Beyond the Lung. Impact of Elexacaftor/Tezacaftor/Ivacaftor on Sinonasal Disease in Children With Cystic Fibrosis
Isabelle Sermet-Gaudelus and François Simon are co-last authors.
Funding: This work was supported by funding from the Vaincre La Mucoviscidose (RC20240503466) and CFF Award # (003341121).
ABSTRACT
Background
Elexacaftor/tezacaftor/ivacaftor (ETI) is a Cystic Fibrosis Transmembrane Conductance Regulator (CFTR) therapy that improves pulmonary function and chronic rhinosinusitis (CRS) in cystic fibrosis (CF) adults with at least one copy of the F508del CFTR mutation. The purpose of this study is to evaluate the impact of ETI on CRS symptoms in children and adolescents with CF.
Methods
The MODUL-CF observational study is a multicenter prospective cohort study enrolling CF children with at least 1 F508del mutation in France. Subjects answered a standardized questionnaire on nasal obstruction and smell and were invited to complete the Sinus and Nasal Quality of Life Survey (SN-5) questionnaire prior to ETI therapy and at 1, 3, 6, and 12 months ETI. Part of the cohort underwent sinus computerized tomography (CT) within an ancillary study, scored by the Lund–Mackay CT score (LMKS).
Results
Of 391 subjects, 94 (24.0%) were aged between 6 and 12 years, and 297 were adolescents. Sixty-four (16.3%) reported nasal obstruction at baseline. One hundred and sixty-three completed the SN-5 questionnaire at M0, 181 at M12, and 123 at M0 and M12. Mean SN-5 global score baseline value was similar to that of a healthy pediatric control cohort. SN-5 global score, nasal obstruction, sinus infection, and emotion domain subscores improved significantly at 1 month and this was sustained over 12 months. LMKS improved significantly in 43 patients who underwent sinus CT at M0 and M12.
Conclusion
ETI improves sinonasal symptoms and related quality of life (QOL), including emotion domain, in children and adolescents with CF.
Cystic fibrosis (CF) is a life-limiting autosomal recessive hereditary disorder associated with mutations in the Cystic Fibrosis Transmembrane Conductance Regulator (CFTR) gene [1]. The most prevalent mutation, p.Phe508del (F508del thereafter), is observed in around 85% of patients with cystic fibrosis (pwCF). Absence or dysfunction of the CFTR protein leads to production of a thick, adhesive mucus that clogs the ducts of organs with progressive pulmonary obstruction and exocrine pancreatic insufficiency [1]. In the upper airways, this results in dehydration of the fluid lining the surface of the nasal and sinuses epithelium, local hypoxia, inflammation, and finally abnormal mucociliary clearance. These viscous secretions obstruct sinus ostia and induce bacterial overgrowth [2]. Upper airway disease adversely impacts lower airway CF disease, as the sinuses serve as a reservoir for Staphylococcus aureus, Pseudomonas aeruginosa, or Aspergillus sp. and facilitate lung infection [3, 4].
The triple combination of CFTR-modulating drugs elexacaftor/tezacaftor/ivacaftor (ETI) strongly modifies the prognosis of this life-limiting disease [1]. A recent meta-analysis reported significant improvements in adults with CF regarding their sinonasal symptoms, as well as rhinology care and sinus surgery [5]. This is associated with decreased nasal crusting, polyposis, less mucosal inflammation, and a reduction in Pseudomonas and Burkholderia species in the upper airway [6-15]. Sinus imaging shows a reduction in sinus opacification, mucosal thickening, and mucopyoceles [8, 11, 12, 16–18]. Although such observations are particularly interesting in the context of CF natural history, there are still few studies about sinonasal disease and chronic rhinosinusitis (CRS) in school-children and adolescents with CF [19-24], and even less regarding impact of ETI on upper airway [8, 11, 25, 26].
To fill this gap, we investigated experience of sinonasal-related symptoms in a large, unselected cohort of school-aged children and adolescents with CF at initiation and during the year after starting ETI. This was based on patient reported outcome of sinonasal symptoms, impact on quality of life (QOL) using the pediatric Sinus and Nasal Quality of Life Survey (SN-5) score [27, 28] and scoring of their sinus computerized tomography (CT) using the Lund–Mackay CT score (LMKS) [29].
