The endothelial glycocalyx—All the same? No, it is not
Abstract
The endothelial glycocalyx covers the lumen of blood vessels throughout the body and plays an important role in endothelial homeostasis. Advances in electron microscopy techniques have provided clues to better understand the structure and composition of identical vascular endothelial glycocalyx. The morphology and thickness of the endothelial glycocalyx differ from organ to organ. The content of the endothelial glycocalyx covering the vascular lumen differs even in the brain, heart, and lungs, which have the same continuous capillaries. Various types of inflammation are known to attenuate the endothelial glycocalyx; however, we found that the morphology of the glycocalyx damaged by acute inflammation differed from that damaged by chronic inflammation. Acute inflammation breaks the endothelial glycocalyx unevenly, whereas chronic inflammation leads to the overall shortening of the endothelial glycocalyx. The same drug has different effects on the endothelial glycocalyx, depending on the location of the target blood vessels. This difference in response may reflect not only the size and shape of the endothelial glycocalyx but also the different constituents. In the cardiac tissue, the expression of glypican-1, a core protein of the endothelial glycocalyx, was enhanced. By contrast, in the pulmonary tissue, the expression of heparan sulfate 6-O-sulfotransferase 1 and endothelial cell-specific molecule-1 significantly increased in the treatment group compared with that in the no-treatment group. In this review, we present the latest findings on the evolution of the vascular endothelial glycocalyx and consider the microstructural differences.
INTRODUCTION
Blood vessels are located throughout the body and deliver oxygen, nutrients, and electrolytes to the organs and tissues. The blood vessels, which comprise arteries, arterioles, capillaries, venules, and veins, are components of the circulatory system. The aorta from the heart branches six to eight times to form small arteries, which in turn branch two to five times to form capillaries with diameters ranging from 5 to 20 μm. Capillaries comprise a single layer of endothelial cells without a muscle layer.1 All vascular lumen, including the capillaries, are lined with complexes of glycoproteins and polysaccharides called the glycocalyx. The glycocalyx is a gel-like layer with a bush-like structure that lines the lumen of blood vessels. The endothelial glycocalyx is in constant contact with blood, which carries substances throughout the body, and is a wall that separates the blood from tissues. The endothelial glycocalyx not only functions as a physical wall separating blood and tissues but also possesses various functions, such as the regulation of vascular permeability,2 maintenance of smooth blood flow,3 mechanotransduction in the blood vessels,4 inhibition of blood coagulation,5, 6 and leukocyte adhesion.7 The endothelial glycocalyx covers the vascular lumen and plays a pivotal role in the physiological functions of endothelial cells. Advances in the electron microscopy analysis of the endothelial glycocalyx have facilitated the understanding of the endothelial glycocalyx morphology in detail and helped detect changes under various conditions. Visualizing morphological changes in the glycocalyx could lead to discoveries.
In this review, we report on the structure, function, and medicinal reactions of the endothelial glycocalyx following injury.
STRUCTURE OF THE VASCULAR ENDOTHELIAL GLYCOCALYX
The endothelial glycocalyx comprises glycoproteins and polysaccharides on the vascular endothelial surface. The glycocalyx is a network of proteoglycans and glycoproteins that are directly bound to the endothelial cell luminally, and glycans, such as hyaluronan and thrombomodulin, are indirectly bound to the cell membrane.8
Proteoglycans are complex carbohydrates composed of polysaccharides and proteins, of which syndecans and glypicans are representative.8, 9 Syndecans are a small family of heparan sulfate proteoglycans, which are type I transmembrane glycoproteins. They consist of an N-terminal polypeptide with multiple glycosaminoglycan chains attached to specific serine residues in the protein core, a single transmembrane domain, and a C-terminal cytoplasmic domain comprising three domains. There are four members of syndecans in mammals, of which syndecan-1, -2, and -4 are present on vascular endothelial cells.10 Glypicans are heparan sulfate proteoglycans anchored to the cell membrane by glycosylphosphatidylinositol linkages.11, 12 There are six types of glypicans in mammals. All glypican members preserve the localization of 14 cysteine residues by conserving their three-dimensional structure.11 Only glypican-1 is present in vascular endothelial and smooth muscle cells.13 There is a difference in the structures that constitute these core proteins. Syndecan-2 and -4 have only heparan sulfate, whereas syndecan-1 contains both heparan sulfate and chondroitin sulfate in the extracellular domains.12 Glypicans comprise heparan sulfate in their extracellular domains.12
