Enantioselective Cobalt-Catalyzed Preparation of Trifluoromethyl-Substituted Cyclopropanes†
Bill Morandi
Laboratorium für Organische Chemie, ETH Zürich, 8093 Zürich (Switzerland), Fax: (+41) 1-632-1328 http://www.carreira.ethz.ch
Search for more papers by this authorDr. Brian Mariampillai
Laboratorium für Organische Chemie, ETH Zürich, 8093 Zürich (Switzerland), Fax: (+41) 1-632-1328 http://www.carreira.ethz.ch
Search for more papers by this authorCorresponding Author
Prof. Dr. Erick M. Carreira
Laboratorium für Organische Chemie, ETH Zürich, 8093 Zürich (Switzerland), Fax: (+41) 1-632-1328 http://www.carreira.ethz.ch
Laboratorium für Organische Chemie, ETH Zürich, 8093 Zürich (Switzerland), Fax: (+41) 1-632-1328 http://www.carreira.ethz.chSearch for more papers by this authorBill Morandi
Laboratorium für Organische Chemie, ETH Zürich, 8093 Zürich (Switzerland), Fax: (+41) 1-632-1328 http://www.carreira.ethz.ch
Search for more papers by this authorDr. Brian Mariampillai
Laboratorium für Organische Chemie, ETH Zürich, 8093 Zürich (Switzerland), Fax: (+41) 1-632-1328 http://www.carreira.ethz.ch
Search for more papers by this authorCorresponding Author
Prof. Dr. Erick M. Carreira
Laboratorium für Organische Chemie, ETH Zürich, 8093 Zürich (Switzerland), Fax: (+41) 1-632-1328 http://www.carreira.ethz.ch
Laboratorium für Organische Chemie, ETH Zürich, 8093 Zürich (Switzerland), Fax: (+41) 1-632-1328 http://www.carreira.ethz.chSearch for more papers by this authorWe are grateful to the Swiss National Foundation and the SSCI for a fellowship to B. Morandi and a NSERC post-doctoral fellowship to B. Mariampillai. Dr. Schweizer is acknowledged for X-ray analysis.
Graphical Abstract
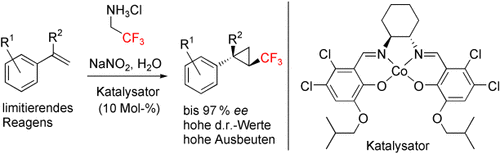
Leichter Zugang auf Wasser: Eine Cobalt-katalysierte asymmetrische Synthese von Trifluormethylcyclopropanen wurde entwickelt, die mit einer breiten Palette von Styrolen hohe Enantiomerenüberschüsse ergibt (siehe Schema). Die Reaktion bietet einen neuen Zugang zu enantiomerenangereicherten CF3-Bausteinen.
Supporting Information
Detailed facts of importance to specialist readers are published as ”Supporting Information”. Such documents are peer-reviewed, but not copy-edited or typeset. They are made available as submitted by the authors.
| Filename | Description |
|---|---|
| ange_201004269_sm_miscellaneous_information.pdf1.5 MB | miscellaneous_information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1Reviews on asymmetric cyclopropanations:
- 1aH. Lebel, J.-F. Marcoux, C. Molinaro, A. B. Charette, Chem. Rev. 2003, 103, 977–1050;
- 1bH. Pellissier, Tetrahedron 2008, 64, 7041–7095.
- 2Selected examples with other diazo compounds:
- 2aS. Zhu, J. V. Ruppel, H. Lu, L. Wojtas, X. P. Zhang, J. Am. Chem. Soc. 2008, 130, 5042–5043;
- 2bJ. R. Denton, H. M. L. Davies, Org. Lett. 2009, 11, 787–790;
- 2cJ. R. Denton, D. Sukumaran, H. M. L. Davies, Org. Lett. 2007, 9, 2625–2628.
- 3For general reviews on fluorine chemistry see:
- 3aP. Kirsch, Modern Fluoroorganic Chemistry: Synthesis Reactivity, Applications, Wiley-VCH, Weinheim 2004;
10.1002/352760393X Google Scholar
- 3bM. Schlosser, Angew. Chem. 2006, 118, 5558–5572;
10.1002/ange.200600449 Google ScholarAngew. Chem. Int. Ed. 2006, 45, 5432–5446;
- 3cC. Isanbor, D. O’Hagan, J. Fluorine Chem. 2006, 127, 303–319;
- 3dK. Müller, C. Faeh, F. Diederich, Science 2007, 317, 1881–1886;
- 3eS. Purser, P. R. Moore, S. Swallow, V. Gouverneur, Chem. Soc. Rev. 2008, 37, 320–330.
- 4Reviews on asymmetric trifluoromethylations:
- 4aJ.-A. Ma, D. Cahard, Chem. Rev. 2008, 108, PR 1–PR43;
- 4bN. Shibata, S. Mizuta, H. Kawai, Tetrahedron: Asymmetry 2008, 19, 2633–2644;
- 4cT. Billard, B. R. Langlois, Eur. J. Org. Chem. 2007, 891–897.
