Association between ambient temperature and chronic rhinosinusitis
M. Ramanathan and Z. Zhang contributed equally and are co-senior authors.
Abstract
Background
Chronic exposure to particulate matter air pollution (PM2.5) is associated with chronic rhinosinusitis (CRS). Elevated ambient temperature may increase PM2.5 levels and thereby exacerbate sinonasal symptoms. This study investigates the association between high ambient temperature and the risk of CRS diagnosis.
Methods
Patients with CRS were diagnosed at Johns Hopkins hospitals from May to October 2013–2022, and controls were matched patients without CRS meanwhile. A total of 4752 patients (2376 cases and 2376 controls) were identified with a mean (SD) age of 51.8 (16.8) years. The effect of maximum ambient temperature on symptoms was estimated with a distributed lag nonlinear model (DLNM). Extreme heat was defined as 35.0°C (95th percentile of the maximum temperature distribution). Conditional logistic regression models estimated the association between extreme heat and the risk of CRS diagnosis.
Results
Exposure to extreme heat was associated with increased odds of exacerbation of CRS symptoms (odds ratio [OR] 1.11, 95% confidence interval [CI] 1.03–1.19). The cumulative effect of extreme heat during 0–21 lag days was significant (OR 2.37, 95% CI 1.60–3.50) compared with the minimum morbidity temperature (MMT) at 25.3°C. Associations were more pronounced among young and middle-aged patients and patients with abnormal weight.
Conclusions
We found that short-term exposure to high ambient temperature is associated with increased CRS diagnosis, suggesting a cascading effect of meteorological phenomena. These results highlight climate change's potentially deleterious health effects on upper airway diseases, which could have a significant public health impact.
1 INTRODUCTION
Chronic rhinosinusitis (CRS) is an inflammatory condition of the nose and paranasal sinuses, diagnosed by the presence of at least two of the following cardinal symptoms for at least 12 consecutive weeks and confirmed by endoscopic or radiographic findings1: nasal obstruction/congestion/blockage; anterior or posterior (mucopurulent) nasal drainage; loss or decreased sense of smell; facial pressure/pain/fullness. Studies have shown that CRS is associated with depression, impaired cognitive function, sleep disturbances, and poor quality of life.1 CRS varies by country, with a prevalence of 6.9%–27.1% in Europe,2 2.1%–13.8% in the United States,3-6 and 4.8%–9.7%7 in China. Recently, our group and others have demonstrated that environmental particulate matter air pollution, specifically PM2.5, is associated with the development of CRS.8 These studies highlight the increasing role of environmental factors in the development of CRS.
With the progress of global warming, the health effects of high ambient temperature are receiving increasing attention.9-13 A meta-analysis of 266 studies showed that a 1°C increase was positively associated with increased cardiovascular disease-related mortality across all diagnoses considered.9 A meta-analysis of over 1.7 million mental health-related deaths demonstrated that a 1°C temperature rise was associated with a significant increase in morbidity of, such as, mood disorders, organic mental disorders, schizophrenia, neurotic, and anxiety disorders.10 In addition, studies have indicated that high ambient temperatures increase the risk of asthma and chronic obstructive pulmonary disease (COPD).11, 12 However, little is known about the impact of high ambient temperature on sinonasal diseases.
In this large outpatient-based case–control study, we focused on investigating the association between high ambient temperature exposure and the risk of developing CRS in patients who visited the Johns Hopkins hospitals in the Baltimore–Washington metropolitan area. We hypothesized that a positive association exists between high ambient temperature exposure and the development of CRS.
2 MATERIALS AND METHODS
2.1 Study population
Patients aged 18 years or older diagnosed with CRS for the first time by a board-certified otolaryngologist during the warm season between January 1, 2013 and December 31, 2022, within the Johns Hopkins Health System were included in this study. Patients with a history of environmental allergies were excluded. CRS (International Classification of Diseases 10th Revisions [ICD-10] code J32) was the primary outcome, and its subtypes including chronic maxillary, ethmoidal, frontal, sphenoidal sinusitis, multipart sinusitis (presence in at least two sinuses), and pansinusitis (presence in all four sinuses) were the secondary outcomes. The diagnosis was made using nasal endoscopy and confirmed with computed tomography (CT) scans. The CRS onset or exacerbation time was the time at diagnosis.
