Single-Molecule Covalent Chemistry with Spatially Separated Reactants†
Tudor Luchian
Department of Medical Biochemistry & Genetics, The Texas A&M University System Health Science Center, 440 Reynolds Medical Building, TAMU 1114, College Station, TX 77843-1114, USA, Fax: (+1) 979-847-9481
These authors contributed equally to this work.
Search for more papers by this authorSeong-Ho Shin
Department of Medical Biochemistry & Genetics, The Texas A&M University System Health Science Center, 440 Reynolds Medical Building, TAMU 1114, College Station, TX 77843-1114, USA, Fax: (+1) 979-847-9481
Department of Chemistry, Texas A&M University, College Station, TX 77843, USA
These authors contributed equally to this work.
Search for more papers by this authorHagan Bayley
Department of Medical Biochemistry & Genetics, The Texas A&M University System Health Science Center, 440 Reynolds Medical Building, TAMU 1114, College Station, TX 77843-1114, USA, Fax: (+1) 979-847-9481
Department of Chemistry, Texas A&M University, College Station, TX 77843, USA
Search for more papers by this authorTudor Luchian
Department of Medical Biochemistry & Genetics, The Texas A&M University System Health Science Center, 440 Reynolds Medical Building, TAMU 1114, College Station, TX 77843-1114, USA, Fax: (+1) 979-847-9481
These authors contributed equally to this work.
Search for more papers by this authorSeong-Ho Shin
Department of Medical Biochemistry & Genetics, The Texas A&M University System Health Science Center, 440 Reynolds Medical Building, TAMU 1114, College Station, TX 77843-1114, USA, Fax: (+1) 979-847-9481
Department of Chemistry, Texas A&M University, College Station, TX 77843, USA
These authors contributed equally to this work.
Search for more papers by this authorHagan Bayley
Department of Medical Biochemistry & Genetics, The Texas A&M University System Health Science Center, 440 Reynolds Medical Building, TAMU 1114, College Station, TX 77843-1114, USA, Fax: (+1) 979-847-9481
Department of Chemistry, Texas A&M University, College Station, TX 77843, USA
Search for more papers by this authorThis work was supported by the Office of Naval Research, the U.S. Department of Energy, the Multidisciplinary University Research Initiative (ONR-1999), and the National Institutes of Health. We thank Sean Conlan for help with molecular modeling.
Graphical Abstract
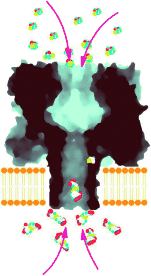
Proteinporen als Nanoreaktoren konnten kürzlich eingesetzt werden, um die reversible kovalente Chemie einer einfachen Reaktion auf Einzelmolekül-Ebene anhand des Stroms durch die Pore zu beobachten. Jetzt ist es gelungen, eine mehrstufige Reaktion zu beobachten, bei der zwei der Reaktanten durch die Membran, welche die Pore enthält, getrennt sind und in die Reaktionszone diffundieren (siehe Bild).
References
- 1M. Grandbois, M. Beyer, M. Rief, H. Clausen-Schaumann, H. E. Gaub, Science 1999, 283, 1727–1730.
- 2A. Ishijima, H. Kojima, T. Funatsu, M. Tokunaga, H. Higuchi, H. Tanaka, T. Yanagida, Cell 1998, 92, 161–171.
- 3H. P. Lu, L. Xun, X. S. Xie, Science 1998, 282, 1877–1882.
- 4X. Zhuang, H. Kim, M. J. B. Pereira, H. P. Babcock, N. G. Walter, S. Chu, Science 2002, 296, 1473–1476.
- 5M. J. Levene, J. Korlach, S. W. Turner, M. Foquet, H. G. Craighead, W. W. Webb, Science 2003, 299, 682–686.
- 6H. Bayley, P. S. Cremer, Nature 2001, 413, 226–230.
- 7D. C. Jacobs, Nature 2003, 423, 488–489.
- 8J. A. Mindell, H. Zhan, P. D. Huynh, R. J. Collier, A. Finkelstein, Proc. Natl. Acad. Sci. USA 1994, 91, 5272–5276.
- 9T. Luchian, S.-H. Shin, H. Bayley, Angew. Chem. 2003, 115, 1970–1973;
10.1002/ange.200250666 Google ScholarAngew. Chem. Int. Ed. 2003, 42, 1926–1929.
- 10D. C. J. Jaikaran, G. A. Woolley, J. Phys. Chem. 1995, 99, 13 352–13 355.
- 11S.-H. Shin, T. Luchian, S. Cheley, O. Braha, H. Bayley, Angew. Chem. 2002, 114, 3859–3861;
Angew. Chem. Int. Ed. 2002, 41, 3707–3709.
