Single-Layered Hybrid Materials Based on 1D Associated Metalorganic Nanoribbons for Controlled Release of Pheromones
Abstract
A new family of stable layered organic–inorganic materials has been prepared, in one-step solvothermal process. They are based on an ordered nickel cluster-type nanoribbons separated from each other by specific alkyl (heptyl- or dodecyl-) arylic mono-carboxylate moieties acting as molecular spacers, perpendicular to the 1D inorganic chains. These organic spacers contain hydrocarbon tails with different length which control the separation level between inorganic 1D sub-units, inhibiting the 3D growth of conventional DUT-8-type metal–organic frameworks (MOFs). The lamellar nature of the materials formed was studied and confirmed by different characterization techniques, showing the structural location of individual organic and inorganic building units. They have been successfully used as a long-lasting biodegradable and water-proof materials for controlled release of chemicals, such as pheromones for sustainable treatment of insect plagues.
Nanometric sheets are fundamental structural units to obtain novel families of versatile lamellar materials, their preparation, study, and applicability being a true challenge in materials science. They are useful since it is possible to take advantage of their capacity to be modified and used as integral parts of new hybrid nanocomposite solids. Inorganic sheets, based on metallic oxides, are essential structural units which are provide different types of lamellar solids with several long-order topologies, spatial arrangement, and physico-chemical characteristics.1, 2, 3 These layered materials can be modified by the spatial alteration of the individual layers through swelling, intercalation, or exfoliation processes to obtain more accessible solids,4-6 and it is possible to incorporate additional functions during post-synthesis treatments.7-9 In the case of metalorganic structures, such as metal–organic frameworks (MOFs), the formation of modifiable layered precursors is not frequent, and usually 3D structures based on the coordination binding between metallic nodes and rigid bi-carboxylate linkers are obtained.10, 11 Preparation of modifiable layered metalorganic precursors formed by individual organic–inorganic layers obtained by direct synthesis, is a matter of interest, and has been studied in 2D coordination polymers.12, 13 Incipient studies related with the formation of MOF nanosheets by modulation of growth kinetics have also been reported.14 Nevertheless, the existence of 3D conventional MOFs which exhibited lamellar structural tendency in the spatial distribution of their structural building units, could be indicative of the possibility to obtain lamellar metalorganic structures.15-21
Taking the above into account, we have succeeded herein in the preparation of different layered organic–inorganic materials, in a one-step process. The materials are based on ordered associated nickel-cluster-type nanoribbons, similar to sub-networks detectable in 3D DUT-8 materials, separated by specific alkyl arylic mono-carboxylate moieties acting as molecular spacers, and which are perpendicular to the inorganic clusters. These specific organic spacers contain hydrocarbon tails with different lengths that control the separation between the 1D inorganic sub-units, inhibiting the 3D growth of conventional DUT-8-type MOFs. These lamellar hybrid materials appeared to be solid micelles and suggested they should be investigated as containers for the controlled release of non-polar active principles (semiochemicals), offering a real possibility for field applications as an alternative to the massive use of pesticides.22-26
The methodology was based on the use of organic spacers with only one carboxylate reactive point that interacts with inorganic metallic nodes through stable coordination bonds. In our case, alkyl benzene monocarboxylate compounds with hydrocarbon chains of different length (ethyl (EB), heptyl (HB), and dodecyl (DB)), in all cases in the para conformation, were used as molecular spacers (Scheme S1 in the Supporting Information). These organic spacers were employed instead of conventional rigid arylic dicarboxylate linkers. Solvothermal processes facilitated the preparation of ordered lamellar hybrid materials based on associated 1D nickel-metalorganic nanoribbons separated by the alkyl benzene monocarboxylate spacers, perpendicular to the inorganic nodes. When desired, a post-synthesis treatment of the layered structures with non-polar solvents allowed the expansion, dispersion, and exfoliation of the metalorganic 1D subunits (Scheme 1). Representation of the individual organic–inorganic nanoribbons is in Scheme S2 in the Supporting Information, highlighting the different basal space obtained between the associated layers, as a function of the organic spacers’ length which act as effective growing inhibitors of the 3D metalorganic structure. The individual 1D organic–inorganic subunits could be formed by consecutive edge-sharing NiO4(OH)2 octahedral, forming inorganic chains (Scheme 1), which are separated by alkyl benzene monocarboxylate ligands, located in both sides of metallic nodes.
