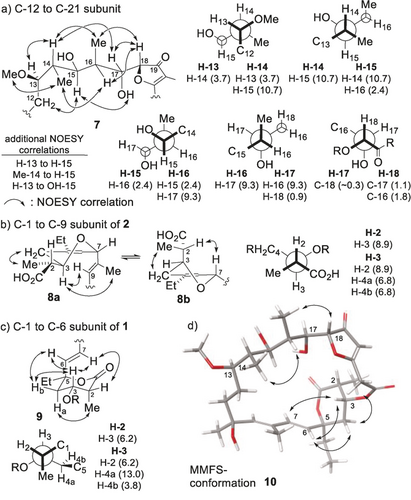
Key data for stereochemical assignment of the tuscolid/tuscorons; data for 1 given in (a) and (c), except for heteronuclear couplings, given for 6. MMFS=Merck molecular force field static.
Finally, correlation of these subunits and the centers at C-10 and C-18 was effectuated by molecular modeling (Macromodel: Monte Carlo searches using a generalized water solvent model) of the possible stereochemical permutations. The calculated conformation 10 of tuscolid isomer 1 resulted in a close match of calculated and observed NMR data. No low-energy conformers for other C17,C18 configurations were found in accordance with the spectral data, thus confirming this assignment. Finally, the absolute configuration was derived by Mosher ester analysis of the OH-15 of tuscoron A (2; Figure 1). Structure 1 summarizes the assignment of the full relative and absolute configuration of tuscolid. Based on the common biogenesis and close similarity of the spectral data, the stereochemistry of tuscorons A–E (3–6) was assigned accordingly (all Figure 1).
For validation of our proposal, key biosynthetic intermediate tuscoron E was selected together with tuscoron D as synthetic targets. A synthesis of tuscoron E was also required for definite structural proof. Our synthetic approach relied on three building blocks, that is, furanone 11, polypropionate 12, as well as lactones 13 and 14 for the construction of tuscorons D and E, respectively (Scheme 1). For connection, a challenging cross-coupling to forge the hindered C-7/C-8 bond and a modular Mukaiyama aldol reaction for attachment of the Western fragment were planned to enable a stereodivergent access to all stereoisomers for further stereochemical proof.
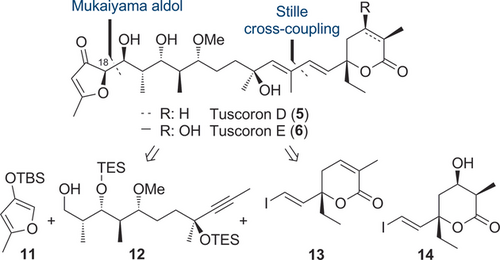
Retrosynthetic analysis of tuscorons D and E.
As shown in Scheme 2, synthesis of the central polypropionate fragment 12 began with early introduction of the C-10 tertiary allylic alcohol. It was generated from geraniol (15) by a sequence involving Sharpless epoxidation, Appel reaction, and double elimination of the resulting chloride 16, with subsequent methylation of the resulting alkyne and TBS protection.7 Inspired by a related transformation,8 it proceeded in a reliable fashion giving 17 in high yields (64 % from 15). After quantitative Sharpless dihydroxylation and solid-supported periodate cleavage,9 the resulting aldehyde 18 was elongated by an anti-aldol coupling with Roche ester derived ketone 19, giving 20 with high selectivity (dr 96:4), purely based on substrate control. After 1,3-syn-reduction and OH-13 methylation, the hydroxy group at C-15 was protected as an acetate (21). Ester migration to the primary alcohol during TBAF-mediated TBS deprotection was then effectively used for selective introduction of a TES ether at OH-15, followed by liberation of the primary alcohol, to give 12 in a reliable sequence. Notably, introduction of a TES ether was required for stereochemical control in the Mukaiyama coupling.
