Controlled Living Ring-Opening-Metathesis Polymerization by a Fast-Initiating Ruthenium Catalyst†
Tae-Lim Choi
Arnold and Mabel Laboratories of Chemical Synthesis, California Institute of Technology, Division of Chemistry and Chemical Engineering, MC 164-30 Pasadena, CA 91125, USA, Fax: (+1) 626-564-9297
Search for more papers by this authorRobert H. Grubbs Prof.
Arnold and Mabel Laboratories of Chemical Synthesis, California Institute of Technology, Division of Chemistry and Chemical Engineering, MC 164-30 Pasadena, CA 91125, USA, Fax: (+1) 626-564-9297
Search for more papers by this authorTae-Lim Choi
Arnold and Mabel Laboratories of Chemical Synthesis, California Institute of Technology, Division of Chemistry and Chemical Engineering, MC 164-30 Pasadena, CA 91125, USA, Fax: (+1) 626-564-9297
Search for more papers by this authorRobert H. Grubbs Prof.
Arnold and Mabel Laboratories of Chemical Synthesis, California Institute of Technology, Division of Chemistry and Chemical Engineering, MC 164-30 Pasadena, CA 91125, USA, Fax: (+1) 626-564-9297
Search for more papers by this authorThe authors would like to thank the National Science Foundation for generous support of this research, and D. Benitez, D. P. Sanders, J. P. Morgan, and O. A. Scherman for helpful discussions, and A. Hejl for the generous supply of catalyst 4.
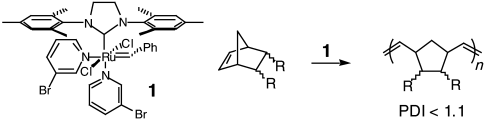
Graphical Abstract
Hoch aktiver Katalysator, ultraschnelle Initiation: Der Katalysator 1 vermittelt die lebende Ringöffnungspolymerisation von Norbornen- und 7-Oxonorbornen-Derivaten (siehe Schema). Das hohe Verhältnis ki/kp, das dabei mit 1 erreicht wird, führt zu besonders guter Steuerung des Molekulargewichts und zu einem niedrigen Polydispersitätsindex (PDI).
References
- 1For recent reviews on ROMP, see:
- 1aB. M. Novak, W. Risse, R. H. Grubbs, Adv. Polym. Sci. 1992, 102, 47;
- 1bK. J. Ivin, J. C. Mol, Olefin Metathesis and Metathesis Polymerization, Academic Press, San Diego, CA, 1997;
- 1cR. H. Grubbs, E. Khosravi, Mater. Sci. Technol. 1999, 20, 65;
- 1dM. R. Buchmeiser, Chem. Rev. 2000, 100, 1565;
- 1eU. Frenzel, O. Nuyken, J. Polym. Sci. Part A 2002, 40, 2895.
- 2
- 2aR. R. Schrock, Acc. Chem. Res. 1990, 23, 158;
- 2bG. C. Bazan, R. R. Schrock, H. N. Cho, V. C. Gibson, Macromolecules 1991, 24, 4495.
- 3
- 3aS. Kanaoka, R. H. Grubbs, Macromolecules 1995, 28, 4707;
- 3bP. Schwab, R. H. Grubbs, J. W. Ziller, J. Am. Chem. Soc. 1996, 118, 100;
- 3cM. Weck, P. Schwab, R. H. Grubbs, R. H. Macromolecules 1996, 29, 1789.
- 4
- 4aT. Weskamp, W. C. Schattenmann, M. Spiegler, W. A. Herrman, Angew. Chem. 1998, 110, 2631;
10.1002/(SICI)1521-3757(19980918)110:18<2631::AID-ANGE2631>3.0.CO;2-J Google ScholarAngew. Chem. Int. Ed. 1998, 37, 2490;10.1002/(SICI)1521-3773(19981002)37:18<2490::AID-ANIE2490>3.0.CO;2-X CAS PubMed Web of Science® Google Scholar
- 4bT. Weskamp, F. J. Kohl, W. Hieringer, D. Gleich, W. A. Herrman, Angew. Chem. 1999, 111, 2573;
10.1002/(SICI)1521-3757(19990816)111:16<2573::AID-ANGE2573>3.0.CO;2-A Google ScholarAngew. Chem. Int. Ed. 1999, 38, 2416;10.1002/(SICI)1521-3773(19990816)38:16<2416::AID-ANIE2416>3.0.CO;2-# CAS PubMed Web of Science® Google Scholar
- 4cJ. Huang, E. D. Stevnes, S. P. Nolan, J. L. Petersen, J. Am. Chem. Soc. 1999, 121, 2675.
- 5M. Scholl, S. Ding, C. W. Lee, R. H. Grubbs, Org. Lett. 1999, 1, 953.