1 Methods
1.1 Study Population and Study Design
Study participants were prospectively enrolled in the French observational multicenter real-world study MODUL-CF (NCT 04301856, IRB 00011928). Prior to inclusion in the national cohort, informed consent was obtained from all parents. Patients had a confirmed diagnosis based on a CF causing genotype with F508del on at least one allele. They were compared to pediatric healthy controls enrolled in the validation study of the SN-5 French translation study (NCT04117646, IRB/CPP Ouest V 2019-A00387-50) [28].
Evaluation at baseline (M0) included weight, sweat test, percentage predicted of forced expiratory volume in 1 s (ppFEV1) and forced vital capacity (ppFVC). Longitudinal data were collected at scheduled visits (1 month (M1), 3 months (M3), 6 months (M6), and 12 months (M12)), except sweat test which was only performed at M1.
1.2 Sinonasal Symptom Evaluation
The adolescents and children completed sinonasal symptoms evaluation, at baseline (covering at least 12 weeks prior questionnaire) and follow up visits (covering time since the last study visit). This was done as much as possible by themselves, or alternatively, with the help of the nursing staff blinded to the clinical outcomes.
They answered a set of questions on nasal obstruction (absent, partial, total) and smell loss prior to initiation of ETI therapy and at each study visit. Nasal obstruction and smell loss were deemed specific of potential sinonasal disease improvement upon ETI. We did not include questions about nasal drainage because this item was considered too allergy and season dependent, nor facial pain, experienced very rarely [23].
Patients were invited to complete the SN-5 questionnaire recently validated in a French cohort [28]. SN-5 is composed of symptom clusters linked to 5 domains (sinus infection, nasal obstruction, allergy symptoms, emotional distress, and activity limitations), and an overall 10 point visual-analog scale (VAS). Each domain is assigned a score ranging from 1 to 7 (never, exceptionally, rarely, occasionally, often, almost all the time, all the time) and the global SN-5 score is the mean of all domains score out of a maximum (worse) of 7 [27]. We recently validated SN-5's French translation, including 37 healthy pediatric controls, whose mean global score was 1.8 ± 0.9 [28]. We thus considered a SN-5 score of 1.8 or below as normal and a SN-5 score > 3.5 as clinically meaningful. This upper threshold was based on previous validated studies [28, 30–33], showing increased frequency of chronic sinonasal disease [28, 31], allergic rhinitis [32], and radiological sinus disease [33]. The Minimal Clinically Important Difference (MCID) was defined as half the standard deviation of our control healthy population, for example, 0.45 [28].
Overall, symptom of nasal obstruction was considered at a first level by the questionnaire, as part of the routine care (self-report nasal obstruction) and by the SN-5 questionnaire (nasal obstruction domain).
Obstruction self-report was our primary end-point. SN-5 was a secondary end-point, as we anticipated that not all participants would answer at all time points in this multicenter study
Summary
- School-age children and adolescents with cystic fibrosis (CF) downplay their sinonasal symptoms.
- Sinus and Nasal Quality of Life Survey (SN-5) instrument detects clinically relevant sinus disease in pediatric CF patients.
- Elexacaftor/tezacaftor/ivacaftor improves sinonasal-related quality of life in children and adolescents with CF.
- Elexacaftor/tezacaftor/ivacaftor improves sinus opacification in children and adolescents with CF.
- Change in sinus opacification may be considered as an objective noninvasive surrogate biomarker for the detection of response to Cystic Fibrosis Transmembrane Conductance Regulator (CFTR) modulators.
1.3 Sinus CT Scan Evaluation
Thin-section low-dose noncontrast maxillofacial CT scan were performed at both baseline (M0) and 1-year (M12) visits in a subgroup of patients. They were reviewed by an otolaryngologist to determine the LMKS. This staging system scores each sinus as: 0 (no opacity), 1 (partial opacity), or 2 (complete opacity) [29]. The total score, modified to account for sinus aplasia, ranges from 0 (absence of any sinus opacity) to 24 (complete opacity of all sinuses).
