dP/dtmax: An underestimated prognostic factor in large animal infarction model
Abstract
The present study aims to establish a reproducible large animal experimental unit using a minipig model to monitor cardiac function changes. A 90-min closed-chest balloon occlusion of the left anterior descending branch of the coronary artery was used to induce myocardial infarction in Pannon minipigs. To monitor the cardiac function, measurements were made by cardiac magnetic resonance imaging (cMRI), invasive pressure monitoring, and a Pulse index Continuous Cardiac Output (PiCCO) hemodynamic system at 0, 72, and 720 h during the follow-up period. End-diastolic and end-systolic volumes (EDV, ESV), left ventricular ejection fraction (LVEF) obtained by cMRI evaluation, global ejection fraction and aortic dP/dtmax obtained by the invasive method, were recorded and compared. The 72- and 720-h EDV data showed a significant increase (p = 0.012, <0.001) compared to baseline, and the Day 30 data showed a significant increase compared to Day 3 (p = 0.022). The ESV 72 h after the infarction showed a significant increase (p = 0.001) compared to baseline, which did not change significantly by Day 30 (p = 0.781) compared to Day 3. EDV and ESV were significantly negatively correlated with aortic dpmax, and ESV was significantly correlated with LVEF. For LVEF and dPmax, a significant (p < 0.001 and p = 0.002) worsening was demonstrated at Day 3 compared to baseline, which was no longer statistically detectable for LVEF at Day 30 (p = 0.141), while the difference for dPmax was maintained (p = 0.002). The complementary use of PiCCO hemodynamic measurements in large animal models makes the previously used methodologies more robust and reliable.
1 INTRODUCTION
Cardiovascular disease (CVD) is one of the leading causes of death worldwide, accounting for 31% of all deaths, affecting nearly 17.9 million people annually.1 Acute myocardial infarction (AMI), and its complication, ischemic heart failure (HF), is one of the principal causes of death and permanent disability worldwide.2 To reduce the size of myocardial infarction (MI) and prevent heart failure, novel therapies are needed to protect the heart, for which translational large animal model experiments are essential. In this field, porcine models have played an important role in recent decades. Nowadays pigs, rather than dogs and monkeys, are the preferred experimental animals for non-rodent models, because the similarity between human and porcine organs, with a high degree of similarity in the skin, cardiovascular, gastrointestinal, and urinary excretory systems.
Within the pig breed, the use of minipigs is becoming more widespread compared to traditional domestic pigs because minipigs are smaller at maturity, have slower growth rates, are easy to handle, and their genetic and microbiological status is considered homogeneous. An additional advantage of using adult minipigs, in addition to their size stability, is that they can be used to model cardiovascular disorders and diseases more effectively than juvenile conventional domestic pigs. The most commonly studied pathologies are typically found in older age groups, and it is advisable to observe the clinical responses in adult animals. In this way, the regenerative capacity of the young organism has less influence on the value of the model.
In biomedical research, besides commercial fattening pigs, several minipig breeds, such as Göttingen, Hanford, Yucatan, Sinclair, Minnesota, CLAWN, Ohmini, NIBS etc., have been used for decades.3, 4 The Yucatan minipig is a naturally occurring breed, gray in color and with an adult weight of 70–80 kg. This breed is a little difficult to handle.4 Tibetan minipigs are 12–14 kg at the age of 3 months old, when they are normally used in experiments.5 These breeds have various features, with a decrease in adult size being the main breeding goal.
In many countries, minipigs are bred purely for research purposes, using the Asian pot-bellied breeds and even normal-sized pigs. In vivo and post-mortem studies of Sinclar, Hanford, and Yucatan minipigs show that their circulatory systems and related biochemical parameters make them suitable for testing human devices and related pharmaceutical and biochemical research.6
The most important and well-regarded breed of laboratory swine is the Göttingen minipig, which has proved to be a relevant laboratory animal for decades.7 In cardiovascular research, Göttingen minipigs have several advantages, such as genetically stable features and a heart with anatomical and functional features similar to the human heart.7 Based on the Göttingen minipig, a valid non-rodent cardiac model was developed, which is a valid pharmacology model for safety testing in the case of cardiovascular diseases.8 The Göttingen minipig was not developed primarily for the purpose of cardiovascular modeling, and therefore cardiovascular anatomical and functional uniformity was not a focus during breeding programs. For this reason, frequent instances of sinus arrhythmia and ECG irregularities can occur.9
Based on data gathered from the literature, the use of minipigs in animal experiments also can contribute to improvement in the 3Rs during research activities. The general expectation of a well-designed animal experiment is to produce definite results, which is inconceivable without high quality laboratory animals with both health and breeding certificates.10 To ensure the reliability and validity of preclinical cardiovascular porcine models, breeders must provide high quality, healthy experimental animals. Thus, the voluntary participation of breeders is necessary to develop breeding programs for homogeneous populations with uniform cardiovascular systems,9 and this was the key-point in the establishment of the Pannon Minipig herd.
