Taxonomic variation in the supraorbital region of catarrhine primates
Funding information: Arts and Humanities Research Council/London Arts and Humanities Partnership, Grant/Award Number: AH/L503873/1
Abstract
Objectives
This study aimed to test the taxonomic utility of the catarrhine supraorbital region using 3D geometric morphometrics, with the aim of establishing its potential use in elucidating the position of more debated hominin groups.
Materials and Methods
230 3D coordinates were used to record the supraorbital morphology of two datasets: one containing 460 non-hominin catarrhine primates from species and subspecies of Gorilla, Pan, Papio, and Macaca; and the other containing 55 Pleistocene hominins from Homo, Australopithecus, and Paranthropus. Principal component analyses in tangent, form, and allometry-free shape space were used to assess differentiation of taxa, with biological distinctiveness of taxa being established using step-wise discriminant analysis with subsampling.
Results
Results indicated that the recorded supraorbital morphology could be used to separate non-hominin catarrhine primate genera, species, and subspecies, although accuracy was found to decrease with decreasing Linnaean rank. In addition, analyses in tangent space were found to produce the highest accuracy when classifying primates of known taxonomy. Biological distinctiveness of the middle and later Homo species was comparable to or higher than that of the non-hominin primates, and relatively lower for the earlier groups of Homo.
Discussion
This study indicates that the supraorbital region preserves taxonomic information that can be used to delineate between closely related groups, both within hominins and wider catarrhine primates. Therefore, this region may be used to provide insight when assessing the taxonomic affiliation of disputed hominin specimens.
1 INTRODUCTION
Assessment of craniofacial morphology is a primary method in establishing the taxonomy of hominin fossils (Athreya, 2006, 2009; Cramon-Taubadel, 2013; Lieberman, 1995, 2011; Lieberman, 2000). Fossil species play a dual role in paleontology: they are a basis for the study of evolutionary processes giving rise to current groups (along with extant species); and they allow us to record past levels of biodiversity, and link this to changing factors such as geographic expansion and climate change (Tattersall, 1986). As such, the ability to identify and delineate different taxonomic groups is an important aspect of paleontology (Tattersall, 1986). Classification of fossil specimens is intrinsically linked to the available evidence (Stringer, Howell, & Melentis, 1979), however the fossil record only preserves small, incomplete samples of hominins from which to build our understanding (Simpson, 1961). In addition, the fossil record typically only preserves hard-tissue evidence, yet many taxonomic differences in extant animals are only apparent in behavioral or soft-tissue evidence (Simpson, 1961; Smith, 1994; Tattersall, 1986, 1992), making application of popular taxonomic criteria to the fossil record extremely difficult.
Paleoanthropologists use morphology to differentiate between hominin groups (Kimbel, 1991; Smith, 1994; Tattersall, 2005; Wood, 2010), for instance through the application of a morphological species concept. Such a concept can be applied by comparing levels of within- and between-group variation in skeletal morphology of fossil specimens to that of individuals of known taxonomic classification. In paleoanthropology, non-hominin primates, and especially the Catarrhini, are generally used as reference taxa. Researchers have proposed the use of multiple model taxa when attempting to address taxonomic questions (Harvati, Frost, & McNulty, 2004), partly due to the great ecological and biological diversity found in animals, and primates in particular (Ackermann, 2002; Baab, 2008; Jiménez-Arenas, Palmqvist, & Pérez-Claros, 2011; O'Higgins & Dryden, 1993; Schaefer, Mitteroecker, Gunz, Bernhard, & Bookstein, 2004; Wood, Li, & Willoughby, 1991).
The inclusion of non-human apes provides an opportunity to model taxonomic variation in groups with different levels of sexual dimorphism, which is relevant to paleoanthropology as studies have indicated that some extinct hominins may have been more sexually dimorphic than recent Homo sapiens (Garvin et al., 2017; Lockwood, 1996, 1999; Plavcan, 2012; Richmond & Jungers, 1995; Royer, Lockwood, Scott, & Grine, 2009). Non-human apes share a close relationship to Homo, with the Pan and Homo clades diverging approximately 6–9 Mya (Dos Reis et al., 2018; Langergraber et al., 2012; Perelman et al., 2011; Schrago & Voloch, 2013; Wilkinson et al., 2011), and the divergence between Gorilla and the Pan/Homo clade being dated to between 6 and 19 Mya (Glazko & Nei, 2003; Langergraber et al., 2012).