- 5
- 5aB. Morandi, E. M. Carreira, Angew. Chem. 2010, 122, 950–953;
10.1002/ange.200905573 Google ScholarAngew. Chem. Int. Ed. 2010, 49, 938–941;
- 5bB. Morandi, E. M. Carreira, Angew. Chem. Int. Ed. 2010, 49, 4294–4296; Angew. Chem. 2010, 122, 4390–4392.
- 6
- 6aRef. [2a];
- 6bY. Chen, J. V. Ruppel, X. P. Zhang, J. Am. Chem. Soc. 2007, 129, 12074–12075;
- 6cY. Chen, K. B. Fields, X. P. Zhang, J. Am. Chem. Soc. 2004, 126, 14718–14719;
- 6dS. Zhu, J. A. Perman, X. P. Zhang, Angew. Chem. 2008, 120, 8588–8591; Angew. Chem. Int. Ed. 2008, 47, 8460–8463; S. Zhu, J. A. Perman, X. P. Zhang, Angew. Chem. 2008, 120, 8588–8591;
- 6eT. Fukuda, T. Katsuki, Synlett 1995, 825–826;
- 6fT. Fukuda, T. Katsuki, Tetrahedron 1997, 53, 7201–7208;
- 6gT. Fukuda, T. Katsuki, Tetrahedron Lett. 1997, 38, 3443–3446;
- 6hT. Niimi, T. Uchida, R. Irie, T. Katsuki, Tetrahedron Lett. 2000, 41, 3647–3651.
- 7
- 7a Organic Reactions in Water (Ed.: ), Blackwell, Oxford, 2007;
10.1002/9780470988817 Google Scholar
- 7bC.-J. Li, T.-H. Chan, Organic Reactions in Aqueous Media, Wiley, New York, 1997;
- 7cS. Kobayashi, Pure Appl. Chem. 2007, 79, 235; we have already reported reactions in aqueous media:
- 7dO. Soltani, M. A. Ariger, E. M. Carreira, Org. Lett. 2009, 11, 4196–4198;
- 7eO. Soltani, M. A. Ariger, H. Vazquez-Villa, E. M. Carreira, Org. Lett. 2010, 12, 2893–2895.
- 8
- 8aR. P. Wurz, A. B. Charette, Org. Lett. 2002, 4, 4531–4533;
- 8bA. G. M. Barrett, D. C. Braddock, I. Lenoir, H. Tone, J. Org. Chem. 2001, 66, 8260–8263;
- 8cJ. R. Fulton, V. K. Aggarwal, J. de Vicente, Eur. J. Org. Chem. 2005, 1479–1492.
- 9P. Le Maux, S. Juillard, G. Simonneaux, Synthesis 2006, 1701–1704.
- 10
- 10aM. Palucki, N. S. Finney, P. J. Pospisil, M. L. Güler, T. Ishida, E. N. Jacobsen, J. Am. Chem. Soc. 1998, 120, 948–954;
- 10bE. N. Jacobsen, W. Zhang, M. L. Güler, J. Am. Chem. Soc. 1991, 113, 6703–6704;
- 10cS. K. Koehn, S Gronert, J. T. Aldajaei, Org. Lett. 2010, 12, 676–679;
- 10din the kinetic resolution of epoxides, the use of a Cr–salen complex that includes a 3,3′,5,5′-tetrachloro-substituted ligand was reported: A. Berkessel, E. Ertürk, Adv. Synth. Catal. 2006, 348, 2619–2625.
- 11CCDC 783661 (9) contains the supplementary crystallographic data for this paper. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/data_request/cif.
- 12For a discussion of the trans-axial effect see:
- 12aY. Chen, X. P. Zhang, Synthesis 2006, 1696–1700;
- 12bT. Ikeno, M. Sato, H. Sekino, A. Nishizuka, T. Yamada, Bull. Chem. Soc. Jpn. 2001, 74, 2139–2150.
- 13S. Narayan, J. Muldoon, M. G. Finn, V. V. Fokin, H. C. Kolb, K. B. Sharpless, Angew. Chem. 2005, 117, 3339–3343; Angew. Chem. Int. Ed. 2005, 44, 3275–3279.
- 14A. Klapars, X. Huang, S. L. Buchwald, J. Am. Chem. Soc. 2002, 124, 7421–7428. CCDC 784005.
- 151,1-Methylstyrene afforded the product in excellent yield (91 %) and good d.r. (5:1) under standard reaction conditions but we have been unable to determine the enantioselectivity by chiral SFC.
- 16Absolute configuration established by analogy to that observed for the derivative shown in Scheme 3.
Citing Literature
This is the
German version
of Angewandte Chemie.
Note for articles published since 1962:
Do not cite this version alone.
Take me to the International Edition version with citable page numbers, DOI, and citation export.
We apologize for the inconvenience.