The Pannon Minipig breed originated as a result of several years of selection in Kaposvár aimed at creating a homogeneous Hungarian stock for research purposes. Based on previous cardiovascular research in Kaposvár, the need for an in-house-bred, cost effective minipig, which could also be used for chronic animal studies became an urgent task.
The objective of our research is to gather data about the Pannon minipig as an animal model for myocardial infarctions and establish physiological characteristics and differences and develop the breed-specific elements of experimental methodology.
AMI can be induced in porcine model by closure of the coronary artery using open- or closed-chest surgical techniques. In order to be able to model the most common human infarct case (percutaneous intervention followed by revascularization), the closed-chest intravascular balloon ischemia/reperfusion pig model is most suitable. The main advantage of the percutaneous intervention is the reduction of complications during surgery and it is also preferential from an animal welfare point of view, although the angiographic device is specifically required.11 Although cardiac necro enzyme levels can be used to detect myocardial damage caused by ischemia in pigs, and even in human medicine,12 the functional changes of the left ventricle can be monitored profoundly using imaging procedures. The most effective imaging method to measure changes in AMI is still debated. Ultrasound is a non-invasive method, but also needs general anesthesia in pigs. Speckle-tracking echocardiography is more common and easier to implement than cardiac magnetic resonance imaging (cMRI), though cMRI is considered as the reference method for analysis of ventricular function and mass, because it supplies higher reproducibility and superior image quality with less interference from anatomical structures in the chest.13, 14 Today, one of the most commonly used models for AMI is the 90-min closed-chest balloon occlusion followed by reperfusion, monitoring the lesions with late contrast-enhanced MRI scans and further therapeutic management of the disease. The interventional approach to myocardial infarction development is standardized, as is the examination of the damaged myocardium with cMR imaging, and human cardiac scan sequences are adapted by the researchers to the pig. The “gold standard” in this is the late enhanced gadolinium contrast scan, which can be used to determine the location and size of the infarct and the extent of edema. The cMRI method also provides ventricular functional data, such as left ventricular ejection fraction (LVEF) – the main parameter of pump function, from the moving image series.
Accurately performing and evaluating cMRI measurements in pigs is not always straightforward. The shape and thoracic position of the heart and the lenght of the ventricles often differ from human anatomy, and therefore the longitudinal axis of the heart can be difficult to determine and align during imaging. At the same time, the optimal imaging of the apex and the slices in the plane of the valves in short-axis images of the left ventricle can be problematic.
Consequently, the accurate segmentation of image slices using a software package optimized for the human heart can be difficult, even for the experienced analyst, and significant differences in derived parameters can arise between evaluators. As the left ventricular volumes (LVV), and especially the left ventricular ejection fraction (LVEF) values, are currently the basis of many decision mechanisms for the efficacy of a new molecule in preclinical drug trials, the additional use of a relatively simple method is justified. The use of reliable hemodynamic parameters in pig trials can provide clinically relevant, accurate, and objective data.