The observer was blinded to clinical outcomes and scans were presented in a random order. If there was uncertainty regarding any of the scores (anatomic variant, mucosal thickening, or polyps), a second evaluator assessed the CT scan.
1.4 Statistical Analysis
Continuous variables are presented as mean and standard deviation (SD), while categorical values are expressed as numbers and percentages calculated over the nonmissing values. Missing data were not replaced. Comparisons between M0 and subsequent visits were performed with Wilcoxon signed-rank tests with continuity correction for quantitative variables and MacNemar tests for qualitative variables to account for the longitudinal feature of the data. Comparisons between independent subgroups were performed by Wilcoxon rank sum tests for quantitative variables and chi-squared tests or Fisher tests for qualitative variables. A generalized linear mixed-effects model for repeated measures (MMRM) with time considered as a continuous covariate was used to assess whether the decrease over time of proportion of children/adolescents with CF (cawCF) was statistically significant. Correlations between quantitative variables were estimated by the Spearman ρ coefficient. All analyses were performed with R version 4.3.2. under Rstudio.
2 Results
2.1 CF Patient Characteristics
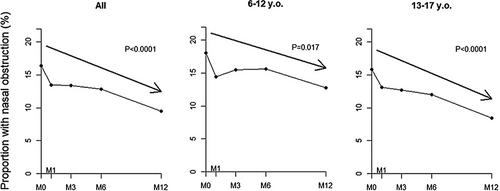
The baseline characteristics of the 391 cawCF enrolled are summarized in Table 1. They presented with a mild CF respiratory disease as assessed by a mean ppFEV1 of 82.2 (19.2)%. As expected, at 1-year ETI, the sweat chloride concentration decreased significantly by 46.1 (24.2) mmol/L, cawCF gained 13.8 (13.6)% ppFEV1, and 0.21 (0.48) weight z-score (p < 0.001 for each; Table 1). Ninety-four patients (24.0%) were aged between 6 and 12 years, and 297 between 13 and 17 years. School-age children had a milder respiratory disease, as assessed by a significantly better ppFEV1 (Supporting Information Table 1). Two hundred and nine patients had been previously treated with Lumacaftor/Ivacaftor (LI) and had a slightly but significantly decreased sweat chloride, better weight z-score and respiratory function tests (RFT) at baseline (Supporting Information Table 2). These differences disappeared after 1-year ETI, except for sweat chloride which remained significantly lower in cawCF previously treated with LI (Supporting Information Tables 1 and 2).
| M0 | M12 | ||
|---|---|---|---|
| n | 391 | 391 | p-value |
| Demographic and cystic fibrosis (CF) disease characteristics | |||
| Age | 14.2 (1.9) | 15.2 (1.9) | — |
| Sex (male) | 198 (50.6%) | — | — |
| F508del (+/+) | 226 (57.8%) | — | — |
| Previous treatment with LI | 209 (53.5%) | — | — |
| Sweat chloride (mmol/L) |
90.7 (22.0) n = 349 |
43.8 (21.4) n = 254 |
< 0.001 |
| Weight z-score | −0.65 (0.99) | −0.42 (1.00) | < 0.001 idem |
| ppFEV1 (%) | 82.2 (19.2) | 95.8 (15.6) | < 0.001 |
| ppFVC (%) | 89.5 (16.4) | 98.7 (14.2) | < 0.001 |
| Sinonasal symptoms and imaging | |||
| Nasal obstruction (yes) | 64 (16.4%) | 37 (9.5%) | 0.004 |
| SN-5 |
2.0 (1.0) n = 163 |
1.8 (0.8) n = 181 |
0.009 |
| Smell loss |
4 (1.0%) n = 383 |
2 (0.5%) n = 390 |
0.37 |
| LMKS |
11.3 (5.1) n = 43 |
4.2 (2.9) n = 43 |
< 0.001 |
- Continuous and categorical values expressed respectively as mean (standard deviation) and number (percentage).
- Abbreviations: CT, computed tomography; LI, lumacaftor/ivacaftor; LMKS, Lund–Mackay score; ppFEV1, percentage predicted of forced expiratory volume in 1 s; ppFVC, percentage predicted of forced vital capacity; SN-5, Sinus and Nasal Quality of Life Survey.