Control participants were selected from patients who underwent nasal endoscopy and facial CT scan or magnetic resonance imaging (MRI) at the otolaryngology department. The controls were excluded if they had symptoms including nasal polyps, loss of smell, or any other conditions or known risk factors associated with CRS, such as neurodegenerative disease, or previous traumatic brain injury. One control was matched for each identified CRS case according to age, sex, and race/ethnicity using the nearest-neighbor matching strategy.
Clinical characteristics, such as the date of admission, primary diagnosis code (based on ICD-10), race/ethnicity, preexisting medical conditions, demographic information, and socioeconomic status (SES), were extracted from the EPIC medical records system for all participants. We used the US Census Bureau's American Community Survey to determine each participant's median household income (assigned to the patient's residence zip code tabulation area [ZCTA]).14 We conducted the inflation adjustment for the median ZCTA household income to 2016 US dollars.
Our study protocol was approved by the Johns Hopkins University School of Medicine Institutional Review Board. Informed consent was optional because, except for zip codes, no patient-identifying information was collected.
2.2 Exposure assessment
We obtained daily maximum and minimum temperatures from the National Oceanic and Atmospheric Administration (NOAA).15 We assigned the measurements from the nearest metrological station to each participant as the exposure. Daily relative humidity data were retrieved from the North American Regional Reanalysis (NARR)16 and paired with each participant according to zip code. We defined the warm season as May to October, referring to previous studies and local hydrological characteristics.17, 18 We defined extreme heat as days with a daily maximum temperature over and equal to the 95th percentiles of the warm season's daily maximum temperature distribution (35.0°C [95.0°F]) and reported the odds ratio (OR) for days of extreme heat relative to the minimum morbidity temperature (MMT). We identified the MMT according to the temperature percentile (bounded between the 1st and 99th percentiles) associated with the lowest rate of CRS based on the overall cumulative exposure–response association.19 Regarding the expression of lag days, lag 0 represents the measurement from the day of diagnosis; lag 0–21 represents the average measurements from the day of diagnosis to the prior 21st day; and the meanings of lag 0–3, lag 0–14, and lag 0–28 are analogous.
2.3 Covariates
Covariates included national holidays, 4-day moving averages (lag 0–3) of daily mean relative humidity, body mass index (BMI), alcohol consumption status, smoking status, hypertension, diabetes, and ZCTA median household income. The national holidays were retrieved from the Federal Reserve Board.20 BMI was calculated as weight in kilograms divided by height in meters squared, with BMI from 18.5 to 25 defined as normal weight, BMI under 18.5 defined as underweight, BMI from 25 to 30 defined as overweight, and BMI over 30 defined as obesity. We classified alcohol consumption as current alcohol users and nonusers. We classified the smoking status as never smokers, current smokers, and former smokers. Hypertension and diabetes were binary variables determined by medical history. We adjusted for ZCTA median household income to reflect patients’ SES. Median household income may indicate the general standard of living in the area, as well as surrogate for patients’ preference to use air conditioner when temperatures rise to uncomfortable levels.
2.4 Statistical analysis
We applied a matched case–control design to investigate the association between ambient temperature and the risk of CRS. Conditional logistic regression models were used, and the ORs and 95% confidence intervals (CIs) were obtained by adjusting for covariates and potential confounders.
As the effects of ambient temperature on health outcomes are often reported as nonlinear21-24 and delayed,25, 26 we modeled the temperature–CRS association using a distributed lag nonlinear model (DLNM). The DLNM combines the “basis” functions for a nonlinear exposure–response association and a lag–response association (estimating delayed effects over a lag period), so both dimensions can be estimated simultaneously.27 Specifically, we used a quadratic B-spline to model the exposure–response association and a natural cubic B-spline with two internal knots placed at equal intervals on the log scale of lags to model the lag–response association.28 We chose a lag period of 21 days to fully account for any potential delayed effects of ambient temperature.29
We conducted subgroup analyses to identify potentially susceptible subgroups and to assess whether the associations between ambient temperature and CRS varied by age, sex, race/ethnicity, BMI, tobacco use status, and alcohol use status groups (all converted to binary variables). Also, as CRS is often clinically classified into those with and those without nasal polyps (CRSwNP and CRSsNP),1 we also performed subgroup analyses of these two groups. We conducted sensitivity analyses to assess the robustness of our findings. First, we changed the lag period from 21 days to 14 or 28 days. Second, we applied different knots for the DLNM, including (1) modeling the exposure–response association using a quadratic B-spline with one or two internal knots and (2) modeling the lag–response association using a natural cubic B-spline with three internal knots placed at equal intervals on the log scale of lags. Furthermore, in addition to using the daily maximum temperature, we also used the daily minimum temperature and daily mean temperature to check whether our results were affected by the choice of exposure measurement.