It is important to recognize that left ventricular (LV) dysfunction in animal model experiments is often mild to moderate, as a balance must be struck between the extent of ischemic damage and the size of the resulting infarct versus the animals' propensity for fatal arrhythmias. Although all models result in acute hemodynamic changes in the first 24 h, consistent with acute MI, the longer-term (1–4 weeks) effect influences key hemodynamic indicators of the heart failure. Therefore, the majority of large animal models of coronary ischemia should be considered as models of AMI with or without mild-to-moderate LV dysfunction rather than as models of heart failure per se.15
The impairment of LV contractility can also be determined by the change in dP/dtmax values.16, 17 In addition, the first derivative of the aortic pressure peak, Ao_dP/dtmax, can also be used to assess the change in LV contractility if true preload independence is achieved.18, 19
The benefits of using the PiCCO (Pulse index Continuous Cardiac Output) system have been described in several cases of septic shock, acute respiratory distress syndrome, and necrotizing pancreatitis.20 The PiCCO system integrates a wide range of static and dynamic hemodynamic data with a combination of trans-cardiopulmonary thermodilution and pulse contour analysis. Its use requires intra-arterial and central venous catheterization, which may limit its clinical application; however, this is not a difficulty in the porcine model.21
In cardiovascular diseases, the additive PiCCO hemodynamic monitoring system can be used to detect left ventricular remodeling after MI, giving knowledge of the volumetric parameters. Also in animal model experiments, induced ischemia leads to LV remodeling characterized by altered end-systolic and end-diastolic volumes (ESV, EDV) and pressures (ESP, EDP). As a prognostic factor, ESV is the primary predictor of survival after myocardial infarction and is more accurate than ejection fraction.22
During the evaluation of cMRI images the accurate segmentation of slices can be challenging even for a trained analyst. Because of this challenge, significant variation can occur in the values of derived parameters. A recently published meta-analysis suggests that there is a lack of standardization in image analysis for cMRI. Pooled results show significant differences in left ventricular parameters by sex, age, and ethnicity. Other published studies have reported that the accuracy of left ventricular volume and left ventricular ejection fraction (LVEF) measurements is challenged by the delineation of the mitral valve and apical plane on short-axis images, which is expected to contribute an uncertainty of 8% to LVEF.
A model with measurable hemodynamic data would reduce the number of elements in the most-criticized large animal studies, in line with the 3R rule (Replace, Reduce, and Refine).
The present study aims to adapt the standardized closed-chest ischemia/reperfusion acute MI model developed in meat-type pig breeds to adult Pannon minipigs and to complement the gold standard cMRI with a hemodynamic parameter characterizing contractility, which will allow a more accurate and objective assessment of the cardiac function changes accompanying the infarction.
2 METHODS
All institutional and national guidelines for the care and use of laboratory animals were followed and approved by the appropriate institutional committees.
The experimental protocol and the ethical guidelines of this study were approved by the Somogy County Government Office under license number SOI/31/00161-7/2020.
A total of 24 female Pannon minipigs weighing 55–90 kg and aged 15–24 months were used in the experiment. When determining the number of animals, our aim was to create an experimental design that can be analyzed using appropriate statistical methods. In our study, we performed statistical analysis of the quantitative data from the experimental endpoints. Each animal was also its own statistical control. because The ANOVA method and the so-called “E” value (degree of freedom) were used to establish the sample size, because there are no available previously published data for effect size and standard deviation values for Pannon minipigs. The rate of sample reduction was calculated as 30%. The sample size was approved by the Scientific Ethics Council for Animal Experiments and by the Government Office for Somogy County, applying the 3R method.
The animals were supplied from a herd free from Aujeszky's disease, brucellosis, PRRS, and leptospirosis, and an African swine fever-free area. The pigs were fed a high fiber, low energy feed (“Agroszasz experimental pig feed” containing 88.2% dry matter, 14.01% crude protein, 2.57% crude fat, 10.13% crude fiber, 11.35 MJ/kg digestible energy) at 3% of body weight per day. Bedding straw was provided as manipulable material and as a hiding place. Animals were housed in free-range groups to allow them to exercise natural behavioral patterns.
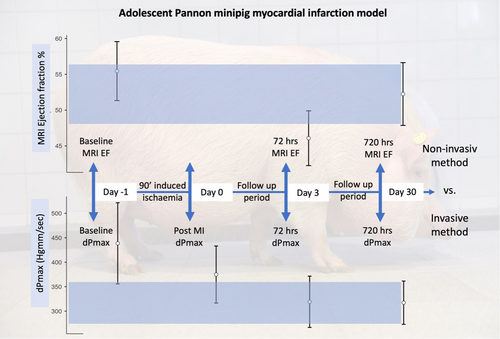
2.1 cMRI examinations
We performed baseline cardiac MRI before the infarct induction and viability tests on Days 3 and 30 during the follow-up period. Imaging was performed after 12 h of fasting. Animals were sedated with a mixture of 12 mg/kg ketamine hydrochloride (Narkamon 100 mg/mL 50 mL inj., Bioveta), 1 mg/kg xylazine (CP-Xylazine 2% 50 mL inj., CP-Pharma GmbH) and 0.04 mg/kg atropine sulfate (Atropinum-sulfuricum 0.1% inj., Egis). After endotracheal intubation (5.0–6.0 tube diameter), the anesthesia was maintained by inhalation of 1.5–2.5 vol% isoflurane (Isoflutek 1000 mg/g, Karizoo) and 2 L/min of oxygen gas mixture. The MRI scan was performed with Siemens Magnetom Vision Plus 1.5T equipment, with ECG control. To improve image quality, cine scans in the long- and short-axis planes of the heart were performed with intravenous administration of 1.5 mg/kg atracurium besylate (Tracrium 50 mg/mL inj., GlaxoSmithKline) with respiratory arrest and artificial ventilation (10 mL/kg 13/min). Image analysis was performed separately by three investigators (GR, KD, and VA) using the freely available Segment software.23 For the evaluation, consensus results of MR parameters were used (Table S1).