Papionins, as members of Cercopithecoidea, are more distantly related to hominins. Nevertheless, numerous researchers have suggested that they are appropriate models for the study of our evolutionary past (Baab, 2008; Delson, 1978; DeVore, 1963; Frost, Marcus, Bookstein, Reddy, & Delson, 2003; Harvati et al., 2004; Jolly, 1970; Jolly, 2001; Zinner, Groeneveld, Keller, & Roos, 2009). Their suitability arises from many characteristics, including: the numerous homologous traits they share with hominins; their phylogenetic distance to our own group, which may highlight possible parallelisms in primate evolution; their increased range in comparison to non-human apes, both geographically and ecologically; the existence of multiple species which diverged at similar time-depths and in broadly analogous habitats to hominins; the occurrence of hybridization across different taxonomic boundaries; and the occurrence of relatively great biodiversity and craniofacial variation in some groups (Ackermann & Bishop, 2010; Alberts & Altmann, 2001; Baab, 2008; Frost et al., 2003; Harvati et al., 2004; Jolly, 2001; Pan & Oxnard, 2002; Pan & Oxnard, 2004; Pan, Oxnard, & Milne, 2002; Zinner et al., 2009; Zinner, Wertheimer, Liedigk, Groeneveld, & Roos, 2013).
This paper aimed to test whether the morphology of the supraorbital region can be used to detect taxonomic boundaries in primates, particularly at the species and subspecies level. The supraorbital region is one of the best-preserved in key periods of the hominin fossil record, potentially due to the robusticity of this area. It has also been suggested to document a moderate phylogenetic signal (McNulty, 2005; Smith, 2009; Weidenreich, 1947), and displays established phenetic differences between primate taxa (Aiello & Dean, 1990; Hublin et al., 2017; Lahr & Wright, 1996; Lieberman, 2000; Lieberman, 2011; Russell, 1985; Schwartz & Tattersall, 2010; Smith & Ranyard, 1980), indicating its potential usefulness for assessing the taxonomy of extinct hominins. Reference non-hominin primates included members of Gorilla, Pan, Papio, and Macaca. Following a test of the taxonomic information content of the supraorbital region in extant non-hominin primates, the same methods were applied to a dataset of Pleistocene hominin fossils, to test whether supraorbital morphology could provide valid insights into the hominin fossil record and, ultimately, the taxonomy of more debated hominin groups.
2 MATERIALS AND METHODS
2.1 Sample
Only adult specimens were included in this study. Adult status was assessed dentally, by the full eruption of the third molars (both maxillary and mandibular, if present), and cranially, by full fusion of the basioccipital-basisphenoidal synchondrosis (Wood et al., 1991). When assessment of the fusion of the basioccipital-basisphenoidal synchondrosis could not be conducted, dental maturity was used as the sole criterion. Specimens showing evidence of pathology or trauma in the cranium were excluded. Specimens with detailed geographical locations were favored, although some with unknown origin were included when available specimen numbers were low.
2.2 Non-hominin primate dataset
This study used a dataset of non-hominin primates consisting of 460 adult specimens from 10 species within Gorilla, Pan, Papio, and Macaca (see Table 1 and Supporting Information SI-1). Previous researchers and collectors did not always recognize current subspecific distinctions within the non-hominin apes, therefore geographical information was used to establish subspecies categories of Pan and Gorilla specimens, using data on current taxonomic distribution from the IUCN (2017). Due to the relatively recent taxonomic separation of Papio cynocephalus and Papio kindae, geographic data from the IUCN was used to inform classification, although this was not possible for all specimens. Sex information was taken from collection material, and the ratio of males to females across the sample was approximately equal (225 females, 226 males, nine unknown). Sample sizes varied between groups due to unequal representation of the included taxa in the various collections used. A maximum of 50 individuals were selected from each taxon. All non-hominin primate data was taken from original specimens, either in the form of 3D laser scans collected with a NextEngine Desktop Laser Scanner, or surface models generated from available CT data using 3D Slicer (Fedorov et al., 2012; see SI-1 and SI-2 in Supporting Information).
