A Well-Defined Complex for Palladium-Catalyzed Aerobic Oxidation of Alcohols: Design, Synthesis, and Mechanistic Considerations†
David R. Jensen
Department of Chemistry, University of Utah, 315 S. 1400 East, Salt Lake City, UT 84112, USA, Fax: (+1) 801-581-8433
Search for more papers by this authorMitchell J. Schultz
Department of Chemistry, University of Utah, 315 S. 1400 East, Salt Lake City, UT 84112, USA, Fax: (+1) 801-581-8433
Search for more papers by this authorJaime A. Mueller
Department of Chemistry, University of Utah, 315 S. 1400 East, Salt Lake City, UT 84112, USA, Fax: (+1) 801-581-8433
Search for more papers by this authorMatthew S. Sigman Prof. Dr.
Department of Chemistry, University of Utah, 315 S. 1400 East, Salt Lake City, UT 84112, USA, Fax: (+1) 801-581-8433
Search for more papers by this authorDavid R. Jensen
Department of Chemistry, University of Utah, 315 S. 1400 East, Salt Lake City, UT 84112, USA, Fax: (+1) 801-581-8433
Search for more papers by this authorMitchell J. Schultz
Department of Chemistry, University of Utah, 315 S. 1400 East, Salt Lake City, UT 84112, USA, Fax: (+1) 801-581-8433
Search for more papers by this authorJaime A. Mueller
Department of Chemistry, University of Utah, 315 S. 1400 East, Salt Lake City, UT 84112, USA, Fax: (+1) 801-581-8433
Search for more papers by this authorMatthew S. Sigman Prof. Dr.
Department of Chemistry, University of Utah, 315 S. 1400 East, Salt Lake City, UT 84112, USA, Fax: (+1) 801-581-8433
Search for more papers by this authorThis work was supported by the National Institutes of Health (NIGMS no. RO1 GM63540). D.R.J. is supported by an ACS Division of Organic Chemistry Graduate Fellowship sponsored by the Schering–Plough Research Institute. Pd salts were provided by Johnson Matthey. The crystal-structure analysis was performed by Atta Arif.
Graphical Abstract
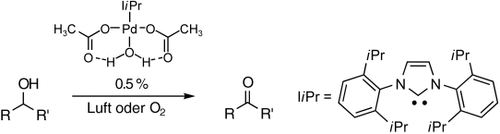
Ein Hauch frischer Luft: In Gegenwart von 0.5–0.1 Mol-% Katalysator konnte eine Vielfalt von Alkoholen oxidiert werden – in einigen Fällen sogar an der Luft (siehe Schema). Mechanistische Schlüsse können anhand einer Kristallstruktur, die auf ungewöhnliche Wasserstoffbrücken zwischen dem koordinierten Wasser und Acetatliganden hinweist, und eines starken kinetischen Isotopeneffekts gezogen werden.
Supporting Information
Supporting information for this article is available on the WWW under http://www.wiley-vch.de/contents/jc_2001/2003/z51997_s.pdf or from the author.
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1For recent reviews, see
- 1aR. A. Sheldon, I. W. C. E. Arends, G. ten Brink, A. Dijksman, Acc. Chem. Res. 2002, 35, 774;
- 1bR. A. Sheldon, I. W. C. E. Arends, A. Dijksman, Catal. Today 2000, 57, 157.
- 2For examples, see
- 2aM. J. Schultz, C. C. Park, M. S. Sigman, Chem. Commun. 2002, 3034;
- 2bN. Kakiuchi, Y. Maeda, T. Nishimura, S. Uemura, J. Org. Chem. 2001, 66, 6620;
- 2cK. Hallman, C. Moberg, Adv. Synth. Catal. 2001, 343, 260;
- 2dG. ten Brink, I. W. C. E. Arends, R. A. Sheldon, Science 2000, 287, 1636;
- 2eT. Nishimura, T. Onoue, K. Ohe, S. Uemura, J. Org. Chem. 1999, 64, 6750;
- 2fK. P. Peterson, R. C. Larock, J. Org. Chem. 1998, 63, 3185.
- 3For the use of Pd-catalyzed alcohol oxidation for kinetic resolution, see
- 3aS. K. Mandal, D. R. Jensen, J. S. Pugsley, M. S. Sigman, J. Org. Chem. 2003, 68, 4600;
- 3bJ. T. Bagdanoff, E. M. Ferreira, B. M. Stoltz, Org. Lett. 2003, 5, 835;
- 3cD. R. Jensen, M. S. Sigman, Org. Lett. 2003, 5, 63;
- 3dE. M. Ferreira, B. M. Stoltz, J. Am. Chem. Soc. 2001, 123, 7725;
- 3eD. R. Jensen, J. S. Pugsley, M. S. Sigman, J. Am. Chem. Soc. 2001, 123, 7475.