2.2 Invasive measurements and infarction-induction
Three healthy minipigs were used to develop the methodology for invasive hemodynamic monitoring and collecting breed-specific PiCCO baseline data. Data from these animals were not used for analysis.
In the ischemia–reperfusion (IR) model animals, a 6 F introducer was inserted into the femoral artery after surgical exploration, and 5000 IU of heparin was administered through the introducer following baseline imaging.
For the invasive hemodynamic monitoring and recording of specific baseline values, a 4F size transpulmonary thermodilution catheter (PiCCO, PULSION Medical Systems SE, Munich, Germany) was passed through the already inserted introducer and was connected to the PiCCO hemodynamic monitor. In addition, a 7F size central venous catheter was inserted into the right jugular vein. All hemodynamic parameters were indexed by body surface area values, which were determined from previously performed CT scans.
The PiCCO system uses pulse contour analysis according to a modified version of the Wesseling algorithm. This pulse-contour algorithm analyses the actual shape of the pressure wave, as well as the part of the pressure wave below the systole.24, 25
Baseline measurements were taken before induction of the infarct, and after switching to a guide catheter, a balloon catheter 2.5–2.75 mm in diameter and 8–15 mm long was inserted into the left anterior descending branch (LAD) and inflated at 5–6 atm for 90 min to occlude blood flow after the second diagonal origin (Picture 1).
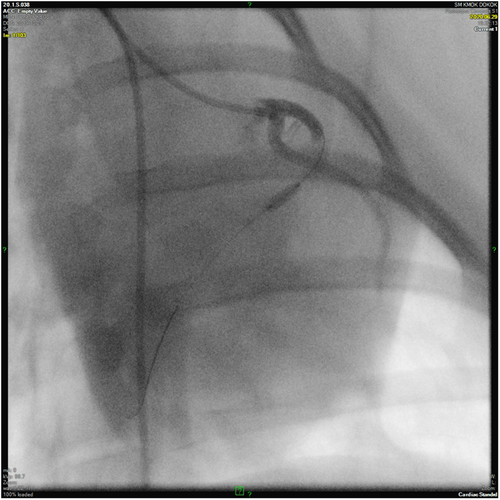
The balloon-occlusion, reperfusion and ventricular function were monitored by control angiography followed by invasive hemodynamic measurements. After the balloon cathteter withdrawal, the intravascular introducer was removed, the femoral artery was ligated, and wound closure was performed according to professional standards. After wound closure, the surgical site was sprayed with an aluminum-containing polymer spray and all animals were given broad-spectrum antibiotics and long-acting NSAIDs. Animals were awakened under constant supervision and extubated upon return of pharyngeal reflex. They were then kept in isolated cages in a temperature-controlled preparation room until complete recovery.
2.3 Follow-up period
The method for the follow-up time points was adapted from the standardized infarction model on meat-type pigs. Control MRI scans and PiCCO measurements were performed on Days 3 and 30 post-infarction. The preparation of the scans was the same as for the pre-infarction MRI scans. To assess myocardial perfusion and viability, gadobutrol (Gadovist 1 mmol/mL, Bayer) contrast agent was administered intravenously at 0.16 mmol/kg, and measurements were performed to characterize the left ventricular function, perfusion, and viability. At the end point of the experiment, the animals were gently euthanized.
2.4 Platelet aggregation inhibition
On the day before the AMI induction, animals were given a single oral dose of 300 mg clopidogrel and 500 mg aspirin dissolved in their drinking water after a short fast, and from the first day onwards all animals received 100 mg aspirin and 75 mg clopidogrel orally mixed with feed daily.
