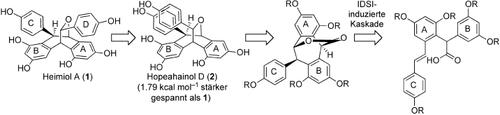
Total Syntheses of Heimiol A, Hopeahainol D, and Constrained Analogues†
Corresponding Author
Prof. Dr. Scott A. Snyder
Department of Chemistry, Columbia University, Havemeyer Hall, 3000 Broadway, New York, NY 10027 (USA) http://www.columbia.edu/cu/chemistry/groups/snyder/
Department of Chemistry, Columbia University, Havemeyer Hall, 3000 Broadway, New York, NY 10027 (USA) http://www.columbia.edu/cu/chemistry/groups/snyder/Search for more papers by this authorNathan E. Wright
Department of Chemistry, Columbia University, Havemeyer Hall, 3000 Broadway, New York, NY 10027 (USA) http://www.columbia.edu/cu/chemistry/groups/snyder/
Search for more papers by this authorJason J. Pflueger
Department of Chemistry, Columbia University, Havemeyer Hall, 3000 Broadway, New York, NY 10027 (USA) http://www.columbia.edu/cu/chemistry/groups/snyder/
Search for more papers by this authorSteven P. Breazzano
Department of Chemistry, Columbia University, Havemeyer Hall, 3000 Broadway, New York, NY 10027 (USA) http://www.columbia.edu/cu/chemistry/groups/snyder/
Search for more papers by this authorCorresponding Author
Prof. Dr. Scott A. Snyder
Department of Chemistry, Columbia University, Havemeyer Hall, 3000 Broadway, New York, NY 10027 (USA) http://www.columbia.edu/cu/chemistry/groups/snyder/
Department of Chemistry, Columbia University, Havemeyer Hall, 3000 Broadway, New York, NY 10027 (USA) http://www.columbia.edu/cu/chemistry/groups/snyder/Search for more papers by this authorNathan E. Wright
Department of Chemistry, Columbia University, Havemeyer Hall, 3000 Broadway, New York, NY 10027 (USA) http://www.columbia.edu/cu/chemistry/groups/snyder/
Search for more papers by this authorJason J. Pflueger
Department of Chemistry, Columbia University, Havemeyer Hall, 3000 Broadway, New York, NY 10027 (USA) http://www.columbia.edu/cu/chemistry/groups/snyder/
Search for more papers by this authorSteven P. Breazzano
Department of Chemistry, Columbia University, Havemeyer Hall, 3000 Broadway, New York, NY 10027 (USA) http://www.columbia.edu/cu/chemistry/groups/snyder/
Search for more papers by this authorWe thank the NSF (CHE-0619638) for an X-ray diffractometer and Prof. Gerard Parkin, Wesley Sattler, and Aaron Sattler for performing all of the crystallographic analyses. We also thank Adel ElSohly for performing the calculations. Financial support was provided by Columbia University, the National Institutes of Health (R01GM84994), Bristol-Myers Squibb, Eli Lilly, the NSF (Predoctoral Fellowships to N.E.W. and S.P.B.), the ACS Division of Organic Chemistry (summer fellowship to J.J.P.), and the Research Corporation for Science Advancement (Cottrell Scholar Award to S.A.S.).
Graphical Abstract
Supporting Information
Detailed facts of importance to specialist readers are published as ”Supporting Information”. Such documents are peer-reviewed, but not copy-edited or typeset. They are made available as submitted by the authors.
| Filename | Description |
|---|---|
| ange_201103575_sm_miscellaneous_information.pdf1.3 MB | miscellaneous_information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1J.-F. F. Weber, I. A. Wahab, A. Marzuki, N. A. Thomas, A. A. Kadir, A. H. A. Hadi, K. Awang, A. A. Latiff, P. Richomme, J. Delaunay, Tetrahedron Lett. 2001, 42, 4895–4897.
- 2
- 2aS. Atun, N. Aznam, R. Arianingrum, Y. Takaya, N. Masatake, J. Phys. Sci. 2008, 19( 2), 7–21;
- 2bE. H. Sahidin, L. D. Juliawaty, Y. M. Syah, L. Din, E. L. Ghisalberti, J. Latip, I. M. Said, S. A. Achman, Z. Naturforsch. C 2005, 60, 723–727.
- 3H. M. Ge, W.-H. Yang, J. Zhang, R.-X. Tan, J. Agric. Food Chem. 2009, 57, 5756–5761.
- 4For an excellent review on total syntheses of colchicine, see: T. Graening, H.-G. Schmalz, Angew. Chem. 2004, 116, 3292–3318;
10.1002/ange.200300615 Google ScholarAngew. Chem. Int. Ed. 2004, 43, 3230–3256.
- 5Calculations were performed with the B3LYP functional in conjunction with the 6–31G(d) basis set. Electronic energies are from single-point calculations on the optimized geometrics.
- 6Using thermodynamic strain energy to dictate synthetic planning is rare, though kinetic energies have been used to great effect with one example being calculation of atropisomeric equilibrations to determine the ordering of bond constructions for the vancomycin aglycon: D. L. Boger, S. Miyazaki, S. H. Kim, J. H. Wu, S. L. Castle, O. Loiseleur, Q. Jin, J. Am. Chem. Soc. 1999, 121, 10004–10011.