| Taxon | Abbr. | Count | % | Total | ||||
|---|---|---|---|---|---|---|---|---|
| Female | Male | Unknown | Female | Male | Unknown | |||
| Gorilla beringei beringei | GBB | 6 | 8 | 42.9% | 57.1% | 14 | ||
| Gorilla beringei graueri | GBG | 23 | 18 | 56.1% | 43.9% | 41 | ||
| Gorilla gorilla gorilla | GGG | 25 | 25 | 50.0% | 50.0% | 50 | ||
| Gorilla gorilla diehli | GGD | 9 | 9 | 50.0% | 50.0% | 18 | ||
| Pan paniscus | PP | 21 | 18 | 4 | 48.8% | 41.9% | 9.3% | 43 |
| Pan troglodytes troglodytes | PTT | 25 | 25 | 50.0% | 50.0% | 50 | ||
| Pan troglodytes schweinfurthii | PTS | 16 | 11 | 3 | 53.3% | 36.7% | 8.8% | 30 |
| Pan troglodytes verus | PTV | 6 | 10 | 37.5% | 62.5% | 16 | ||
| Pan troglodytes ellioti | PTE | 2 | 3 | 40.0% | 60.0% | 5 | ||
| Papio anubis | PA | 17 | 25 | 40.5% | 59.5% | 42 | ||
| Papio cynocephalus sensu latoa | PC? | 3 | 2 | 60.0% | 40.0% | 5 | ||
| Papio cynocephalus | PC | 2 | 6 | 25.0% | 75.0% | 8 | ||
| Papio kindae | PK | 10 | 13 | 43.5% | 56.5% | 23 | ||
| Macaca fascicularis | MFa | 24 | 25 | 49.0% | 51.0% | 49 | ||
| Macaca fuscataa | MFu? | 3 | 6 | 33.3% | 66.7% | 9 | ||
| Macaca fuscata fuscata | MFuF | 3 | 1 | 2 | 50.0% | 16.7% | 33.3% | 6 |
| Macaca fuscata yakui | MFuY | 5 | 2 | 71.4% | 28.6% | 7 | ||
- Note: Abbr. indicates group abbreviations. Data for sex was taken from museum records.
- a Some Macaca fuscata and Papio cynocephalus sensu lato specimens could not be assigned a subspecies classification.
2.3 Hominin dataset
A Pleistocene hominin dataset was also used in this study, and consisted of 55 specimens from Paranthropus, Australopithecus, and Homo (see Table 2 and Supporting Information SI-1). Earlier Homo and Late Australopithecus specimens, from purported species such as Homo rudolfensis and Homo habilis, were included, along with a reconstruction of Dinaledi Hominin 1 (DH1; Homo naledi). This specimen has been dated to the Middle Pleistocene (Dirks et al., 2017; Hawks & Berger, 2016), although its morphology indicates a closer relationship to Early Pleistocene hominins (Berger et al., 2015; Laird et al., 2017; Schroeder et al., 2017). Homo erectus (sensu lato) specimens from three subgroups (Homo georgicus, Homo ergaster, and Homo erectus sensu stricto) were also included, allowing modeling of craniofacial variation in a widespread and generally well-accepted hominin species with a considerable life span (over 1.5 million years [Antón, 2003]). Middle Pleistocene hominins (MPH) were included, although, while these hominins have previously been classified as Homo heidelbergensis sensu lato, they have an unresolved taxonomy, possibly constituting multiple species (Buck & Stringer, 2014; Harvati, 2007; Hublin, 2013; Stringer, 2012). As such, they were included to avoid inflating the morphological distance between earlier and later Homo species, but were not considered as a taxon for the purposes of this study. Late Pleistocene fossils included both Homo neanderthalensis and Homo sapiens, providing a model of variation between two sister-species. Hominin data were taken from research-quality casts of specimens, although original data were available in a number of cases (see Table 2), either from 3D laser scans of the original fossil, or from surface models generated from available CT data (see Supporting Information SI-1 and SI-2).