The glycosaminoglycans that bind to the extracellular domain of the core protein are heparan sulfate and chondroitin sulfate. Heparan sulfate is a linear polysaccharide composed of repeated hexuronic acid and d-glucosamine disaccharides.14 Heparan sulfate can polymerize to form two or more disaccharide units, catalyzed by exostosin glycosyltransferases 1/2 (EXT1/2). The structures of heparin sulfate are diversified by sulfation patterns.15 Hyaluronan, a linear polysaccharide in the extracellular matrix composed of repeating N-acetylglucosamine and glucuronic acid, does not directly bind to the cell membrane.16, 17 Hyaluronan is differentiated from other glycosaminoglycans because it is the only nonsulfate glycosaminoglycan.18
Glycoproteins, such as proteoglycans, are directly bound to the cell membrane. The major glycoproteins are endothelial cell adhesion molecules and components of the coagulation and fibrinolysis systems. The three families of cell adhesion molecules present in the endothelial glycocalyx are the selectin, integrin, and immunoglobulin superfamilies.8, 9 These adhesion molecules are shorter than proteoglycans and are usually coated with proteoglycans; however, when proteoglycans are detached due to inflammation, they are involved in blood cell adhesion.19
EACH STRUCTURE OF THE ENDOTHELIAL GLYCOCALYX LOOKS ALIKE BUT IS NOT THE SAME
Using an electron microscope, endothelial structures can be classified into three types of capillaries: continuous, fenestrated, and sinusoidal.20-22 The difference in structure can be confirmed using scanning electron microscopy or transmission electron microscopy. However, it is impossible to observe the endothelial glycocalyx using conventional fixation methods because of its fragility and instability.23 Detection of the endothelial glycocalyx using electron microscopy requires careful staining with lanthanum nitrate using a perfusion pump.24, 25 This electrical staining approach revealed that the endothelial structures and the endothelial glycocalyx differed depending on the organ and its function.
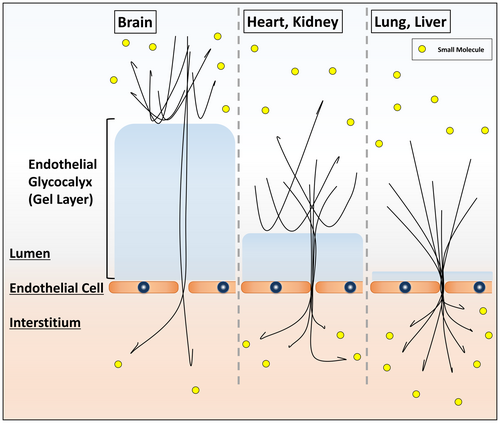
Continuous capillaries are the most common type of structure and are found in the lungs, heart, brain, skeletal muscle, and nerves, where endothelial cells must be tightly interlocked. Conventional scanning electron microscopy examination of the luminal side of the cardiac, pulmonary, and cerebral capillary endothelia revealed the presence of an uninterrupted endothelium and continuous basal lamina.25-27 Electron staining with lanthanum nitrate clarified that the moss- or broccoli-like vascular endothelial glycocalyx of each endothelial lumen was paved without any interruption in the endothelial lumen. However, they are not uniform and appear distinctly different depending on the organ. The pulmonary endothelial glycocalyx is a thin layer that does not narrow the capillary lumen. The cerebral endothelial glycocalyx is a thick layer that primarily occupies the lumen. In comparison with these, the cardiac endothelial glycocalyx can be described as an intermediate layer. Transmission electron microscopy can quantify and confirm the presence of the glycocalyx on the surface of endothelial cells and revealed that the percentages of endothelial glycocalyx area in the heart, lungs, and brain were 15.1%, 3.7%, and 40.1%, respectively.25, 27
The structure of the endothelial glycocalyx has a great influence on the function of the organ, and it is even possible that its morphology is shaped according to the organ's requirements. To maintain homeostasis in the brain, the blood–brain barrier blocks harmful substances from entering the brain, thereby protecting the normal functioning of cerebral cells.28 The endothelial glycocalyx, which dominates the cerebral vascular endothelium, plays an important role in the blood–brain barrier blockade. Experimentally, the endothelial glycocalyx prevents the penetration of large molecules into the brain parenchyma compared with small molecules.29 Furthermore, the thickness of the endothelial glycocalyx determines the difference in substance permeability depending on the organ27 (Figure 1).