Descriptive statistics were calculated using the mean (standard deviation [SD]) for continuous variables and frequency count (percentage) for categorical variables. Statistical analyses were conducted using R (version 4.1.0; R Development Core Team) and STATA (version 16.0; Stata Corp.) from June to December 2022. P-values were two sided, and p < 0.05 was considered statistically significant.
3 RESULTS
Our analysis included a total of 4752 participants, 56.9% of whom were women, with an average age of 51.8 (16.8) years (Table 1). The year distribution of these 2367 CRS cases was similar to the trend of average daily maximum temperature from 2013 to 2022 (Figure 1). After matching, the age, sex, and race/ethnicity composition of the control group was identical with that of the case group. In terms of other characteristics, patients with CRS were more likely to be overweight and obese and live in areas with higher household incomes. However, they were less likely to smoke or drink alcohol or have been diagnosed with hypertension or diabetes.
| CRS patients | Controls | p-value | |
|---|---|---|---|
| n | 2376 | 2376 | |
| Age, years | 51.7 (16.7) | 51.9 (16.9) | 0.684 |
| Male | 1024 (43.1) | 1024 (43.1) | 1.000 |
| Race/ethnicity | 0.999 | ||
| White | 635 (26.7) | 633 (26.6) | |
| African American | 513 (21.6) | 516 (21.7) | |
| Hispanic/Latino | 116 (4.9) | 114 (4.8) | |
| Other | 1112 (46.8) | 1113 (46.8) | |
| Body mass index, kg/m2 | <0.001 | ||
| Normal weight, 18.5 to <25 | 758 (31.9) | 920 (38.7) | |
| Underweight, <18.5 | 35 (1.5) | 103 (4.3) | |
| Overweight, 25 to <30 | 778 (32.7) | 632 (26.6) | |
| Obesity, ≥30 | 805 (33.9) | 721 (30.3) | |
| Current smoking status | <0.001 | ||
| Never smoker | 1804 (75.9) | 1299 (54.7) | |
| Current smoker | 129 (5.4) | 386 (16.2) | |
| Former smoker | 443 (18.6) | 691 (29.1) | |
| Current alcohol user | 803 (33.8) | 982 (41.3) | <0.001 |
| Hypertension | 550 (23.1) | 645 (27.1) | 0.002 |
| Diabetes | 156 (6.6) | 227 (9.6) | <0.001 |
| Nasal polyps | 214 (9.0) | 0 (0.0) | <0.001 |
| Median household income, US$ | 89129 (34996) | 76819 (32322) | <0.001 |
| Max temperature (lag 0–21)a | 27.8 (3.9) | 27.6 (3.7) | 0.058 |
| Min temperature (lag 0–21)b | 16.8 (3.9) | 16.6 (3.9) | 0.290 |
| Mean temperature (lag 0–21)c | 22.2 (3.8) | 22.1 (3.7) | 0.214 |
| Relative humidity (lag 0–3)d | 75.7 (7.0) | 75.9 (6.8) | 0.434 |
- Note: Values are mean (standard deviation [SD]) for continuous variables and n (%) for categorical variables. Values were calculated using the Mann–Whitney U-test for continuous variables and the chi-square test for categorical variables.
- Abbreviation: CRS, chronic rhinosinusitis.
- a 22-day moving average (lag 0–21) of daily maximum temperature (°C).
- b 22-day moving average (lag 0–21) of daily minimum temperature (°C).
- c 22-day moving average (lag 0–21) of daily mean temperature (°C).
- d 4-day moving average (lag 0–3) of daily mean relative humidity.
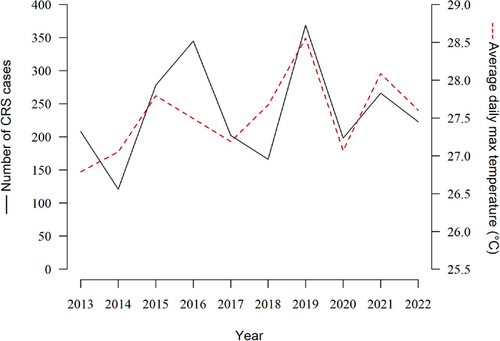
The year distribution of chronic rhinosinusitis (CRS) cases and the trend of average daily maximum temperature from 2013 to 2022.