- 4For mechanistic studies, see
- 4aJ. A. Mueller, M. S. Sigman, J. Am. Chem. Soc. 2003, 125, 7005;
- 4bJ. A. Mueller, D. R. Jensen, M. S. Sigman, J. Am. Chem. Soc. 2002, 124, 8202;
- 4cB. A. Steinhoff, S. S. Stahl, Org. Lett. 2002, 4, 4179;
- 4dB. A. Steinhoff, S. R. Fix, S. S. Stahl, J. Am. Chem. Soc. 2002, 124, 766;
- 4eG. ten Brink, I. W. C. E. Arends, R. A. Sheldon, Adv. Synth. Catal. 2002, 344, 355;
- 4fS. S. Stahl, J. L. Thorman, R. C. Nelson, M. A. Kozee, J. Am. Chem. Soc. 2001, 123, 7188.
- 5A notable exception is the phenathrolene-based catalyst.[2d] In this catalyst, a bidentate ligand is used. Since the solvent is H2O at 100 °C and 30 atm, the oxidation precedes by dissociation of anionic ligands.
- 6For a review of NHC ligands, see W. A. Hermann, Angew. Chem. 2002, 114, 1342;
Angew. Chem. Int. Ed. 2002, 41, 1290.
10.1002/1521-3773(20020415)41:8<1290::AID-ANIE1290>3.0.CO;2-Y CAS PubMed Web of Science® Google Scholar
- 7For evidence of acetate acting as a base, see ref. [4b].
- 8CCDC-213715 contains the supplementary crystallographic data for this paper. These data can be obtained free of charge via www.ccdc.cam.ac.uk/conts/retrieving.html (or from the Cambridge Crystallographic Data Centre, 12 Union Road, Cambridge CB2 1EZ, UK; fax: (+44) 1223-336-033; or [email protected]). A yellow prism-shaped crystal 0.30×0.23×0.18 mm in size was mounted on a glass fiber with traces of viscous oil and then transferred to a Nonius KappaCCD diffractometer equipped with MoKα radiation (λ=0.71073 Å). Ten frames of data were collected at 150(1) K with an oscillation range of 1 deg/frame and an exposure time of 20 s/frame. Indexing and unit-cell refinement based on all observed reflection from those ten frames indicated a monoclinic P lattice. A total of 12 906 reflections (λmax=27.5°) were indexed, integrated, and corrected for Lorentz, polarization, and absorption effects using DENZO-SMN and SCALEPAC. Post refinement of the unit cell gave a=9.59400(10), b=19.1341(3), c=17.1480(3) Å, β=92.3451(5)°, and V=3145.27(8) Å3. Axial photographs and systematic absences were consistent with the compound having crystallized in the monoclinic space group P21/a. The structure was solved by a combination of direct methods and heavy atom using SIR 97. All of the non-hydrogen atoms were refined with anisotropic displacement coefficients. Hydrogen atoms were located and refined isotropically except those on the C31 atom which were assigned isotropic displacement coefficients U(H)=1.5 U(C methyl), and their coordinates were allowed to ride on their respective carbon atoms using SHELXL97. The weighting scheme employed was w=1/[σ2(F
 )+(0.0257 P)2+2.171 P] where P=(F
)+(0.0257 P)2+2.171 P] where P=(F +2 F
+2 F )/3. The refinement converged to R1=0.0303, wR2=0.0726, and S=1.027 for 6402 reflections with I>2σ(I), and R1=0.0353, wR2=0.0758, and S=1.027 for 7162 unique reflections and 517 parameters. The maximum Δ/σ value in the final cycle of the least-squares was 0.001, and the residual peaks on the final difference-Fourier map ranged from −0.594 to 0.869 e Å−3.
)/3. The refinement converged to R1=0.0303, wR2=0.0726, and S=1.027 for 6402 reflections with I>2σ(I), and R1=0.0353, wR2=0.0758, and S=1.027 for 7162 unique reflections and 517 parameters. The maximum Δ/σ value in the final cycle of the least-squares was 0.001, and the residual peaks on the final difference-Fourier map ranged from −0.594 to 0.869 e Å−3.
- 9For a homogeneous Pd catalyst using air, see ref. [2c]. For a heterogeneous Pd catalyst using air, see ref. [2b].
- 10The H2O molecule binds when the complex is formed and not during recrystallization, as evidenced by identical 1H NMR spectra for the complex formed in situ and after recrystallization.
- 11The hydrogen atoms of the water molecule were located and found to be within the van der Waal radii of the acetate and the water. See Supporting Information for details.
- 12See ref. [4b,c,e] and G. Noronha, P. M. Henry, J. Mol. Catal. A 1997, 120, 75.
- 13For the reversible formation of PdII–hydride species under acidic conditions, see C. Amatore, A. Jutand, G. Meyer, I. Carelli, I. Chiarotto, Eur. J. Inorg. Chem. 2000, 1855.
Citing Literature
This is the
German version
of Angewandte Chemie.
Note for articles published since 1962:
Do not cite this version alone.
Take me to the International Edition version with citable page numbers, DOI, and citation export.
We apologize for the inconvenience.