The endothelial cells of fenestrated capillaries found in the renal glomeruli have a characteristic morphology in which small pores of 60–80 nm are regularly arranged. However, lanthanum nitrate staining revealed that the negatively charged glycocalyx narrowed and almost blocked the pores to a width of 15 nm, which is the actual condition in capillaries.25, 30 Moreover, the glycocalyx covers the surface of podocytes.25 Thus, albumin and larger molecules are not normally filtered into the tubular fluid.30 The percentage of the capillary endothelial glycocalyx area is 16.7%, which is the same as that of the cardiac vascular area. Sinusoidal capillaries are found in hematopoietic organs such as the spleen and bone marrow, besides the liver. There are irregular oval pores over the endothelial lumen due to weak connections between the endothelial cells. The basement membrane is not well developed, and compared with other types of capillaries, the lumen is wide, and blood flow is slow. Therefore, the passage of substances is easy, and the blood communicates with the Disse space (perisinusoidal space) through this hole and exchanges substances such as bilirubin.1 The endothelial glycocalyx–covered sinusoidal capillaries narrow the large pore, but it is insufficient to close the hole and is similar to that in the renal capillaries. The percentage of endothelial glycocalyx area in capillaries is 3.7% that of the pulmonary vasculature.25 It is believed that the renal endothelial glycocalyx exists to block the small pores so that the necessary substances do not exit the capillaries, whereas the hepatic vasculature does not close the pores, to facilitate the exchange of substances.
ACUTE AND CHRONIC INFLAMMATION ARE ALIKE IN APPEARANCE BUT QUITE DIFFERENT IN NATURE
The endothelial glycocalyx is an unstable and fragile structure that is damaged by various stimuli. In acute conditions, such as sepsis,31 ischemia–reperfusion injury,32 trauma,33, 34 acute respiratory distress syndrome,35 and burns,36 and in chronic diseases, such as diabetes mellitus,37 chronic kidney disease,38 and hyperlipidemia,39 there are some findings where the endothelial glycocalyx appears disrupted. Electron microscopy revealed that the manner of glycocalyx damage differed depending on the disease group.
In a recent study by Sampei et al.,40 C57BLKS/J Iar−m+/+leprdb/(db/+) mice were intraperitoneally administered saline or lipopolysaccharide (LPS; 15 mg/kg), as healthy or acute endotoxemia models, and C57BLKS/J Iar−+ leprdb/leprdb (db/db) were used as chronic diabetes mellitus models, in investigating the effects of inflammation. Because of its anatomical characteristics, the endothelial glycocalyx is most exposed to exogenous pathogen-associated molecular patterns and endogenous damage-associated molecular patterns during bacterial, fungal, or viral infection, accompanied by massive inflammatory mediators, and it also serves as a barrier to prevent the spreading of inflammation.41 The endothelial glycocalyx is disrupted throughout the blood vessels. The vascular endothelial glycocalyx appears to be lined throughout the pulmonary microvessels in healthy mice (Figure 2A). In the chronic condition group, the characteristics of the glycocalyx were that it appeared to be short, with grass-like turf (Figure 2B). However, in the acute inflammation group, the vascular endothelial glycocalyx was destroyed, and the endothelial cell surface was exposed to the lumen of the vascular endothelium (Figure 2C).
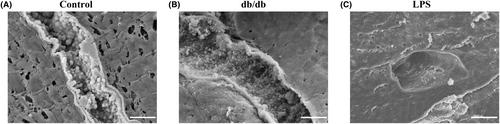
There are several possible mechanisms underlying this phenomenon. First, in the acute condition group, although inflammation occurred throughout the body, possibly unevenness occurred at the vascular endothelium level. Numerous spherical structures within the blood vessels have been reported.26 Even if some of the core proteins are peeled off, the adjoining endothelial glycocalyx is bound to glycosaminoglycans and hyaluronans; therefore, it is thought that they are not completely peeled off and form clumps. In the chronic condition group, there may have been an imbalance between injury and synthesis caused by inflammation. We advocate that the existence of the vascular endothelial glycocalyx is related to the balance of these two factors: inflammatory injury and synthesis of the endothelial glycocalyx.
Patients with diabetes mellitus are constantly exposed to hyperglycemia, and endothelial damage occurs through several mechanisms. The endothelial glycocalyx is decreased, and plasma hyaluronan and hyaluronidase levels are increased in patients with type 1 diabetes mellitus.42 It has been demonstrated that activated neutrophils induce endothelial damage in type 2 diabetes mellitus.43 The expression of EXT1, an essential gene for the synthesis of heparan sulfate; Csgalnact1, an essential gene for the synthesis of chondroitin sulfate; and VCAN, which encodes versican as the core protein of the glycocalyx in db/db mice, were significantly lower than those in healthy mice.40 This could be one reason the vascular endothelial glycocalyx remained short in the chronic diabetes group.
THE MEDICINAL REACTION OF THE ENDOTHELIAL GLYCOCALYX MIGHT BE DIFFERENT FOR EACH ORGAN
Recent findings have revealed that identical drugs may have different effects on the endothelial glycocalyx, depending on the organ.44 There are several possible mechanisms through which drugs act beneficially on the endothelial glycocalyx. Two factors, namely inflammatory injury and synthesis of the endothelial glycocalyx, clarify these factors.