| Specimen | Species | Abbr. | Subgroup | Abbr. |
|---|---|---|---|---|
| Chancelade | Homo sapiens | HS | Anatomically modern human | AMH |
| Furfooz I | Homo sapiens | HS | Anatomically modern human | AMH |
| Keilor | Homo sapiens | HS | Anatomically modern human | AMH |
| Oberkassel I | Homo sapiens | HS | Anatomically modern human | AMH |
| Oberkassel II | Homo sapiens | HS | Anatomically modern human | AMH |
| Abri Pataud | Homo sapiens | HS | Anatomically modern human | AMH |
| Brno II | Homo sapiens | HS | Anatomically modern human | AMH |
| Cro-Magnon I | Homo sapiens | HS | Anatomically modern human | AMH |
| Cro-Magnon II | Homo sapiens | HS | Anatomically modern human | AMH |
| Dolní Věstonice III | Homo sapiens | HS | Anatomically modern human | AMH |
| Mladeč 1a | Homo sapiens | HS | Anatomically modern human | AMH |
| Mladeč 2 | Homo sapiens | HS | Anatomically modern human | AMH |
| Předmostí III | Homo sapiens | HS | Anatomically modern human | AMH |
| Předmostí IV | Homo sapiens | HS | Anatomically modern human | AMH |
| Zhoukoudian UC.101 | Homo sapiens | HS | Anatomically modern human | AMH |
| Zhoukoudian UC.102 | Homo sapiens | HS | Anatomically modern human | AMH |
| Border Cave 1 | Homo sapiens | HS | Early modern human | EMH |
| Jebel Irhoud 1 | Homo sapiens | HS | Early modern human | EMH |
| Herto | Homo sapiens | HS | Early modern human | EMH |
| Liujiang | Homo sapiens | HS | Early modern human | EMH |
| Omo 1 | Homo sapiens | HS | Early modern human | EMH |
| Qafzeh 9 | Homo sapiens | HS | Early modern human | EMH |
| Skhūl Va | Homo sapiens | HS | Early modern human | EMH |
| Tabun I | Homo neanderthalensis | HN | ||
| La Quina H5 | Homo neanderthalensis | HN | ||
| Spy 1a | Homo neanderthalensis | HN | ||
| La Chapelle | Homo neanderthalensis | HN | ||
| Guattari | Homo neanderthalensis | HN | ||
| Gibraltar 1b | Homo neanderthalensis | HN | ||
| Le Moustier 1 | Homo neanderthalensis | HN | ||
| Amud 1 | Homo neanderthalensis | HN | ||
| Krapina C | Homo neanderthalensis | HN | ||
| Krapina E | Homo neanderthalensis | HN | ||
| Saint-Césaire I | Homo neanderthalensis | HN | ||
| Shanidar I | Homo neanderthalensis | HN | ||
| Shanidar V | Homo neanderthalensis | HN | ||
| Bodoa | Middle Pleistocene hominin | MPH | ||
| Kabwe (Broken Hill) 1b | Middle Pleistocene hominin | MPH | ||
| Petralona | Middle Pleistocene hominin | MPH | ||
| Sima de los Huesos 5 (SH5) | Middle Pleistocene hominin | MPH | ||
| Solo VI | Homo erectus sensu lato | HEsl | Homo erectus sensu stricto | HE |
| Sangiran 17 | Homo erectus sensu lato | HEsl | Homo erectus sensu stricto | HE |
| Zhoukoudian XII | Homo erectus sensu lato | HEsl | Homo erectus sensu stricto | HE |
| KNM-ER 3773 | Homo erectus sensu lato | HEsl | Homo ergaster | HEr |
| KNM-ER 3883 | Homo erectus sensu lato | HEsl | Homo ergaster | HEr |
| Dmanisi D4500 | Homo erectus sensu lato | HEsl | Homo georgicus | HG |
| Dmanisi D2282 | Homo erectus sensu lato | HEsl | Homo georgicus | HG |
| Dinaledi hominin 1 (DH1) | Homo naledi | HNa | ||
| KNM-ER 1813 | Homo habilis | HHa | ||
| OH 24 | Homo habilis | HHa | ||
| KNM-ER 1470 | Homo rudolfensis | HRu | ||
| Sts 5a | Australopithecus africanus | AAfr | ||
| KNM-WT 17000 | Paranthropus aethiopicus | ParA | ||
| KNM-ER 406 | Paranthropus boisei | ParB | ||
| KNM-ER 732 | Paranthropus boisei | ParB | ||
| OH 5 | Paranthropus boisei | ParB |
- Note: Abbr. indicates group abbreviations. Homo sapiens were separated into two subgroups based on their morphology. Older specimens not showing the full suite of Homo sapiens craniofacial traits were classified within Early Modern Humans (EMH), while later specimens showing modern craniofacial traits were classified as Anatomically Modern Humans (AMH).
- a Indicates specimens where surface models were generated from available CT data.
- b Indicates specimens where surface models were collected from the original fossil.
2.4 Reconstruction
Given the nature of the fossil record, it is unsurprising that most fossil hominin specimens included in this study were not intact across the region of interest, and some specimens had suffered postdepositional distortion. While efforts were made to select only the most well-preserved extant non-hominin primate specimens, this was not possible for members of the less numerous taxa, and a number of extant non-hominin primates in the final sample had missing data. While the simplest way to deal with missing data is to remove the affected landmarks from the dataset, this is unrealistic in larger samples. The alternative is to reconstruct the missing data so that the incomplete specimens may still be included in the analysis (Gunz, 2005). Each specimen was assessed individually in order to apply the most appropriate method given the above considerations. Details of the reconstruction methods and reference specimens can be found in Supporting Information SI-3. Effects of reconstruction methods and reference specimens were assessed (Supporting Information SI-4). These were found to have a nonsignificant impact on intragroup variation.