The calculation of the annual average daily maximum temperature covered the areas where the participants were primarily sourced (Maryland, Virginia, Pennsylvania, and Washington DC) but only included data during the warm season (May to October).
The average daily maximum temperature of the case group and the control group were 27.8°C (82.0°F) and 27.6°C (81.7°F), respectively (Table 1). We found that the cumulative effects of temperature over 21 days on the risk of CRS were significant at both high and low temperatures, compared with the MMT at 25.3 (Figure 2). Specifically, there was a significant effect of extreme heat (35.0°C, 95th percentiles of temperature distribution), with an OR of 2.37 (95% CI, 1.60–3.50). According to the overall lag effects of extreme heat (35.0°C) on the risks of CRS, we found it evident that the temperature–CRS association was nonlinear (Table 2 and Figure S1(a)). Generally, the effect of high temperature reached a small peak after 3 days of exposure, and there was a significant increase in the risk of CRS exacerbation after approximately 2 weeks. For the secondary outcome, we found that cumulative temperature exposure was associated with a higher risk of maxillary sinusitis at both high and low temperatures (Figures S1 and S2), with a significant effect after 1 week of exposure compared with the MMT.
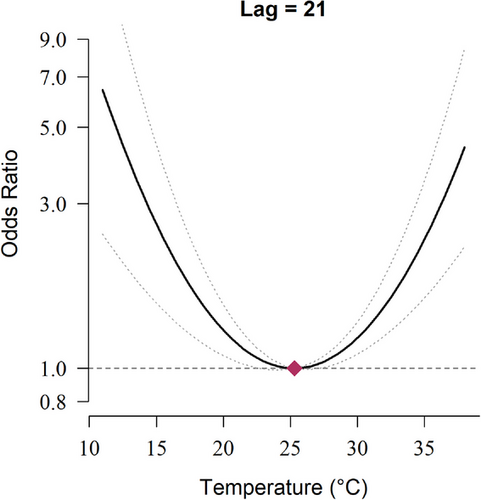
The cumulative exposure–response curve for the association between daily maximum temperature and the risk of chronic rhinosinusitis over lag 0–21.
The cumulative exposure–response curve was calculated using a quadratic B-spline with no internal knots to model the exposure–response association and a natural cubic B-spline with two internal knots placed at equal intervals on the log scale of lags to model the lag–response association. The reference exposure level was set at the temperature of minimum morbidity (25.3°C). Odds ratios (ORs) were adjusted for 4-day moving averages (lag 0–3) of daily mean relative humidity, whether the lag 0 day was a federal holiday, body mass index, hypertension, diabetes, current smoking status, current alcohol consumption status, and household income.
| Any CRS | Maxillary sinusitis | Frontal sinusitis | Ethmoidal sinusitis | Sphenoidal sinusitis | Multipart sinusitis | Pansinusitis | |
|---|---|---|---|---|---|---|---|
| (n = 967) | (n = 639) | (n = 277) | (n = 329) | (n = 169) | (n = 255) | (n = 67) | |
| lag0 |
1.00 (0.85, 1.17) |
1.10 (0.88, 1.39) |
0.94 (0.66, 1.35) |
1.26 (0.92, 1.71) |
0.87 (0.53, 1.42) |
1.24 (0.92, 1.67) |
1.05 (0.51, 2.14) |
| lag1 |
1.03 (0.96, 1.11) |
1.07 (0.96, 1.19) |
1.01 (0.85, 1.20) |
1.19 (1.03, 1.39) |
0.98 (0.77, 1.24) |
1.17 (1.01, 1.36) |
0.96 (0.69, 1.33) |
| lag3 |
1.05 (0.98, 1.13) |
1.04 (0.94, 1.16) |
1.07 (0.90, 1.27) |
1.11 (0.96, 1.28) |
1.09 (0.87, 1.37) |
1.09 (0.94, 1.25) |
0.91 (0.64, 1.29) |
| lag5 |
1.04 (0.98, 1.09) |
1.05 (0.97, 1.13) |
1.04 (0.92, 1.18) |
1.07 (0.96, 1.18) |
1.07 (0.91, 1.25) |
1.05 (0.95, 1.16) |
0.97 (0.76, 1.24) |
| lag7 |
1.02 (0.98, 1.06) |
1.06 (1.00, 1.11) |
1.01 (0.93, 1.10) |
1.04 (0.97, 1.11) |
1.02 (0.91, 1.15) |
1.03 (0.97, 1.10) |
1.03 (0.86, 1.23) |
| lag13 |
1.03 (0.99, 1.07) |
1.06 (1.00, 1.12) |
1.00 (0.91, 1.10) |