- p-values are derived from Wilcoxon signed-rank tests with continuity correction. Wilcoxon rank sum test; Pearson's chi-squared test; Fisher's exact test.
2.2 Sinonasal Symptoms at Baseline
All patients completed the first questionnaire on nasal obstruction and smell loss. Sixty-four (16.4%) reported nasal obstruction prior ETI initiation. This symptom was qualified as moderate/partial by 48, severe/total by 3, while 13 could not reliably qualify the degree of obstruction. Baseline characteristics of the patients with nasal obstruction self-report did not differ from those who did not report this symptom regarding the CF disease (Supporting Information Table 3). Only four patients (1.0%) reported smell disorders (Table 1). Nasal obstruction frequency did not differ between the children and the adolescent cohort (Supporting Information Table 1). Patients previously treated with LI tended to report less nasal obstruction as compared to those who had not received CFTR modulator treatment (12.9% vs. 20.3%; p = 0.05; Supporting Information Tables 2 and 3).
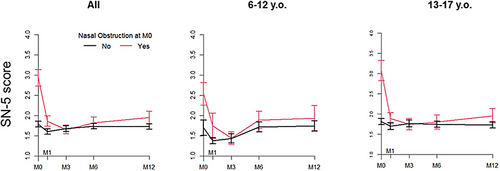
Only 163 out of the 391 cawCF entirely completed the SN-5 questionnaire at M0. They did not differ from those who did not complete the score regarding CF disease (Supporting Information Table 4). At M0, mean SN-5 global score was 2.0 ± 1.0 and VAS 8.3 ± 1.7 (Supporting Information Table 5). Only 12 patients out of the 163 who fully completed the questionnaire (7.4%), reported a score above the clinically significant level of 3.5. These baseline values, as well as those of the domain subscores, were not significantly different from those of the French healthy pediatric control cohort (Supporting Information Table 5) and did not differ according to age or previous treatment with LI (Supporting Information Tables 1 and 2). Nasal obstruction, sinus infection, and emotion were the domains the most correlated with the SN-5 global score (Supporting Information Figure 1).
The patients self-reporting nasal obstruction at the standardized questionnaire had a significantly higher SN-5 global score at M0 as compared to those without complaint (2.93 (1.2) vs. 1.79 (0.82), p < 0.001; Supporting Information Table 5). Patients with SN-5 in the worse upper quartile (SN-5 global score > 2.6) were not significantly different from those in the lower quartiles regarding age and severity of CF disease, although they tended to have worse RFTs (Supporting Information Table 6). As a whole, SN-5 was correlated with ppFEV1 at baseline (Spearman rho = −0.2, p = 0.007), suggesting that SN-5 score reflected pulmonary disease severity in this cohort.
2.3 ETI Improves Sinonasal Symptoms
Thirty-four out cawCF (53.1%) self-reported improvement of nasal obstruction from baseline at 12 months ETI (p < 0.001) at the standardized questionnaire and seven with no nasal obstruction at baseline reported this symptom at M12 (Table 1). This improvement significantly increased over time in both school-age children and adolescents as indicated in Figure 1. This profile was similar in both age cohorts.
One hundred and sixty cawCF (40.9%) completed the SN-5 score at M0 and at least one follow-up visit, including 123 (31.5%) at baseline and M12. These 123 patients did not differ from the whole population (Supporting Information Table 7). At the whole population level, SN-5 global score improved significantly at M1 (SN-5 change: −0.4 (1.05), p < 0.001), and this remained stable over time until M12 (Supporting Information Table 8). This global score change remained below the MCID of 0.45, but interestingly the sinus infection and nasal obstruction domain scores displayed an absolute change above this value. VAS also improved by 0.38 (1.89); p = 0.01) at 1-month ETI. VAS and SN-5 were significantly correlated (r = −0.61, p < 0.0001; Supporting Information Figure 2).
This improvement was mainly observed in patients self-reporting nasal obstruction at baseline (Figure 2 and Supporting Information Tables 5 and 8) where SN-5 dropped from 2.93 (1.2) at M0 to 1.86 (0.74) at M1 (mean change: −1.08 (1.28); p < 0.001), reaching at M3 a value not significantly different from that observed in patients without nasal obstruction.