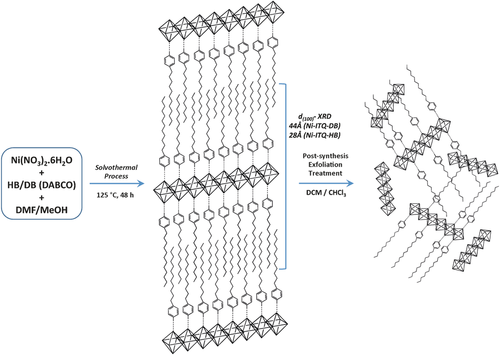
Synthesis of layered hybrid materials: Ni-ITQ-HB or Ni-ITQ-DB and exfoliated solids.
The X-ray diffraction (XRD) patterns of the hybrid materials showed that lamellar organization was achieved when longer alkyl benzene monocarboxylate linkers (HB and DB) are used, as shown by the (100) low-angle diffraction peak, which is characteristic of layered solids formed by individual sheets perpendicularly disposed to a axis (Figure 1). The presence of further peaks, assigned to (200) and (300) diffraction orders, would confirm that the highly regular separation of the successively layered organic–inorganic sheets (Scheme 1). When a shorter alkyl arylic spacer (EB) was used, the material obtained (Ni-ITQ-EB) exhibited poor crystallinity and lamellar structuration was not achieved. In contrast, regular lamellar materials were obtained with heptyl- (HB) and dodecyl- (DB) benzene monocarboxylic acids, named as Ni-ITQ-HB and Ni-ITQ-DB solids. They show one intense low angle (100) diffraction peak centered at approximately 3.1 and 2.0 2θ degrees, corresponding to basal spaces of around 28 Å and 44 Å, respectively. Considering that the organic spacers used —HB and DB—have molecular lengths of 13.8 Å and 20.0 Å, respectively, and that they were probably perpendicularly located in both sides of 1D inorganic NiO4(OH)2 chains, it would be possible to estimate that the thickness of each individual 1D nickel-chain was close to 4–5 Å (Scheme 1). This result would imply the formation of lamellar hybrid materials based on ordered associated 1D nanoribbons of [NiO6] octahedral separated by long alkyl arylic spacers. XRD peaks observed at higher than 20 2θ degrees, in the Ni-ITQ-DB pattern, were assigned to nickel oxyhydroxide species present in the 1D inorganic chains.
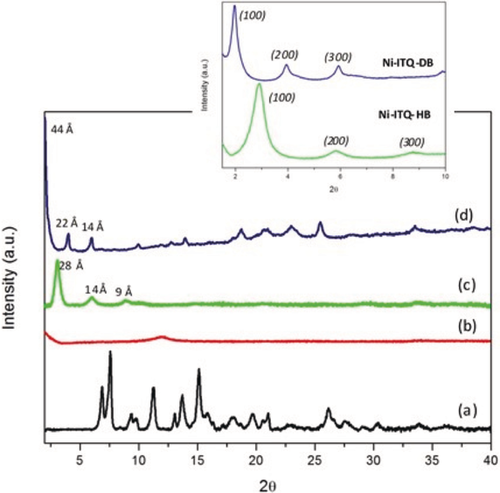
XRD patterns of layered hybrid materials and of 3D Ni-MOF: a) DUT-8(Ni), b) Ni-ITQ-EB, c) Ni-ITQ-HB, and d) Ni-ITQ-DB. Inset: XRD patterns of Ni-ITQ-HB and Ni-ITQ-DB materials at lower 2θ angles.