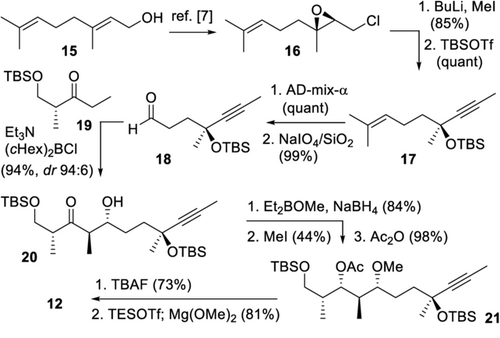
Synthesis of central polypropionate 12. TBSOTf=tert-butyldimethylsilyl trifluoromethanesulfonate, AD-mix-α=asymmetric dihydroxylation mixture alpha, (cHex)2BCl=dicyclohexylboron chloride, TBAF=tetra-n-butylammonium fluoride, TESOTf=triethylsilyl trifluoromethanesulfonate.
The joint synthesis of the eastern fragments 13 and 14 was accomplished by a diversification of the common precursor 24 (Scheme 3). Zirconium-induced carboalumination10-12 of commercial 22 with addition of formaldehyde gave allylic alcohol 23, and the tertiary alcohol was obtained by an analogous sequence as above. Notably, innovative use of Et3Al in the initial zirconium-enabled carboalumination presents an extension of this method. For tuscoron D fragment 13, 24 was deprotected, oxidized, and elongated by a Still–Gennari olefination13 with phosphonate 27.14 After saponification, both resulting diastereomers 29 (E/Z=2.1:1) were cyclized by a Yamaguchi protocol, involving a dynamic E/Z isomerization.15 Finally, iodide 13 was obtained by hydrostannylation and tin–iodine exchange. For tuscoron E fragment 14, joint intermediate 24 was protected and oxidized to aldehyde 25, which was homologated by a Masamune anti-aldol16 reaction with 26 proceeding with high selectivity (dr 19:1) towards 28,17 after auxiliary removal and protecting group manipulations. Finally, oxidative lactonization18 and hydrostannylation/tin–iodine exchange gave building block 14.
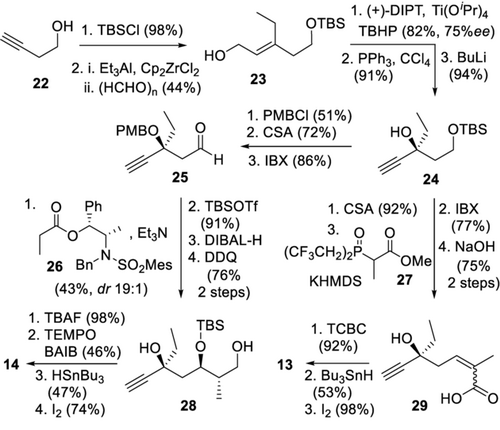
Synthesis of the eastern building blocks. TBS=tert-butyldimethylsilyl, (+)-DIPT=(+)-diisopropyl l-tartrate, PMB=p-methoxybenzyl, CSA=camphorsulfonic acid, IBX=2-iodoxybenzoic acid, DIBAL-H=diisobutylaluminum hydride, DDQ=2,3-dichloro-5,6-dicyano-p-benzoquinone, TEMPO=2,2,6,6-tetramethyl-1-piperidinyloxy, BAIB=bis(acetoxy)iodobenzene, TCBC=2,4,6-trichlorobenzoyl chloride.
Fragment fusion was initiated by a Mukaiyama aldol reaction between ether 11 and aldehyde 31, which were obtained by TBS protection of furanone 3019 and IBX oxidation of polypropionate 12 (Scheme 4). After considerable experimentation, useful selectivity towards the desired isomer 32 was obtained with Me2AlCl in non-polar dichloromethane (DCM), presumably by enforcing an OTES chelation of the aldehyde 31, together with minimization of steric interactions of the α- and β-center,20 and favorable dipole–dipole interactions.21 TES deprotection of sensitive 32 proved delicate, giving decomposition under various conditions. Finally, traces of CSA in DCM targeted triol 32 in acceptable yield, considering its lability. Comparison of the spectral data of all isomers with the natural tuscorons revealed only for the major isomer a close match, while considerable deviations were observed for all other isomers.22 Finally, hydrostannylation and cross-coupling with either 13 or 14, by a protocol reported by Fürstner and co-workers,23 gave tuscorons D (5) and E (6). All spectroscopic data (1H NMR, 13C NMR, MS) and the CD spectra of authentic and synthetic material of 5 were in agreement, thus confirming their architectures, including that of key biosynthetic intermediate 6, and also corroborated the stereochemical proposals of tuscolid/all tuscorons.