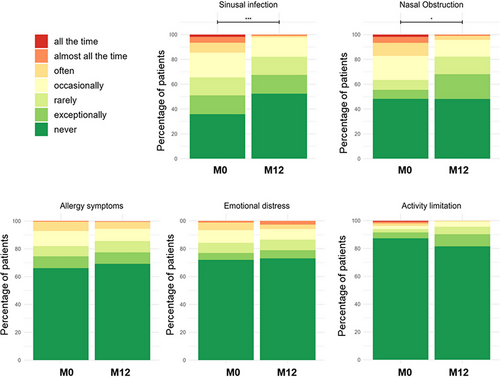
Regarding individual domains, 21 patients out of the 123 patients who positively answered the nasal obstruction item at SN-5 both at M0 and M12 (16.8%) reported it frequently (often, almost all the time, or all the time) at M0 and only eight (6.5%) at M12 (MacNemar test, p ≤ 0.001; Figure 3). The same pattern was observed for sinus infection, 24 patients (6.1%) reporting these symptoms at M0 but only four (1.0%) at M12 (MacNemar test, p ≤ 0.001). Study of the domain subscores showed that not only sinus infection and nasal obstruction improved significantly at M1 (respective change: −0.8 (1.92) and −0.6 (1.88), p < 0.001 for each) but also, emotional distress (−0.39 (1.56), p = 0.002), with a stabilization thereafter. These improvements were more marked in the children with nasal obstruction self-report at baseline (Supporting Information Table 8). The pattern of these changes was not different between the children and the adolescents.
These results were not changed when the analysis was restricted to the 123 patients who completed the SN-5 at M0 and M12 (Supporting Information Table 9 and Supporting Information Figures 3–5).
2.4 ETI Improves Radiologic Score
Forty-three patients underwent sinus CT both at M0 and M12. Although they were significantly older than those who did not undergo CT, they did not have a more severe CF disease (Supporting Information Table 10). Only four CT scans required a second review.
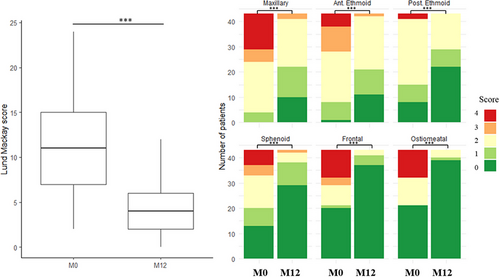
The mean overall LMKS decreased significantly from 11.3 ± 5.1 at M0 to 4.2 ± 2.9 at M12 (p < 0.0001; Figure 4, left panel and Table 1). Improvements were significant for all sinus subgroups (Figure 4, right panel; p < 0.0001 for each region). An example is shown in Supporting Information Figure 6.
There was no correlation between SN-5 scores and LMKS (Supporting Information Figure 7). As a matter of fact, LMKS did not differ significantly between the patients in the upper quartile of SN-5 and those in the lower quartiles (Supporting Information Table 6). Neither baseline LKMS score nor its change differed according to age, previous LI treatment or nasal obstruction self-report at baseline (Supporting Information Tables 1–3).
3 Discussion
Our study shows that the symptom burden of CRS and its impact on QOL was low in this large unselected pediatric population of nearly 400 school-age children and adolescents with CF. In patients with nasal obstruction at baseline, QOL sinonasal specific tool SN-5, showed a clinically relevant improvement after ETI initiation as early as 1 month which was sustained over 12 months. Sinus CT opacification also showed a significant improvement but this did not parallel QOL improvement.
CRS usually appears during childhood and progressively worsens in adolescence [23]. Nasal obstruction is by far the most frequent complaint in children, followed by rhinorrhea, while headache, snoring, sleep disorders, and hyposmia are much less frequent [23]. Little is known about the effects of ETI on the upper respiratory mucosa in the pediatric population. Stapleton et al. showed improvement in the SNOT-22 QOL score in 12 adolescents and young adults as early as 7 days, sustained over 9 months [8]. This was associated with a decrease in nasal crusting and polyps at endoscopy and improvement in sinus CT opacification and thickening. Di Gioia et al. reported similar results in a cohort of 20 adolescents, with pre- and post-treatment CT scan in five of the participants [11]. This supported previous results of two additional pediatric studies showing a significant improvement in SNOT-22 in respectively 23 adolescents/young adults over 22 months ETI and in six children/adolescents over 9 months [25, 26]. Obviously, the small number of patients limits the conclusions of these studies.