The lamellar morphology of Ni-ITQ-HB (Figure 2) and Ni-ITQ-DB materials was confirmed by HRTEM micrographs. The ordered distribution of piled sheets with basal spaces around 30 Å and 44 Å, respectively, can be observed, which are similar to those obtained from X-ray diffractograms. SEM and AFM showed remarkable differences between the layered hybrid materials and the standard 3D DUT-8(Ni). Specifically, 3D DUT-8(Ni) was formed by dense elongated prism-type crystals, while the bi-dimensional character together with a homogenous distribution of ordered layers were detectable in the lamellar hybrid structures, being observable the presence of little plate-type crystallites assembled as bigger flower-like crystals (Figure S1 and Figure S2 in the Supporting Information). Post-synthesis treatments with non-polar solvents allowed the expansion and exfoliation of the ordered lamellar hybrid materials. Lasting and stable colloidal solutions with isolated short ribbons of approximately 5 Å of thickness were identified from HRTEM micrographs (Figure 3). The observable disordered nanowires probably are the inorganic part of each individual metalorganic nanoribbon, that is, the 1D chains based on the associated [NiO6] octahedra (Scheme 1). This behavior showed the expandable capacity of this type of lamellar metalorganic solids and the possibility to obtain individual short Ni-based-ribbons.
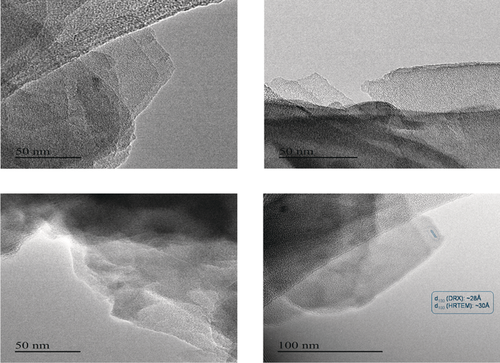
HRTEM images of a Ni-ITQ-HB sample showing the organized nanosheets. In the bottom right image, two consecutive nanosheets with their corresponding basal space are highlighted. d100 (XRD)≈28 Å, d100 (HRTEM)≈30 Å.
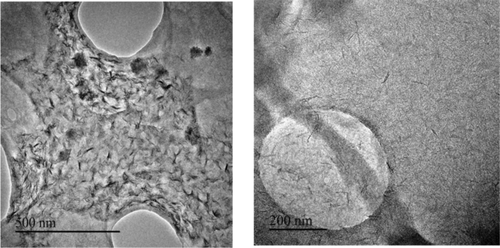
HRTEM images of stable solution derived of post-synthesis treatment of Ni-ITQ-HB sample with dichloromethane.
From elemental CHNS analysis and thermogravimetric curves (Table S1 and Figure S3), it was estimated that the organic content higher in the layered materials than in the standard 3D DUT-8(Ni). The hybrid metalorganic materials show a thermogravimetric feature close to 300–350 °C for the decomposition of para-alkyl benzene monocarboxylate molecules, used as spacers, and of the NiO4(OH)2 units present in the 1D inorganic Ni-chains. Furthermore, the chemical analysis allowed estimating the composition of Ni-ITQ-HB and Ni-ITQ-DB materials that exhibited C/Ni molar ratios between 1.5 and 2. This result implies that the organic linkers were located in both sides of octahedral NiO4(OH)2 units (Scheme 1). 13C CP/MAS NMR and IR spectra of 3D DUT-8(Ni) and lamellar metalorganic materials confirmed the total integrity of the structural linkers (NDC and DABCO) or organic spacers (HB and DB) (Figure S4, Figure S5, and Figure S6).