10.1002/1521-3773(20021004)41:19<3707::AID-ANIE3707>3.0.CO;2-5 CAS PubMed Web of Science® Google Scholar
- 12This is reasonable because the effective concentration of the thiol provided by Cys 117 in the barrel (volume ≈14 000 Å3, as determined with the program HOLE)[27] is ≈0.12 M. In single-molecule terms, the probability of DTT being in the barrel, at a time when DTNB is also in the barrel, is p≈3.6×10−4 (p≈Vbarrel/VDTT; where Vbarrel is the volume inside the barrel and VDTT is the volume of solution occupied by one DTT molecule, based on the external concentration).
- 13At high concentrations of DTT(cis), the reduction in rate most likely arises from depletion of DTNB(trans) by reaction with DTT that has reached the trans unstirred layer.
- 14To check whether the intermediate 2 was indeed formed from DTT, we carried out several controls. For example, in the absence of DTT, the reaction proceeded through the first step, but remained there indefinitely (>60 s; Figure 2 a, upper trace). Further, when DTT was replaced with 50 μM β-mercaptoethanol (βME, cis), a monothiol, the reaction took a different course. The first step with DTNB (50 μM, trans) was observed as before, but it was followed by a step to a level where the system remained for a prolonged period (>60 s), which was distinct from the level representing 2. We interpret the sequence as cleavage of 1 with βME to generate a mixed disulfide that reacts relatively slowly with a second molecule of βME.
- 15G. M. Whitesides, J. E. Lilburn, R. P. Szajewski, J. Org. Chem. 1977, 42, 332–338.
- 16P. Mitchell, Nature 1961, 191, 144–148.
- 17For example, an asymmetrically oriented “molecular triad” was used to transport Ca2+ ions across a lipid bilayer.[28] Intramolecular electron transfer was produced by excitation of the triad with visible light. A hydroquinone carrier in the bilayer was reduced on one side of the membrane, where Ca2+ ions are picked up, and oxidized on the other side, where they are released.
- 18J. M. Wilson, R. J. Bayer, D. J. Hupe, J. Am. Chem. Soc. 1977, 99, 7922–7926.
- 19R. P. Szajewksi, G. M. Whitesides, J. Am. Chem. Soc. 1980, 102, 2011–2026.
- 20J.-H. Ha, S. N. Loh, Nat. Struct. Biol. 1998, 5, 730–737.
- 21U. Lindström, Chem. Rev. 2002, 102, 2751–2772.
- 22S. J. Rowan, S. J. Cantrill, G. R. L. Cousins, J. K. M. Sanders, J. F. Stoddart, Angew. Chem. 2002, 114, 938–993;
10.1002/1521-3757(20020315)114:6<938::AID-ANGE938>3.0.CO;2-K Google ScholarAngew. Chem. Int. Ed. 2002, 41, 898–952; Erratum:10.1002/1521-3773(20020315)41:6<898::AID-ANIE898>3.0.CO;2-E CAS PubMed Web of Science® Google ScholarS. J. Rowan, S. J. Cantrill, G. R. L. Cousins, J. K. M. Sanders, J. F. Stoddart, Angew. Chem. 2002, 114, 1528;10.1002/1521-3757(20020503)114:9<1528::AID-ANGE11111528>3.0.CO;2-Z Google ScholarAngew. Chem. Int. Ed. 2002, 41, 1460.
- 23L.-Q. Gu, S. Cheley, H. Bayley, J. Gen. Physiol. 2001, 118, 481–494.
- 24B. Baumeister, N. Sakai, S. Matile, Org. Lett. 2001, 3, 4229–4232.
- 25B. Baumeister, S. Matile, Macromolecules 2002, 35, 1549–1555.
- 26S. Howorka, L. Movileanu, O. Braha, H. Bayley, Proc. Natl. Acad. Sci. USA 2001, 98, 12 996–13 001.
- 27O. S. Smart, J. G. Neduvelil, X. Wang, B. A. Wallace, M. S. P. Sansom, J. Mol. Graphics 1996, 14, 354–360.
- 28I. M. Bennett, H. M. V. Farfano, F. Bogani, A. Primak, P. A. Liddell, L. Otero, L. Sereno, J. J. Silber, A. L. Moore, T. A. Moore, D. Gust, Nature 2002, 420, 398–401.
Citing Literature
This is the
German version
of Angewandte Chemie.
Note for articles published since 1962:
Do not cite this version alone.
Take me to the International Edition version with citable page numbers, DOI, and citation export.
We apologize for the inconvenience.