Our study, which enrolled nearly 100 children and 300 adolescents, is by far the largest in the CF pediatric population. CRS prevalence, taking as a proxy self-reported nasal obstruction over the last 12 weeks prior ETI, was around 15%, a proportion slightly lower than previous studies [20, 24]. We appraised the impact of sinonasal disease on QOL with SN-5, the single generic tool validated in this age range [27]. Indeed, SN-5 demonstrates good test–retest reliability, internal consistency, and responsiveness to clinical care or endoscopic sinus surgery [30]. Importantly, this tool is anticipated to be more specific than the SNOT-22 as it does not contain items such as “fatigue” or “cough” potentially unrelated to CRS but improved by ETI. This scale has been already used in cawCF and showed a negative correlation with the mean symptom frequency, recent episodes of sinusitis, and number of school days missed due to sinonasal symptoms [19–22, 24].
Our data are consistent with a minimal effect of sinonasal symptoms on QOL, as baseline mean SN-5 global score was in the range of those observed in the healthy pediatric population. Even in patients with chronic nasal obstruction, SN-5 scores were lower than those reported in general pediatric populations with CRS [28, 30]. Because scores started from subnormal values, the mean change of the SN-5 global score induced by ETI fell below the MCID. However, in patients with nasal obstruction and worse scores at baseline, ETI normalized not only the rhinology domains score but also very interestingly, the emotional distress domain. The improvement of the scores after ETI suggests that these symptoms and their impact on QOL were overlooked before ETI. ETI very likely “unmasks” these alterations by enabling children to experience “normal nasal breathing.” This is indirectly suggested by a recent study showing normalization of nasal Nitric Oxide [34]. Altogether, these observations point that sinonasal symptoms play a critical role on psychological dysfunction in cawCF. Change in sinus opacification may be considered as an objective noninvasive surrogate biomarker for the detection of response to CFTR modulators.
CT scans demonstrated a high degree of baseline sinus opacification with a substantial improvement at 1-year ETI. Such results were already shown in a cohort from Germany using magnetic resonance imaging (MRI) [17] and another from the United States with sinus CT analyzed by deep learning [18]. In contrast to QOL, sinus involvement did not fully resolve, indicating persistent sinus disease, as already reported in adults [8, 9, 11–13]. Importantly, both QOL and imaging changes were observed at a similar level in the school-age and in the adolescent population. These observations support the recommendation of the CF Foundation for surveillance visits for children even if asymptomatic [35]. This is all the more important that upper airway disease appears from infancy as shown by a recent MRI study in preschool children unraveling sinus abnormalities and, remarkably, a correlation with lung disease severity [36]. This suggests that change in sinus opacification may be considered as an objective noninvasive surrogate biomarker for the detection of response to CFTR modulators in pediatric patients with mild respiratory disease.
Strengths of this study include a prospective multicenter design in the largest unselected pediatric population published so far, with a comprehensive evaluation based on a QOL tool for CRS validated in this age range, combined with objective metrics of pulmonary and sinus disease performed at the same time. As there is geographic variability in non-CF CRS such as allergic stimuli, inclusion of different sites is representative of the “general” CF French population. Inclusion of children as young as 6 years of age helps inform benefits of ETI in children with minimal respiratory disease. The 1-year follow-up enables to assess persistence of the effect suggesting that changes were related to ETI.