0.99 (0.92, 1.07) |
0.95 (0.84, 1.07) |
1.00 (0.93, 1.07) |
1.04 (0.87, 1.24) |
| lag14 |
1.03 (1.00, 1.07) |
1.05 (1.00, 1.11) |
1.00 (0.92, 1.09) |
0.99 (0.92, 1.06) |
0.94 (0.84, 1.05) |
0.99 (0.93, 1.06) |
1.02 (0.88, 1.20) |
| lag15 |
1.04 (1.01, 1.07) |
1.05 (1.00, 1.10) |
1.01 (0.94, 1.09) |
0.98 (0.92, 1.05) |
0.93 (0.84, 1.03) |
0.99 (0.93, 1.05) |
1.00 (0.87, 1.15) |
| lag21 |
1.11 (1.03, 1.19) |
1.02 (0.91, 1.13) |
1.08 (0.90, 1.30) |
0.97 (0.84, 1.12) |
0.91 (0.70, 1.18) |
0.98 (0.85, 1.13) |
0.84 (0.57, 1.23) |
- Note: The reference exposure level was set at the temperature of minimum morbidity (25.3°C). ORs were adjusted for 4-day moving averages (lag 0–3) of daily mean relative humidity, whether the lag 0 day was a federal holiday, body mass index, hypertension, diabetes, current smoking status, current alcohol consumption status, and household income.
- Abbreviations: CI, confidence interval; CRS, chronic rhinosinusitis; OR, odds ratio.
We conducted subgroup analyses and found that young and middle-aged CRS patients, patients with abnormal weight, and patients without nasal polyps (CRSsNP) were more likely to be affected by ambient temperature (Figure 3). Other factors did not significantly affect the association between ambient temperature and CRS (Figure S3). In the sensitivity analysis, we changed the lag period from 21 days to 14 and 28 days, and the results were consistent (Figure S4). To identify the robustness of the association, we changed the number of internal knots to model the exposure–response association and the lag–response association. We found that the overall lag structures using different DLNM model specifications showed a similar tendency to our main results (Figure S5). When we measured the exposure using daily minimum and mean temperatures instead of maximum temperatures, the results showed a similar association, although the association for daily minimum temperatures was not significant (Figure S6).
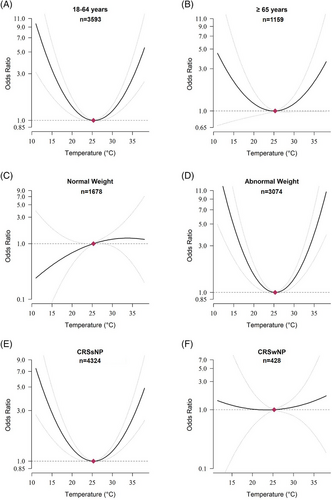
Cumulative exposure–response curves for associations between daily maximum temperature and the risk of chronic rhinosinusitis over lag 0–21 by subgroups.
The cumulative exposure–response curves were calculated using a quadratic B-spline with no internal knots to model the exposure–response association and a natural cubic B-spline with two internal knots placed at equal intervals on the log scale of lags to model the lag–response association. The reference exposure level was set at the temperature of minimum morbidity (25.3°C). Odds ratios were adjusted for 4-day moving averages (lag 0–3) of daily mean relative humidity, whether the lag 0 day was a federal holiday, body mass index, hypertension, diabetes, smoking status, alcohol consumption status, and household income.
4 DISCUSSION
We found a U-shaped association between ambient temperature exposure levels and the risk of developing CRS, with a significant effect of extreme heat during a 21-day lag. The current findings have broad public health implications in the setting of global warming. This study benefits from strengths including a robust patient population (objective testing, otolaryngologist diagnosis) and a novel matching strategy for controls, who had clear sinus imaging.
