Efficient Asymmetry Generation in the Synthesis of Oxo-Rhenium(V) Complex cis-[ReOCl2{OCMe2CMe2OP(OCMe2CMe2O)}py]†
Witold K. Rybak Dr.
Faculty of Chemistry, University of Wrocław, 14 F. Joliot-Curie, 50-383 Wrocław, Poland, Fax: (+48) 71-3757-356
Search for more papers by this authorAnna Skarżyńska Dr.
Faculty of Chemistry, University of Wrocław, 14 F. Joliot-Curie, 50-383 Wrocław, Poland, Fax: (+48) 71-3757-356
Search for more papers by this authorTadeusz Głowiak Prof. Dr.
Faculty of Chemistry, University of Wrocław, 14 F. Joliot-Curie, 50-383 Wrocław, Poland, Fax: (+48) 71-3757-356
Search for more papers by this authorWitold K. Rybak Dr.
Faculty of Chemistry, University of Wrocław, 14 F. Joliot-Curie, 50-383 Wrocław, Poland, Fax: (+48) 71-3757-356
Search for more papers by this authorAnna Skarżyńska Dr.
Faculty of Chemistry, University of Wrocław, 14 F. Joliot-Curie, 50-383 Wrocław, Poland, Fax: (+48) 71-3757-356
Search for more papers by this authorTadeusz Głowiak Prof. Dr.
Faculty of Chemistry, University of Wrocław, 14 F. Joliot-Curie, 50-383 Wrocław, Poland, Fax: (+48) 71-3757-356
Search for more papers by this authorpy=Pyridine.
Graphical Abstract
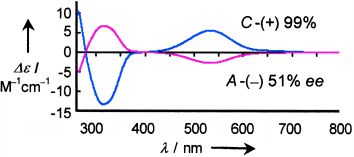
Und das alles ohne Spiegel: Ein einfaches Verfahren zur Gewinnung von Enantiomeren eines Oxorhenium(V)-Komplexes mit einem hohen Enantiomerenüberschuss (99 % ee) aus optisch inaktiven Vorstufen wird vorgestellt. Diese autokatalytische Synthese erfolgt nach der Komplexbildung durch die spontane Trennung des Konglomerats bei der Kristallisation unter Rühren. Die Ergebnisse werden durch Circulardichroismus-Spektroskopie bestätigt (siehe Bild).
Supporting Information
Supporting information for this article is available on the WWW under http://www.wiley-vch.de/contents/jc_2001/2003/z19501_s.pdf or from the author.
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1
- 1aE. L. Eliel, S. H. Wilen, L. N. Mander, Stereochemistry of Organic Compounds, Wiley, New York, 1994;
- 1bA. von Zelewsky, Stereochemistry of Coordination Compounds, Wiley, New York, 1996;
- 1c Comprehensive Asymmetric Catalysis (Ed ), Springer, Berlin, 1999.
- 2
- 2aM. Avalos, P. Babiano, P. Cintas, J. L. Jimenez, J. C. Palacios, L. D. Barron, Chem. Rev. 1998, 98, 2391–2404;
- 2bB. L. Feringa, R. A. van Delden, Angew. Chem. 1999, 111, 3624–3645;
10.1002/(SICI)1521-3757(19991203)111:23<3624::AID-ANGE3624>3.0.CO;2-X Web of Science® Google ScholarAngew. Chem. Int. Ed. 1999, 38, 3418–3438;10.1002/(SICI)1521-3773(19991203)38:23<3418::AID-ANIE3418>3.0.CO;2-V CAS PubMed Web of Science® Google Scholar
- 2cM. Avalos, P. Babiano, P. Cintas, J. L. Jimenez, J. C. Palacios, Tetrahedron: Asymmetry 2000, 11, 2845–2874.
- 3
- 3aI. Bernal, G. B. Kauffman, J. Chem. Educ. 1987, 64, 604–610;
- 3bJ. Jacques, A. Collet, S. H. Wilen, Enantiomers, Racemates and Resolutions, W. Krieger Publishing, Malabar Florida, 1994.
- 4aK. L. Stevenson, J. F. Verdieck, J. Am. Chem. Soc. 1968, 90, 2974–2975;
- 4bB. Norden, Acta Chem. Scand. 1970, 24, 23–25;
- 4cG. L. J. A. Rikken, E. Raupach, Nature 2000, 405, 932–935.
- 5
- 5aR. D. Gillard, F. L. Wimmer, J. P. G. Richards, J. Chem. Soc. Dalton Trans. 1985, 253–258;
- 5bE. H. M. Evans, J. P. G. Richards, R. D. Gillard, F. L. Wimmer, Nouv. J. Chim. 1986, 10, 783–791.
- 6
- 6aD. K. Kondepudi, R. Kaufman, N. Singh, Science 1990, 250, 975–976;
- 6bD. K. Kondepudi, K. L. Bullock, J. A. Digits, J. K. Hall, J. M. Miller, J. Am. Chem. Soc. 1993, 115, 10 211–10 216.
- 7
- 7aK. Asakura, K. Kobayshi, Y. Mizusawa, T. Ozawa, S. Osanai, S. Yoshikawa, Phys. D 1995, 84, 72–78;
- 7bK. Asakura, D. K. Kondepudi, R. Martin, Chirality 1998, 10, 343–348;
- 7cK. Asakura, A. Ikumo, K. Kurihara, S. Osanai, D. K. Kondepudi, J. Phys. Chem. 2000, 104, 2689–2694.