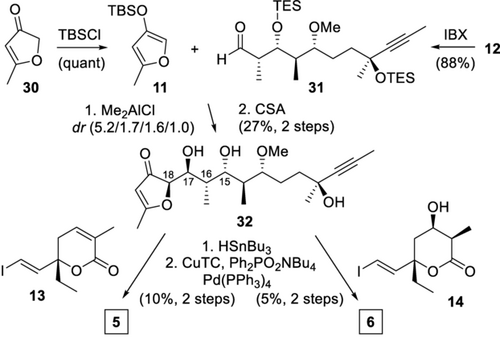
Completion of the total synthesis of tuscorons D and E. CuTC=copper(I) thiophene-2-carboxylate.
These results allow for a detailed formulation of the tuscolid/tuscorone rearrangement. In contrast to a tentative previous hypothesis, a concerted action can be ruled out,1 and a stepwise mechanism has to be formulated. Also, full stereochemical knowledge is now available. The chemically unprecedented cascade is initiated by a decarboxylative macrolactone opening followed by a vinylogous retro-Claisen condensation towards tuscoron E (Scheme 5). The δ-lactone may then be cleaved to give 33, in agreement with the discovery of dehydrated tuscoron C. Finally, an intramolecular SN2′-type endo-cyclisation would close the dihydropyran to tuscoron B (3). Tuscorons A and D (2 and 5) in turn are obtained by water elimination from tuscorons B and E.
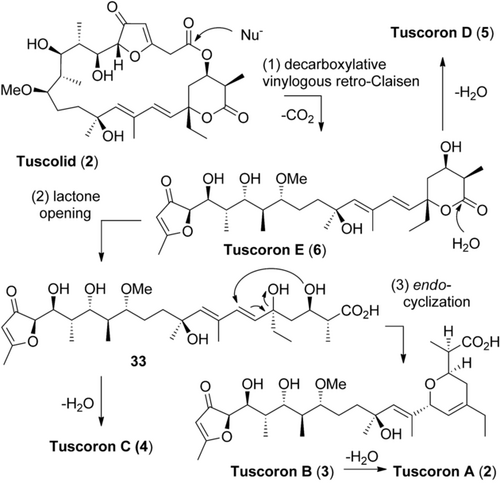
Proposed tuscolid/tuscoron rearrangement.
In summary, full stereochemical assignment of the tuscolid/tuscorons has been accomplished by a combination of high-field NMR studies, modeling, and chemical derivatization. This proposal was unambiguously confirmed by a joint total synthesis of tuscorons D and E by a versatile strategy, which proceeded in 18 steps from geraniol. Key steps involve highly stereoselective aldol reactions, a challenging modular Mukaiyama coupling on highly elaborate fragments, and a late-stage highly hindered and protective-group-free Stille cross-coupling. This first total synthesis of the tuscolid/tuscoron family should be applicable also for the synthesis of other members of this class and enable biological studies of these scarce metabolites. Furthermore, this study provides detailed insight into the biosynthetically and chemically unprecedented tuscolid/tuscoron rearrangement cascade, which should be further investigated.
Acknowledgments
Funding by the DFG (ME 2756/7-1) is gratefully acknowledged. We thank Andreas J. Schneider for excellent HPLC support, Hendrik Schnepel for modeling support, Jutta Niggemann (HZI) for preparation of an extract, and the fermentation service of the HZI. Open access funding enabled and organized by Projekt DEAL.
Conflict of interest
The authors declare no conflict of interest.




