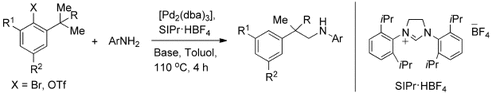
Palladium(0)-Catalyzed Intermolecular Amination of Unactivated C H Bonds†
H Bonds†
Dr. Jun Pan
Department of Chemistry, Room 18-490, Massachusetts Institute of Technology, Cambridge, MA 02139 (USA)
Search for more papers by this authorMingjuan Su
Department of Chemistry, Room 18-490, Massachusetts Institute of Technology, Cambridge, MA 02139 (USA)
Search for more papers by this authorCorresponding Author
Prof. Dr. Stephen L. Buchwald
Department of Chemistry, Room 18-490, Massachusetts Institute of Technology, Cambridge, MA 02139 (USA)
Department of Chemistry, Room 18-490, Massachusetts Institute of Technology, Cambridge, MA 02139 (USA)Search for more papers by this authorDr. Jun Pan
Department of Chemistry, Room 18-490, Massachusetts Institute of Technology, Cambridge, MA 02139 (USA)
Search for more papers by this authorMingjuan Su
Department of Chemistry, Room 18-490, Massachusetts Institute of Technology, Cambridge, MA 02139 (USA)
Search for more papers by this authorCorresponding Author
Prof. Dr. Stephen L. Buchwald
Department of Chemistry, Room 18-490, Massachusetts Institute of Technology, Cambridge, MA 02139 (USA)
Department of Chemistry, Room 18-490, Massachusetts Institute of Technology, Cambridge, MA 02139 (USA)Search for more papers by this authorGenerous financial support from the National Institutes of Health (GM-46059) is gratefully acknowledged. The Bruker 400 MHz instrument used in this work was purchased with funding from the National Institutes of Health (GM 1S10RR13886-01). We are grateful to Georgiy Teverovskiy (MIT) for initial studies on DFT calculations and to Sophie Rousseaux (MIT) for helpful discussions.
Graphical Abstract
Auf die eine oder andere Weise: Die Titelreaktion nichtaktivierter C -H-Bindungen mit Arylaminen wird untersucht (siehe Schema; dba=Dibenzylidenaceton, Tf=Trifluormethansulfonyl). Durch Einstellen der sterischen Eigenschaften des Substrats kann entweder das C-N-Kreuzkupplungsprodukt oder das C-H-Aminierungsprodukt selektiv erhalten werden.
-H-Bindungen mit Arylaminen wird untersucht (siehe Schema; dba=Dibenzylidenaceton, Tf=Trifluormethansulfonyl). Durch Einstellen der sterischen Eigenschaften des Substrats kann entweder das C-N-Kreuzkupplungsprodukt oder das C-H-Aminierungsprodukt selektiv erhalten werden.
Supporting Information
Detailed facts of importance to specialist readers are published as ”Supporting Information”. Such documents are peer-reviewed, but not copy-edited or typeset. They are made available as submitted by the authors.
| Filename | Description |
|---|---|
| ange_201102880_sm_miscellaneous_information.pdf3.1 MB | miscellaneous_information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1
- 1aC. Kibayashi, Chem. Pharm. Bull. 2005, 53, 1375;
- 1bJ. H. Cheng, K. Kamiya, I. Kodama, Cardiovasc. Drug Rev. 2001, 19, 152;
- 1cC. Sanchez, C. Mendez, J. A. Salas, Nat. Prod. Rep. 2006, 23, 1007.
- 2For recent reviews on CH amination, see:
- 2aA. Armstrong, J. C. Collins, Angew. Chem. 2010, 122, 2332;
10.1002/ange.200906750 Google ScholarAngew. Chem. Int. Ed. 2010, 49, 2282;
- 2bP. Thansandote, M. Lautens, Chem. Eur. J. 2009, 15, 5874;
- 2cF. Collet, R. H. Dodd, P. Dauban, Chem. Commun. 2009, 5061;
- 2dH. M. L. Davies, J. R. Manning, Nature 2008, 451, 417;
- 2eA. R. Dick, M. S. Sanford, Tetrahedron 2006, 62, 2439;
- 2fH. M. L. Davies, M. S. Long, Angew. Chem. 2005, 117, 3584;
10.1002/ange.200500554 Google ScholarAngew. Chem. Int. Ed. 2005, 44, 3518;
- 2gP. Muller, C. Fruit, Chem. Rev. 2003, 103, 2905;
- 2hF. Collet, C. Lescot, P. Dauban, Chem. Soc. Rev. 2011, 40, 1926;
- 2iD. N. Zalatan, J. Du Bois, Top. Curr. Chem. 2010, 292, 347.