- 7
- 7aS. A. Snyder, A. L. Zografos, Y. Lin, Angew. Chem. 2007, 119, 8334–8339;
10.1002/ange.200703333 Google ScholarAngew. Chem. Int. Ed. 2007, 46, 8186–8191;
- 7bS. A. Snyder, S. P. Breazzano, A. G. Ross, Y. Lin, A. L. Zografos, J. Am. Chem. Soc. 2009, 131, 1753–1765.
- 8T. Tanaka, T. Ito, K. Nakaya, M. Iinuma, S. Riswan, Phytochemistry 2000, 54, 63–69.
- 9E. J. Corey, M. Chaykovsky, J. Am. Chem. Soc. 1965, 87, 1353–1364.
- 10
- 10aS. E. Snyder, F. A. Aviles-Garay, R. Chakraborti, D. E. Nichols, V. J. Watts, R. B. Mailman, J. Med. Chem. 1995, 38, 2395–2409;
- 10bN. J. Bach, E. C. Kornfeld, N. D. Jones, M. O. Chaney, D. E. Dorman, J. W. Paschal, J. A. Clemens, E. B. Smalstig, J. Med. Chem. 1980, 23, 481–491.
- 11B. O. Lindgren, T. Nilsson, Acta Chem. Scan. 1973, 27, 888–890.
- 12
- 12aM. Curini, F. Epifano, M. C. Marcotullio, F. Montanari, Synlett 2004, 368–370;
- 12bH. Gottam, T. K. Vinod, J. Org. Chem. 2011, 76, 974–977.
- 13S. A. Snyder, D. S. Treitler, A. P. Brucks, J. Am. Chem. Soc. 2010, 132, 14303–14314.
- 14Intriguingly, use of either of these reagents separately did not afford the desired product.
- 15It is worth noting that if 17 was first added to aldehyde 14, no attempt at subsequent haloetherification to reach 20 directly succeeded in any observable yield (although at best only 50 % of material could be advanced to the desired product due to the extra stereogenic center).
- 16Earlier protection of the phenols as benzyl ethers prevented the smooth preparation of alcohol 13 because of issues of additional strain imparted by these groups in forging critical CC bonds.
- 17For the isolation of these molecules, see:
- 17aY. Oshima, Y. Ueno, K. Hisamichi, M. Takeshita, Tetrahedron 1993, 49, 5801–5804;
- 17bT. Tanaka, M. Ohyama, K. Morimoto, F. Asai, M. Iinuma, Phytochemistry 1998, 48, 1241–1243;
- 17cT. Tanaka, I. Iliya, T. Ito, M. Furusawa, K. Nakaya, M. Iinuma, Y. Shitataki, N. Matsuura, M. Ubukata, J. Murata, F. Simozono, K. Hirai, Chem. Pharm. Bull. 2001, 49, 858–862;
- 17dK.-S. Huang, Y.-H. Wang, R.-L. Li, M. Lin, J. Nat. Prod. 2000, 63, 86–89.
- 18For syntheses of this molecule in both protected and nonprotected form, see:
- 18aS. S. Velu, I. Buniyamin, L. K. Ching, F. Feroz, I. Noorbatcha, L. C. Gee, K. Awang, I. B. Wahab, J.-F. F. Weber, Chem. Eur. J. 2008, 14, 11376–11384;
- 18bW. Li, H. Li, Y. Luo, Y. Yang, N. Wang, Synlett 2010, 1247–1250.
- 19Conditions attempted included exposure to acid and heat, as well as the trityl cation to try and establish a dynamic equilibrium.
- 20For recent examples with other unique polyphenolic natural products, see:
- 20aS. A. Snyder, T. C. Sherwood, A. G. Ross, Angew. Chem. 2010, 122, 5272–5276;
10.1002/ange.201002264 Google ScholarAngew. Chem. Int. Ed. 2010, 49, 5146–5150;
- 20bK. C. Nicolaou, Q. Kang, T. R. Wu, C. S. Lim, D. Y.-K. Chen, J. Am. Chem. Soc. 2010, 132, 7540–7548. For an excellent review on synthetic approaches to polyphenolic natural products in general, see:
- 20cS. Quideau, D. Deffieux, C. Douat-Casassus, L. Pouységu, Angew. Chem. 2011, 123, 610–646;
10.1002/ange.201000044 Google ScholarAngew. Chem. Int. Ed. 2011, 50, 586–621. For recent work to prepare oligomeric polyphenols beyond the dimer level, see:
- 20dS. A. Snyder, A. Gollner, M. I. Chiriac, Nature 2011, 474, 461–466;
- 20eK. Ohmori, T. Shono, Y. Hatakoshi, T. Yano, K. Suzuki, Angew. Chem. 2011, 123, 4964–4969; Angew. Chem. Int. Ed. 2011, 50, 4862–4867.
Citing Literature
This is the
German version
of Angewandte Chemie.
Note for articles published since 1962:
Do not cite this version alone.
Take me to the International Edition version with citable page numbers, DOI, and citation export.
We apologize for the inconvenience.