The main limitation is the fact that this study is not a randomized controlled trial. But the use of historical French controls provides context to interpret the data. The participants (adolescents/children) were asked to answer the questionnaires by themselves. It may be that for the younger ones they were helped by the care givers. We did not record this information and are unable to evaluate this potential bias. The patients answered a set of question for smell and nasal obstruction at all the visits but only a part of them completed the SN-5. A respondent bias would infer that more affected individuals answer the questionnaires, which is not the case in our results, hence we think that the risk for this bias is minimal. Indeed, the interpretation did not change when the analysis was restricted to the 123 cawCF who completed the SN-5 both at M0 and M12. The lack of a validated questionnaire to specifically target sinonasal QOL in the CF population makes assessment of this patient population difficult, all the more that it cannot be excluded that improvements in SN-5 may reflect improvement in overall systemic symptoms. Only a subset of children performed sinus CT at baseline and M12. This was based on local practice. There was no difference regarding the main demographic and clinical characteristics of patients who completed sinus CT and those who did not. We thus think that this subgroup analysis is not biased. A final limitation is that the SN-5 is not validated for children older than 12 years. We found no statistically significant difference between the 2 age groups and SN-5 or VAS score. This suggests that the SN-5 instrument is also valid in the adolescent population.
4 Conclusion
There is now an increased interest in detecting and treating sinonasal disease as early as possible to prevent lower airway disease. This study supports monitoring of sinonasal disease based on CF specific sinus-related QOL measures to allow for more personalized therapy and evaluation of response to CFTR modulators.
Acknowledgments
We thank all the families and the children for their participation.
MODUL-CF Study Group
Mahassen Barboura (Paris-Necker); Mélisande Baravalle (Marseille); Audrey Barzic (Roscoff); Lilia Benhalla (Paris-Necker); Khadidja Bennour (Paris-Necker); Katia Bessaci (Reims); Antoine Bessou (Paris-Vaincre la Mucoviscidose); Thiphaine Bihouee (Nantes); Anne-Sophie Bonnel (Versaille); Nesrine Bouleghem (Paris-Necker); Stéphanie Bui (Bordeaux); Frédérique Chedevergne (Paris-Necker);Harriet Corvol (Paris-Trousseau); Laure Cosson (Tours); Laure Couderc (Rouen); Marie-Laure Dalphin (Besancon); Paola De carli (Paris-Vaincre la Mucoviscidose); Eric Deneuville (Rennes); Pierre Foucaud (Paris-Vaincre la Mucoviscidose); Asma Gabsi (Versaille); Elsa Gachelin (La Réunion-Saint-Denis); Fatiha Hassani (Paris-Necker); Veronique Houdouin (Paris-Robert Debré); Frédéric Huet (Dijon); Marie Jamin (Saint-Brieuc); Kadiatou Kaba (Paris-Vaincre la Mucoviscidose); Guillaume Labbe (Clermond-Ferrand); Jane Languepin (Limoges);Muriel Laurans (Caen); Cathy Lerena (Grenoble); Alexia Letierce (Paris-Necker); Clotilde Livrozet (Vannes); Christophe Marguet (Rouen); Laurent Mely (Lyon); Rania Messaoudi (Paris-Necker); Marie Mittaine (Toulouse); Camille Ohlmann (Lyon); Caroline Perisson (La Réunion-Saint-Pierre); Thomas Perrin (Lyon); Carole Piccini-bailly (Nice); Cinthia Rames (Amiens); Philippe Reix (Lyon); Natascha Remus (Creteil); Anna Ronayette (Paris-Vaincre la Mucoviscidose); Djouher Sahki (Paris-Necker); Manuela Scalbert (Dunkerque); Isabelle Sermet (Paris-Necker); Floriane Socchi (Montpellier); Nathalie Stremler (Marseille); Aurélie Tatopoulos (Nancy); Guillaume Thouvenin (Paris-Trousseau); Tom Toin (Lyon); Françoise Troussier (Angers); Laurence Weiss (Strasbourg); Marie Christine Werck-Galois (Lyon); Nathalie Wizla (Lille).
Disclosure
Margaux Petitjean, Alexia Letierce, Anne-Sophie Bonnel, Nathalie Stremler, Romain Luscan, Vincent Couloigner, Katia Bessaci, Laurent Mely, Guillaume Labbe, Mairead Kelly-Aubert, Fatiha Hassani, François Simon have no financial disclosure.
Philippe Reix declares travel fees, grants, and fees for lectures from Vertex Therapeutics.
Eric Deneuville declares travel fees from Vertex Therapeutics.
Christophe Marguet and Isabelle Sermet-Gaudelus declare participation to scientific advisory board at Vertex Therapeutics and are PI in clinical trials of Vertex Therapeutics.