Few studies have explored the relationship between ambient temperature and CRS. Only a recent study found that the associations between mean/highest/lowest temperature, temperature range, and CRS were not statistically significant but did not account for lag effects.30 Existing studies focus more on the effect of ambient temperature on one of the cardinal symptoms of CRS, loss or decreased sense of smell. Animal experiments from blowflies,31 drosophilas,32 and turtles33 have shown a nonlinear association, and high ambient temperature can lead to loss or decreased sense of smell. Evidence from the population is relatively lacking, and no positive association has been found so far.34-36 However, the sample sizes of the existing studies were small, involving only approximately 10 or 50 participants, and did not take the lag effect of ambient temperature on the sense of smell into account.
We found the effect of high temperature on CRS reached a small peak after 3 days of exposure and became significant after 14 days of exposure, suggesting that the effect may be indirect. Elevated ambient temperature may increase PM2.5 levels and thereby exacerbate sinonasal symptoms. The mechanism of the lag effect is unclear, but there may be different pathways for different phenotypes.37 For bacterial CRS and allergic fungal RS, the ambient temperature may impact CRS by affecting the growth of bacteria, fungi, and viruses. For example, the literature indicates that allergic fungal RS occurs mainly in areas with high temperature and high humidity that are conducive to fungal growth.1 For CRS associated with other respiratory diseases, such as asthma, aspirin sensitivity, idiopathic bronchiectasis, and cystic fibrosis, the ambient temperature may influence CRS by affecting the onset of other respiratory diseases. For instance, high temperatures have been shown to increase the risk of adult asthma hospitalizations.11, 38
Subgroup analysis showed that the young and middle-aged group (18–64 years) may have a higher risk of CRS attributable to ambient temperatures than the elderly. This age group difference in susceptibility was consistent with some studies11, 39 and contrary to others.40, 41 Young and middle-aged people were more likely to be exposed to extreme temperatures while working or exercising outdoors, but they also changed their activity habits because of the uncomfortable temperatures. More research is needed to determine the exact differences in temperature sensitivity between age groups. We found that CRS patients with abnormal weight were more susceptible to the effect of ambient temperatures, suggesting that body weight and metabolism may modify the association between ambient temperature and CRS. Further studies are called for to explore the mechanism of this potential modifying effect. We also found that patients without nasal polyps (CRSsNP) were more affected, which needs to be confirmed by studies with a larger sample size of CRSwNP. Among the anatomy-based CRS subtypes, maxillary sinusitis had the highest prevalence due to the ostium being situated high up on the medial wall.42 Our secondary outcome results demonstrated that maxillary sinusitis was most susceptible to extreme heat, suggesting that the effect of ambient temperature on CRS is of great concern.
There are several limitations to our study. First, our study has inherent weaknesses in its retrospective design regarding coding accuracy, potential sampling bias, or inability to determine causal direction. Further experimental studies are needed to confirm the effects. Second, our model did not include participants’ activity patterns (time spent indoors and outdoors) or air conditioner use behavior due to data inaccessibility. Failure to adjust for these variables may have biased the results. To address this shortcoming, we adjusted for household income, which may reflect the overall SES of patients. In addition, changes in participants’ residential address during the study period were not applicable. Therefore, our exposure measurement relied only on ambient temperature mapping to the participants’ initial residential address. Lastly, data on occupational or environmental exposure to allergens or the use of medications were not available.
5 CONCLUSION
In this cross-sectional study, short-term exposure to high ambient temperature was associated with a lag exacerbation of CRS symptoms, suggesting that the effects may be indirect. This finding has broad implications for the proactive response to global warming.
AUTHOR CONTRIBUTIONS
R.D. and Z.Z. designed the study and cleaned the data. R.D. and W.J. performed the analyses. J.M. and Q.Z. checked the codes. S.S. and Z.L. provided technical support for statistical model specifications selection. R.D. and Z.Z. drafted the manuscript. R.D., Z.Z., and M.R. revised the manuscript. All authors reviewed and contributed to the article and approved the submitted version.
ACKNOWLEDGMENTS
The authors thank all participants and physicians who contributed to data collection.
CONFLICT OF INTEREST STATEMENT
The authors declare that they have no competing interests.
AVAILABILITY OF DATA AND MATERIALS
The hyperlinks of publicly available exposure data are included throughout the literature cited. The datasets of patients used and/or analyzed during the current study are available from the corresponding author upon reasonable request.
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
Our study protocol was approved by the Johns Hopkins University School of Medicine Institutional Review Board. Informed consent was not required because, except for zip codes, no patient-identifying information was collected.