2.5 Statistics
Continuous variables are presented as means ± SD. The normality of the distribution was checked with the Shapiro–Wilk test. A mixed model was used for repeated measures. To observe differences, linear mixed effect models were used, where the ID of the animals was used as a random factor. A Holm post hoc test was used to compare different time points. Pearson's correlation test was used between continuous variables in the correlation matrix. A result was considered significant if p < 0.05. All statistical analysis was performed by open-source Jamovi statistical software (version 2.2.1.).26-28
3 RESULTS
Six pigs died during the experiment: one animal as a complication of catheter intervention and five animals because of malignant arrhythmias during infarct induction. These animals developed ventricular fibrillation and ventricular tachycardia after balloon occlusion, which responded poorly to standard medical treatment and led to their rapid death.
The overall parameters of the experimental animals are shown in Table S1.
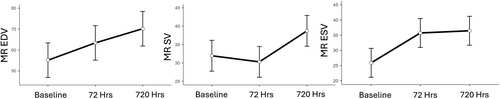
The volumetric changes measured by MRI are illustrated in Figure 1. Holm's post hoc test showed a significant increase in EDV at 72 and 720 h compared to baseline (p = 0.012, <0.001). EDV at Day 30 showed a further significant increase compared to Day 3 (p = 0.022). Stroke volume (SV) at 72 h slightly decreased but by Day 30 had increased significantly (p = 0.001) compared to baseline. ESV showed a significant increase 72 h after infarction (p = 0.001), which was not significantly different on Day 30 (p = 0.781) compared to Day 3.
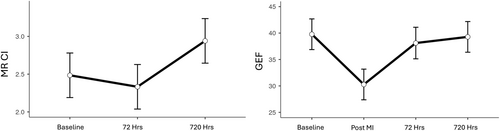
The parameters indicative of left ventricular function obtained by cMRI and hemodynamic methodology, the cardiac index determined by cMRI, and global ejection fraction (GEF) showed a small, non-significant decrease on Day 3 compared to baseline. After reperfusion, cMRI was not feasible, but significant left ventricular function deterioration was measured by invasive methodology (p < 0.001). GEF showed no significant difference from baseline at Day 30, in contrast to the cMRI-based cardiac index (CI), which showed a significant improvement (p = 0.012; Figure 2).
MR-determined EDV and ESV were significantly negatively correlated with aortic dPmax and GEF, and ESV was also correlated with the MR-determined left ventricle ejection fraction. The percentage of scar tissue was significantly correlated with MR EF and ESV (p = 0.006, p = 0.027).
Both LVEF and dPmax showed a significant (p < 0.001 and p = 0.002) deterioration from baseline on Day 3, which was no longer statistically detectable on Day 30 for LVEF (p = 0.141), but remained for dPmax (p = 0.002; Figure 3).
4 DISCUSSION
The most common cause of heart failure is an ischemic lesion, so further development of the preclinical porcine IR model is warranted. The functional, morphological, and histological abnormalities of artificially induced myocardial infarction and heart failure in pigs provide a relevant model for diseases of similar etiology in humans.
In our experiments, we used adult animals of a special breed of minipigs, which allowed us to obtain results free from the disadvantages of the so-called juvenile pig model.
Minipigs, when properly adapted, are well suited for use in cardiovascular experiments, especially when a long follow-up period is required to determine the efficacy of a particular therapeutic procedure. However, a higher incidence of malignant, difficult-to-treat arrhythmias as a consequence of infarction and a higher mortality rate should be expected. Literature data also suggest a better compensatory rate of the minipig heart, compared to meat-type pigs, despite the ventricular motion anomaly.29
Because of the different anatomy of minipigs, cMRI scans are not always optimal for evaluation, especially if the longitudinal axis is not properly captured during imaging. This is a consequence of the limited adaptability of human MR scan sequences to the study of the minipig heart. Therefore, the left ventricular ejection fraction has limited value as a parameter of cardiac performance. This phenomenon justifies the use of hemodynamic measurements in this animal model, which is also essential for the standardization of experimental protocols.