- 8S. Mahurin, M. McGinnis, J. S. Bogard, L. D. Hulett, R. M. Pagni, R. N. Compton, Chirality 2001, 13, 636–640.
- 9A. Szabó-Nagy, L. Keszthelyi, Proc. Natl. Acad. Sci. USA 1999, 96, 4252–4255.
- 10
- 10aT. Głowiak, W. K. Rybak, A. Skarżyńska, Polyhedron 2000, 19, 2667–2672;
- 10bA. Skarżyńska, W. K. Rybak, T. Głowiak, Polyhedron 2001, 20, 2667–2674.
- 11
- 11aN. P. Johnson, F. J. M. Taha, G. W. Wilkinson, J. Chem. Soc. 1964, 2614–2618;
- 11bM. Freni, D. Giusto, P. Romiti, G. Minghetti, Gazz. Chim. Ital. 1969, 99, 286–299;
- 11cA. Guest, C. J. L. Lock. Can. J. Chem. 1971, 49, 603–610.
- 12
- 12aR. R. Holmes, J. Am. Chem. Soc. 1974, 26, 4143–4149;
- 12bR. Burgada, Bull. Soc. Chim. Fr. 1975, 407–424.
- 13Crystal structure determination of cis-C-(+)535-[ReOCl2{OCMe2CMe2OP(OCMe2CMe2O)}py], C17H29Cl2NO5PRe, orthorhombic, space group P212121, a=9.988(2), b=11.655(2), c=18.301(4) Å, V=2130.4(7) Å3, Z=4, ρcalcd=1.919 Mg m−3, μ=6.057 mm−1, F(000)=1208, λ(MoKα)=0.71073 Å, T=90(1) K, crystal dimensions 0.1×0.1×0.15 mm, 2θmax=57.28°, 14 435 reflections collected, 5031 unique; R indices R1=0.0199, wR2=0.0515 (all data) and Flack parameter 0.024(5). For the opposite absolute configuration A: R1=0.0501, wR2=0.1268 and Flack parameter 0.973(6). The data collected on KM4CCD camera diffractometer were corrected for Lorentz and polarization effects. Data reduction and analysis were carried out using the Kuma Diffraction (Wrocław) programs. The structure was solved by means of heavy atom methods using SHELXS-97 and refined by the full-matrix least-squares method on all F2 using SHELXL-97 procedures (G. M. Sheldrick, Universität Göttingen, 1997). Non-hydrogen atoms were refined with anisotropic thermal parameters. Hydrogen atoms were included from the Δρ maps and refined with isotropic thermal parameters. CCDC-167174 (1) contains the supplementary crystallographic data for this paper. These data can be obtained free of charge via www.ccdc.cam.ac.uk/conts/retrieving.html (or from the Cambridge Crystallographic Data Centre, 12 Union Road, Cambridge CB2 1EZ, UK; fax: (+44) 1223-336-033; or [email protected]).
- 14Five different individual crystals grown from highly enriched dextrorotatory (+)535, polycrystalline products (set c in Figure 2) consistently revealed similar Flack parameters and the same C stereochemistry in X-ray analyses, which indicates that there was no racemic twinning of the single crystals and the high optical purity of these polycrystalline products.
- 15The six dextrorotatory-originated crystalline samples, each containing five single crystals, yielded six very similar CD spectra in acetonitrile solution (Experimental Section), corroborating the homochirality of the single crystals and the optical purity of the obtained six crystalline samples.
- 16
- 16aD. K. Kondepudi, J. Laudadio, K. Asakura, J. Am. Chem. Soc. 1999, 121, 1448–1451;
- 16bD. K. Kondepudi, K. Asakura, Acc. Chem. Res. 2001, 34, 946–954.
- 17
- 17aI. Sato, K. Kadowaki, K. Soai, Angew. Chem. 2000, 112, 1570–1572;
Angew. Chem. Int. Ed. 2000, 39, 1510–1512;
10.1002/(SICI)1521-3773(20000417)39:8<1510::AID-ANIE1510>3.0.CO;2-R CAS PubMed Web of Science® Google Scholar
- 17bK. Soai, T. Shibata, I. Sato, Acc. Chem. Res. 2000, 33, 382–390.
- 18
- 18aC. Girard, H. Kagan, Can. J. Chem. 2000, 78, 816–828;
- 18bD. G. Blackmond, Acc. Chem. Res. 2000, 33, 402–411.
- 19D. G. Blackmond, Ch. R. McMilan, S. Ramdechul, A. Schorm, J. M. Brown, J. Am. Chem. Soc. 2001, 123, 10 103–10 104.
- 20The cis complex, C or rac, stirred and boiled in toluene for 5 h, affords the trans complex in 10.5 % or 17.5 % yields, respectively, and the corresponding cis complex with ee +99 % (C) or ee 0 % (rac). After another 15 h reaction time, no further conversion was observed within the experimental error (Supporting Information).
Citing Literature
This is the
German version
of Angewandte Chemie.
Note for articles published since 1962:
Do not cite this version alone.
Take me to the International Edition version with citable page numbers, DOI, and citation export.
We apologize for the inconvenience.