- 3For selected examples of CN bond formation following activation of C
 H bonds, see:
H bonds, see:
- 3aK. Sun, Y. Li, T. Xiong, J. Zhang, Q. Zhang, J. Am. Chem. Soc. 2011, 133, 1694;
- 3bB. Xiao, T.-J. Gong, J. Xu, Z.-J. Liu, L. Liu, J. Am. Chem. Soc. 2011, 133, 1466;
- 3cY. C. Tan, J. F. Hartwig, J. Am. Chem. Soc. 2010, 132, 3676;
- 3dK. H. Ng, A. S. C. Chan, W. Y. Yu, J. Am. Chem. Soc. 2010, 132, 12862;
- 3eK. Inamoto, T. Saito, K. Hiroya, T. Doi, J. Org. Chem. 2010, 75, 3900;
- 3fD. Monguchi, T. Fujiwara, H. Furukawa, A. Mori, Org. Lett. 2009, 11, 1607;
- 3gT. S. Mei, X. S. Wang, J. Q. Yu, J. Am. Chem. Soc. 2009, 131, 10806;
- 3hM. Wasa, J. Q. Yu, J. Am. Chem. Soc. 2008, 130, 14058;
- 3iJ. A. Jordan-Hore, C. C. C. Johansson, M. Gulias, E. M. Beck, M. J. Gaunt, J. Am. Chem. Soc. 2008, 130, 16184;
- 3jW. C. P. Tsang, R. H. Munday, G. Brasche, N. Zheng, S. L. Buchwald, J. Org. Chem. 2008, 73, 7603;
- 3kG. Brasche, S. L. Buchwald, Angew. Chem. 2008, 120, 1958;
10.1002/ange.200705420 Google ScholarAngew. Chem. Int. Ed. 2008, 47, 1932;
- 3lX. Chen, X. S. Hao, C. E. Goodhue, J. Q. Yu, J. Am. Chem. Soc. 2006, 128, 6790;
- 3mW. C. P. Tsang, N. Zheng, S. L. Buchwald, J. Am. Chem. Soc. 2005, 127, 14560;
- 3nQ. Wang, S. L. Schreiber, Org. Lett. 2009, 11, 5178;
- 3oM. Kienle, C. Dunst, P. Knochel, Org. Lett. 2009, 11, 5158;
- 3pT. Kawano, K. Hirano, T. Satoh, M. Miura, J. Am. Chem. Soc. 2010, 132, 6900;
- 3qH. Q. Zhao, M. Wang, W. P. Su, M. C. Hong, Adv. Synth. Catal. 2010, 352, 1301;
- 3rH. G. Wang, Y. Wang, C. L. Peng, J. C. Zhang, Q. A. Zhu, J. Am. Chem. Soc. 2010, 132, 13217;
- 3sS. H. Cho, J. Y. Kim, S. Y. Lee, S. Chang, Angew. Chem. 2009, 121, 9291; Angew. Chem. Int. Ed. 2009, 48, 9127;
- 3tM. Miyasaka, K. Hirano, T. Satoh, R. Kowalczyk, C. Bolm, M. Miura, Org. Lett. 2011, 13, 359;
- 3uS. Guo, B. Qian, Y. Xie, C. Xia, H. Huang, Org. Lett. 2011, 13, 522.
- 4For selected examples of CH amination of activated C
 H bonds which include allylic, benzylic CH bonds, and CH bonds having heteroatoms or electron-withdrawing substituents on the carbon atom, see:
H bonds which include allylic, benzylic CH bonds, and CH bonds having heteroatoms or electron-withdrawing substituents on the carbon atom, see:
- 4aM. M. Diaz-Requejo, T. R. Belderrain, M. C. Nicasio, S. Trofimenko, P. J. Perez, J. Am. Chem. Soc. 2003, 125, 12078;
- 4bM. R. Fructos, S. Trofimenko, M. M. Diaz-Requejo, P. J. Perez, J. Am. Chem. Soc. 2006, 128, 11784;
- 4cR. Bhuyan, K. M. Nicholas, Org. Lett. 2007, 9, 3957;
- 4dG. Pelletier, D. A. Powell, Org. Lett. 2006, 8, 6031;
- 4eJ. L. Liang, S. X. Yuan, J. S. Huang, W. Y. Yu, C. M. Che, Angew. Chem. 2002, 114, 3615;
Angew. Chem. Int. Ed. 2002, 41, 3465;
10.1002/1521-3773(20020916)41:18<3465::AID-ANIE3465>3.0.CO;2-D CAS PubMed Web of Science® Google Scholar
- 4fJ. L. Liang, S. X. Yuan, J. S. Huang, C. M. Che, J. Org. Chem. 2004, 69, 3610;
- 4gC. G. Espino, K. W. Fiori, M. Kim, J. Du Bois, J. Am. Chem. Soc. 2004, 126, 15378;
- 4hK. W. Fiori, J. Du Bois, J. Am. Chem. Soc. 2007, 129, 562;
- 4iY. Zhang, H. Fu, Y. Jiang, Y. Zhao, Org. Lett. 2007, 9, 3813;
- 4jE. Milczek, N. Boudet, S. Blakey, Angew. Chem. 2008, 120, 6931;
10.1002/ange.200801445 Google ScholarAngew. Chem. Int. Ed. 2008, 47, 6825;
- 4kK. J. Fraunhoffer, M. C. White, J. Am. Chem. Soc. 2007, 129, 7274;
- 4lS. A. Reed, M. C. White, J. Am. Chem. Soc. 2008, 130, 3316;
- 4mG. S. Liu, G. Y. Yin, L. Wu, Angew. Chem. 2008, 120, 4811; Angew. Chem. Int. Ed. 2008, 47, 4733.