In animal model experiments, molecular, cellular, and interstitial changes occur after infarction with or without subsequent reperfusion, which may manifest clinically as changes in LV size, shape, and function. This phenomenon is called myocardial remodeling.30
Gaudron and colleagues, studying patients who have had a heart attack, found that in a proportion of patients, left ventricular dilatation develops as a compensatory mechanism in the first 4 weeks after the heart attack. Dilatation is intended to improve left ventricular function, but in 20% of patients, the dilatation becomes non-compensatory, ultimately leading to severe left ventricular dysfunction. In these patients, the reduction in GEF is likely caused by a change in the function of the myocardium, which initially contracted normally.31 The degree of post-infarction ventricular enlargement is related to the extent of the initial myocardial damage, and although an increase in ventricular cavity size helps to restore SV despite persistently low ejection fraction, ventricular dilatation is associated with a reduction in survival. The process of ventricular enlargement may be influenced by three interdependent factors: infarct size, infarct healing, and post-infarct ventricular remodeling.32 Our results support this as well, as post-infarct ventricular remodeling on the 3rd day after infarction is not a major factor. Due to remodeling, we were able to demonstrate a steady increase in SV parallel to the increase in left ventricular end-diastolic volume. By Day 30, we also demonstrated a significant improvement in LVEF and GEF.
ESV showed a significant increase at 72 h after infarction but did not change significantly by Day 30. In our experimental animals, we showed a significant correlation between the size of the formed scar tissue and the ESV values. These results are in line with current literature data: ESV is an important predictor of survival in patients with myocardial infarction and is therefore an important additional parameter for changes in LVEF.22, 33 During our data analysis, we showed a negative significant correlation between ESV change and contractility, as well as left ventricular function, which confirms that the study of contractility in such animal models is of particular importance.
The objective of our study was to obtain an easily reproducible parameter measuring contractility in addition to volumetric data.18, 19 We have demonstrated a continuous decreasing trend in the aorta dP/dtmax, showing a significant decrease from baseline at Day 30. We could demonstrate impaired contractility despite the volumetric compensation seen in remodeling. A similar result was demonstrated by Angeli et al. in a closed-chest IR porcine model of infarction with adjunctive β-blocker treatment.34 Left ventricular enlargement accompanied by a decrease in LVEF, hypokinesis of the infarcted area, and hyperkinesis of the remote area in response to an acute increase in preload was observed during the subacute period of the experiment. These lesions were accompanied by a decrease in diastolic function, reflected by an increase in relaxation time. At 6 weeks after MI induction, LV systolic function was impaired, with a decrease in ventricular contractility and progressive ventricular dilatation, thinning of the infarcted area, compensatory hypertrophy of the distal ventricular areas, reduced LVEF and dP/dtmax. We concluded that there is sufficient justification to investigate the variability of dPmax in these infarction models as a valuable adjunct to current gold standard methodologies. It allows a more accurate and objective assessment of the cardiac function changes that accompany infarction.
5 LIMITATIONS
The experiment has some limiting factors: the anatomical characteristics of the pygmy pig breed used, the relatively small number of animals, the one-gender animal group, the limited number of follow-up time points and the 30-day follow-up period. Several additional trials should be performed, preferably with a longer follow-up period (60–90 days).
6 CONCLUSION
A stable, reproducible, and well-defined translational large animal model is essential for the development and testing of various new therapeutic methods. Adequate experimental protocols and statistical procedures are necessary to analyze changes in cardiac function and left ventricular tissue abnormalities. According to our results, the introduction of hemodynamic measurements in large animal model experiments is strongly recommended.
AUTHOR CONTRIBUTIONS
Rita Garamvölgyi: Conceptualization; investigation; resources; supervision; writing – original draft. Dénes Kőrösi: Data curation; investigation; resources; supervision; writing – review and editing. Ottó Tátrai: Investigation. Emőke Bodor: Investigation. Dániel Fajtai: Data curation; software. Kornélia Farkas: Formal analysis. András Vorobcsuk: Conceptualization; formal analysis; investigation; visualization; writing – review and editing.
ACKNOWLEDGMENTS
The authors would like to express their sincere gratitude to the management and co-workers of Medicopus Nonprofit Ltd. and Kaposi Moritz Somogy County Teaching Hospital for the support during the research process.
FUNDING INFORMATION
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
CONFLICT OF INTEREST STATEMENT
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
ETHICS STATEMENT
No human studies were carried out by the authors for this article. All institutional and national guidelines for the care and use of laboratory animals were followed and approved by the appropriate institutional committees. The protocol and ethical guidelines for this experiment were approved by the Government Office for Somogy County under the license number of SOI/31/00161-7/2020. Twenty-four pigs (Source of supply: Pannon Minipig Ltd. farm registered in the Hungarian state Farm Animal Identification System [ENAR]) from authority monitored breeders were included in the study. Written informed consent was obtained for using the animals in the study from the owner of the animals. The authors complied with ARRIVE guidelines (Animal Research: Reporting In Vivo Experiments).