The hybrid lamellar materials were used as long lasting biodegradable dispensers for controlled release of chemicals, and more specifically pheromones, for sustainable management of insect plagues. The active principle used was 3-(S)-methyl-6-(R,S)-isopropenyl-9-decenyl acetate, that is, the pheromone emitted by the females of the armored scale Aonidiella aurantii (Maskell; California red scale), one of the most damaging pests of citrus plants (Scheme S3).27 In preliminary experiments, a load of 25 wt % (pheromone/ matrix) was estimated as the maximum amount of volatile held by direct adsorption in the materials. Specifically, Ni-ITQ-HB, as a matrix for controlled release, was compared with the same layered material based on aluminum (see experimental part in Supporting Information), while conventional and inexpensive mesoporous silica M41S-type was used as reference matrix.28
Kinetic data showed a very different behavior among the three compared materials. As inferred from Figure 4 a, the standard mesoporous material is not suitable for controlled release because of its high retention level of pheromone (ca. 50 %). Both metalorganic layered materials show a release of the volatile compound, but only the nickel-based material has an acceptable residual pheromone level, around 10 % of the initial load, after sixty days of aeration. Probably, the reason of the high residual level in the aluminum layered hybrid material (ca. 50 %) compared to nickel is the higher Lewis acidity of aluminum, which results in a stronger interaction between the ester moiety of the pheromone and the matrix. Despite the good residual level showed by the Ni-ITQ-HB material, the kinetic results were far from the desirable zero-order, being closer to an exponential desorption rate expression. Since the high level of pheromone initially loaded (25 wt %) can influence the emission behavior, the total amount of the volatile loaded was reduced to 10 wt % in the adsorbed Ni-ITQ-HB material to be comparable with the afore-described prism-type 3D DUT-8(Ni) material.
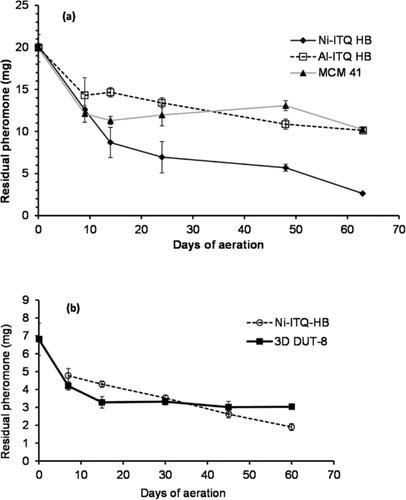
Residual pheromone loading in samples with different initial adsorbed pheromone content: a) 25 wt % and b) 10 wt %.
Kinetic results showed that the lower organic content achieved by the prism-type 3D-DUT-8 material, together with its higher free porous volume, probably promoted the high residual level (with an asymptote at ca. 50 % of content) when compared with the layered material (Figure 4 b). After losing the external surface adsorbed pheromone during the first week of aeration, the release profile of the Ni-layered hybrid material fits a desired zero-order kinetics (R2=0.99), being therefore appropriate for controlled pheromone release. The amount of pheromone released required is specific for each pest and technique, ranging from 60 to 3000 micrograms per device and day, and could be easily adjusted by scaling up the emitters. Taking into account the low residual level of pheromone previously achieved with the Ni-ITQ-HB material (<10 %) and the well stablished comparison between the forced conditions set in the aeration chamber and field conditions, longer dispenser lifespan fitting zero order kinetics for controlled release of pheromones should be achieved with this material in field applications.29
In conclusion, a novel family of layered organic–inorganic materials, based on ordered associated nickel clusters-type nanoribbons, has been prepared in a one-step synthesis. Post-synthesis treatments with different non-polar solvents allowed their easy expansion, and also the generation of exfoliated materials formed by disordered 1D short inorganic chains. This property opens the possibilities for these lamellar hybrid materials to be used as builder precursors to form novel nanocomposites with potential applications in catalysis, adsorption, or separation processes, exploiting their hydrophilic–hydrophobic nature. The excellent kinetics showed in controlling the release of the A. aurantii pheromone makes these materials suitable for controlled liberation of semiochemicals.
Acknowledgements
The support of the European Union (ERC-AdG-2014-671093—SynCatMatch) and the Spanish Government (MAT2014-52085-C2-1-P and Severo Ochoa program SEV-2012-0267) is acknowledged.