The first involves protecting the endothelial glycocalyx from inflammation. Anti-inflammatory agents reduce inflammation and protect the endothelial glycocalyx. Treatment with anti-inflammatory agents in vasculitis model mice reduced the degree of damage to the vascular endothelial glycocalyx. Neutrophil elastase is induced by activated neutrophils to sterilize phagocytosed bacterial and fungal pathogens under septic conditions. However, neutrophil elastase damages the endothelial glycocalyx in tissues that accumulate extravasated neutrophils. Treatment with the neutrophil elastase inhibitor preserves the cardiac and pulmonary endothelial glycocalyx under LPS-induced endotoxemia.45 Recombinant human thrombomodulin (rhTM) treatment also significantly reduces endothelial glycocalyx damage by exerting an anti-inflammatory effect through high mobility group box 1 (HMGB1) adsorption44 that did not affect the endothelial glycocalyx. To identify the effects of rhTM on LPS-induced endotoxemia in mice, gene set enrichment analysis was performed on the hearts and lungs of saline- and rhTM-treated mice. Interestingly, rhTM treatment promoted the synthesis of endothelial glycocalyx in both organs, but there were differences between the groups of upregulated genes. In cardiac tissue, the expression of glypican-1, one of the core proteins of the endothelial glycocalyx, was enhanced in the rhTM-treated group compared with that in the saline-treated group.46 Glypican 1 is a core protein involved in cardiovascular functions. Glypican 1 regulates vascular tone by activating endothelial cell nitric oxide synthase. Flow-induced endothelial cell nitric oxide synthase activation was inhibited in bovine aortic endothelial cells, in which glypican 1 expression was knocked down by glypican 1 short hairpin RNA transfection.47 Glypican 1 is also mediated by heparan sulfate chains and binds to the vascular endothelial growth factor, an angiogenic factor. Glypican 1 addition restored the activity of vascular endothelial growth factor, which was inactivated by the generation of oxidants and free radicals.48 In the pulmonary tissue, the expression of heparan sulfate 6-O-sulfotransferase 1 (HS6ST1) and endothelial cell-specific molecule-1 (ESM-1) was significantly increased in the rhTM-treated group compared with that in the saline-treated group.44 HS6ST1 is an essential component of the lungs because the latter do not develop normally in HS6ST-1–deficient mice.49 The expression of HS6ST1 is associated with the synthetic repair of heparan sulfate.50 Transforming growth factor-β1 is known to increase sulfatase 1 (Sulf1) activity by upregulating Sulf1 mRNA expression, thereby reducing 6-O-sulfation levels.51 Similar reactions may occur during sepsis because the levels increase in this case. ESM-1 is highly localized in pulmonary vascular endothelial cells, where its synthetic secretion also occurs.52 ESM-1 is known to synthesize endocan, a heparan sulfate derived from pulmonary endothelial cells.53
As mentioned previously, the length and shape of the glycocalyx differ depending on the organ. It has been found that these differences are not only due to the size and length but also due to their constituent components. Suzuki et al.1 used several lectins to describe various carbohydrate components of the endothelial glycocalyx. Lectins have specific carbohydrate-binding activity, and this characteristic reveals differences in the carbohydrate-coating structures of the endothelial glycocalyx.1 Concanavalin A (ConA) and Dolichos biflorus agglutinin recognize N-acetylgalactosamine and mannose side chains of glycocalyx glycoproteins, respectively. Immunological staining showed that Dolichos biflorus agglutinin binds to the endothelial glycocalyx of mouse pulmonary capillaries. However, it did not bind to the endothelial glycocalyx of mouse cardiac capillaries.1 These findings indicate that the glycoproteins of the pulmonary endothelial glycocalyx have glycan components that differ from those of the cardiac endothelial glycocalyx. The differences in the endothelial glycocalyx components may contribute to and determine the medicinal reaction of the endothelial glycocalyx.
FUTURE PROSPECTS
The endothelial glycocalyx is a complex of glycoproteins and polysaccharides on the surface of blood vessels. However, its morphology and structure differ depending on the organ, resulting in differences in its function. Furthermore, it has been found that the manner of the endothelial glycocalyx injury differs depending on the type of inflammation. These findings may serve as a fundamental principle for developing anti-inflammatory treatments according to the pathological condition of individual patients and for developing endothelial glycocalyx protection strategies for each organ and inflammation. Appropriate anti-inflammatory therapy may prevent progression to multiple organ failure and improve the patient's long-term and short-term prognoses. This novel therapeutic strategy targeting the vascular endothelium has the potential to be a breakthrough in all diseases that cause vascular endothelial disorders.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflict of interest.
ETHICS STATEMENT
Approval of the Research Protocol: N/A
Informed Consent: N/A
Registry and the Registration No. of the Study/Trial: N/A.
Animal Studies: N/A.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.