- 5For an elegant example of intramolecular CH amination of unactivated C
 H bonds, see: J. J. Neumann, S. Rakshit, T. Droge, F. Glorius, Angew. Chem. 2009, 121, 7024;
Angew. Chem. Int. Ed. 2009, 48, 6892.
H bonds, see: J. J. Neumann, S. Rakshit, T. Droge, F. Glorius, Angew. Chem. 2009, 121, 7024;
Angew. Chem. Int. Ed. 2009, 48, 6892.
- 6
- 6aH. Y. Thu, W. Y. Yu, C. M. Che, J. Am. Chem. Soc. 2006, 128, 9048;
- 6bZ. G. Li, D. A. Capretto, R. Rahaman, C. A. He, Angew. Chem. 2007, 119, 5276; Angew. Chem. Int. Ed. 2007, 46, 5184;
- 6cC. G. Liang, F. Robert-Pedlard, C. Fruit, P. Muller, R. H. Dodd, P. Dauban, Angew. Chem. 2006, 118, 4757; Angew. Chem. Int. Ed. 2006, 45, 4641;
- 6dC. G. Liang, F. Collet, F. Robert-Peillard, P. Muller, R. H. Dodd, P. Dauban, J. Am. Chem. Soc. 2008, 130, 343;
- 6eB. P. Gomez-Emeterio, J. Urbano, M. M. Diaz-Requejo, P. J. Perez, Organometallics 2008, 27, 4126;
- 6fF. Collet, C. Lescot, C. G. Liang, P. Dauban, Dalton Trans. 2010, 39, 10401.
- 7T. E. Barder, S. D. Walker, J. R. Martinelli, S. L. Buchwald, J. Am. Chem. Soc. 2005, 127, 4685.
- 8For reviews on biarylphosphane ligands, see:
- 8aD. S. Surry, S. L. Buchwald, Angew. Chem. 2008, 120, 6438;
10.1002/ange.200800497 Google ScholarAngew. Chem. Int. Ed. 2008, 47, 6338;
- 8bD. S. Surry, S. L. Buchwald, Chem. Sci. 2011, 2, 27.
- 9For recent reviews on N-heterocyclic carbenes, see:
- 9aS. P. Nolan, Acc. Chem. Res. 2011, 44, 91;
- 9bH. Clavier, S. P. Nolan, Chem. Commun. 2010, 46, 841;
- 9cN. Marion, S. P. Nolan, Acc. Chem. Res. 2008, 41, 1440;
- 9dN. Marion, S. Diez-Gonzalez, I. P. Nolan, Angew. Chem. 2007, 119, 3046;
10.1002/ange.200603380 Google ScholarAngew. Chem. Int. Ed. 2007, 46, 2988.
- 10N. Marion, O. Navarro, J. Mei, E. D. Stevens, N. M. Scott, S. P. Nolan, J. Am. Chem. Soc. 2006, 128, 4101.
- 11
- 11aO. Baudoin, A. Herrbach, F. Gueritte, Angew. Chem. 2003, 115, 5914; Angew. Chem. Int. Ed. 2003, 42, 5736;
- 11bJ. Hitce, P. Retailleau, O. Baudoin, Chem. Eur. J. 2007, 13, 792;
- 11cR. Jazzar, J. Hitce, A. Renaudat, J. Sofack-Kreutzer, O. Baudoin, Chem. Eur. J. 2010, 16, 2654.
- 12For a related study on benzocyclobutene formation (mechanism and scope), see: M. Chaumontet, R. Piccardi, N. Audic, J. Hitce, J. L. Peglion, E. Clot, O. Baudoin, J. Am. Chem. Soc. 2008, 130, 15157.
- 13See the Supporting Information for details.
- 14
- 14aM. Brookhart, M. L. H. Green, G. Parkin, Proc. Natl. Acad. Sci. USA 2007, 104, 6908;
- 14bM. Lafrance, S. I. Gorelsky, K. Fagnou, J. Am. Chem. Soc. 2007, 129, 14570;
- 14cS. Rousseaux, M. Davi, J. Sofack-Kreutzer, C. Pierre, C. E. Kefalidis, E. Clot, K. Fagnou, O. Baudoin, J. Am. Chem. Soc. 2010, 132, 10706.
- 15
- 15aL. J. L. Haller, M. J. Page, S. A. Macgregor, M. F. Mahon, M. K. Whittlesey, J. Am. Chem. Soc. 2009, 131, 4604;
- 15bC. E. Kefalidis, O. Baudoin, E. Clot, Dalton Trans. 2010, 39, 10528.
Citing Literature
This is the
German version
of Angewandte Chemie.
Note for articles published since 1962:
Do not cite this version alone.
Take me to the International Edition version with citable page numbers, DOI, and citation export.
We apologize for the inconvenience.