- 6
- 6aA. K. Chatterjee, R. H. Grubbs, Org. Lett. 1999, 1, 1751;
- 6bA. K. Chatterjee, J. P. Morgan, M. Scholl, R. H. Grubbs, J. Am. Chem. Soc. 2000, 122, 3783;
- 6cT.-L. Choi, A. K. Chatterjee, R. H. Grubbs, Angew. Chem. 2001, 113, 1317;
Angew. Chem. Int. Ed. 2001, 40, 1277;
10.1002/1521-3773(20010401)40:7<1277::AID-ANIE1277>3.0.CO;2-E CAS PubMed Web of Science® Google Scholar
- 6dA. K. Chatterjee, T.-L. Choi, R. H. Grubbs, Synlett 2001, 1034;
- 6eT.-L. Choi, C. W. Lee, A. K. Chatterjee, R. H. Grubbs, J. Am. Chem. Soc. 2001, 123, 10 417;
- 6fT.-L. Choi, R. H. Grubbs, Chem. Commun. 2001, 2648;
- 6gC. W. Lee, T.-L. Choi, R. H. Grubbs, J. Am. Chem. Soc. 2002, 124, 3224.
- 7
- 7aM. S. Sanford, M. Ulman, R. H. Grubbs, J. Am. Chem. Soc. 2001, 123, 749;
- 7bM. S. Sanford, J. A. Love, R. H. Grubbs, J. Am. Chem. Soc. 2001, 123, 6543.
- 8
- 8aC. W. Bielawski, R. H. Grubbs, Angew. Chem. 2000, 112, 3025;
10.1002/1521-3757(20000818)112:16<3025::AID-ANGE3025>3.0.CO;2-F Google ScholarAngew. Chem. Int. Ed. 2000, 39, 2903;10.1002/1521-3773(20000818)39:16<2903::AID-ANIE2903>3.0.CO;2-Q CAS PubMed Web of Science® Google Scholar
- 8bC. W. Bielawski, D. Benitez, R. H. Grubbs, Macromolecules 2001, 34, 8610;
- 8cO. A. Scherman, H. M. Kim, R. H. Grubbs, Macromolecules 2002, 35, 5366.
- 9T.-L. Choi, I. M. Rutenberg, R. H. Grubbs, Angew. Chem. 2002, 114, 3995;
Angew. Chem. Int. Ed. 2002, 41, 3839.
10.1002/1521-3773(20021018)41:20<3839::AID-ANIE3839>3.0.CO;2-H CAS PubMed Web of Science® Google Scholar
- 10ROMP with catalyst 3 gives extremely high-molecular-weight polymers that are often insoluble, but low PDIs have been observed in some special cases, see: H. D. Maynard, S. Y. Okada, R. H. Grubbs, Macromolecules 2000, 33, 6239.
- 11J. A. Love, J. P. Morgan, T. M. Trnka, R. H. Grubbs, Angew. Chem. 2002, 114, 4207;
10.1002/1521-3757(20021104)114:21<4207::AID-ANGE4207>3.0.CO;2-G Google ScholarAngew. Chem. Int. Ed. 2002, 41, 4035.10.1002/1521-3773(20021104)41:21<4035::AID-ANIE4035>3.0.CO;2-I CAS PubMed Web of Science® Google Scholar
- 12For ROMP with other fast-initiating catalysts (but slower than 4), see:
- 12aU. Frenzel, T. Weskamp, F. J. Kohl, W. C. Schattenmann, O. Nuyken, W. A. Herrmann, J. Organomet. Chem. 1999, 586, 263;
- 12bC. Slugovc, S. Demel, F. Stelzer, Chem. Commun. 2002, 2572.
- 13ROMP of endo-n-alkyl norbornene dicarboxyimides with catalyst 1 gave polymers with PDI of 1.3, see: E. Khosravi, W. J. Feast, A. A. Al-Hajaji, T. Leejarkpai, J. Mol. Catal. A 2000, 160, 1. Crude solutions of polymers (i.e. without precipitation) were subjected to GPC analysis resulting in clean traces that displayed low PDIs. These traces show that the low PDIs are not a consequence of fractionation of polymers of low molecular weight by precipitation.
- 14This PNB contained 61 % of the cis-olefin isomer and is much higher than PNB produced with 3 (35 % cis), which undergoes chain-transfer reactions.[8 b]
- 15Ten equivalents of 8 were mixed with 4 at −10 °C resulting in complete initiation (the only propagating carbene signal in the corresponding 13C NMR spectrum was observed at δ=18.2 ppm) and 13 % product conversion. Based on the assumption of at least 99 % initiation, a minimum value of ki/kp=19 is calculated by using the Gold equation. See reference [1 b] page 232.
- 16Complexes with other substituted-pyridine ligands can show similar rapid initiation rates A. Hejl, R. Grubbs, unpublished results.
Citing Literature
This is the
German version
of Angewandte Chemie.
Note for articles published since 1962:
Do not cite this version alone.
Take me to the International Edition version with citable page numbers, DOI, and citation export.
We apologize for the inconvenience.