2.4.1 Landmarking
Landmarking was conducted in Stratovan Checkpoint. 230 3D landmarks and surface semilandmarks were used in this study. These included nine landmarks placed around the orbital area, and a mesh of 221 points across the supraorbital region, consisting of 11 control landmarks and 210 automatically generated semilandmarks. Two additional landmark points (Auriculare) were used to guide placement of the mesh but were not included in the final configuration (see Figure 1 and Table 3).
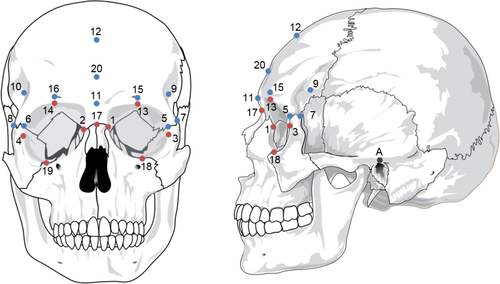
| # | Landmark | Laterality | Definition |
|---|---|---|---|
| A, B | Auriculare | Bilateral | The point vertically above the midpoint of the external auditory meatus on the zygomatic root |
| 1, 2 | Dacryon | Bilateral | The point where a line from Ectoconchion dividing the orbit into two along the long axis intersects with the medial orbital margin |
| 3, 4 | Ectoconchion | Bilateral | The intersection of the most anterior surface of the lateral border of the orbit and a line bisecting the orbit along the long axis |
| 5, 6 | Frontomalare anterior | Bilateral | The point where the frontomalare suture intersects with the lateral orbital margin |
| 7, 8 | Frontomalare posterior | Bilateral | The most posterior point on the frontomalare suture of the zygomatic process |
| 9, 10 | Frontotemporale | Bilateral | The most medial point on the lateral curve of the frontal bone, when viewed from norma verticalis |
| 11 | Glabella | Unilateral | The most anterior point on the frontal bone, between the supraorbital tori, on the midsagittal plane |
| 12 | Mid-frontotemporale | Unilateral | The point where a line between the frontotemporale points intersects with the midsagittal plane |
| 13, 14 | Mid-torus anterior | Bilateral | The most anterior point on the frontal bone directly above the midpoints of the orbit |
| 15, 16 | Mid-torus inferior | Bilateral | The point on the superior orbital margin, at the midpoint of the orbit |
| 17 | Nasion | Unilateral | The point where the nasofrontal suture intersects the midsagittal plane |
| 18, 19 | Orbitale | Bilateral | The most inferior point on the infraorbital margin |
| 20 | Post-toral sulcus | Unilateral | The most inferior point on the region posterior to glabella, in the midsagittal plane |
2.4.2 Analyses
The final configurations of 230 3D points were registered using generalized procrustes analysis (GPA) with a partial Procrustes fit (Rohlf, 1999; Rohlf & Slice, 1990) using the gpagen function in Geomorph (Adams, Otárola-Castillo, & Paradis, 2013). Surface semilandmarks were slid to minimize bending energy during this process. This method is favorable over the minimization of Procrustes distances when samples include multiple taxa with significantly different morphology, as in the present study, as it maintains geometric homology (Gunz & Mitteroecker, 2013). This process was repeated for both the non-hominin primate and hominin datasets, and was conducted in tangent space (a Euclidean approximation of Kendall's shape space), form space (where the effect of scaling was not removed, by scaling the Procrustes shape coordinates by Centroid size [Dryden & Mardia, 1998]), and allometry-free shape space (where the residuals from a regression of size [lnCS] on the Procrustes coordinates were used, using the procD.lm and shape.resid functions in Geomorph). Results from the three shape spaces can be usefully compared to bring to light dissociation between form and shape variation within a sample, and to assess the effect of both isometry and allometry (Mitteroecker & Gunz, 2009; Mitteroecker, Gunz, Windhager, & Schaefer, 2013).
Procrustes shape coordinates resulting from the above GPA were put through principal component analysis (PCA), to reduce the datasets into a few dimensions which summarized the key aspects of variation (Mitteroecker & Gunz, 2009; O'Higgins, 2000). This study used the first two principal components for preliminary assessment and visualization, while including the loadings along the principal components accounting for over 95% of total sample variance combined in subsequent analyses, to avoid misinterpretation of morphological variation. Mean shapes were produced for each taxon, using the Procrustes coordinates and the mshape function in Geomorph, and were visualized as 3D scatterplots in SPSS 25. Mean pair-wise Procrustes distances were calculated within and between taxa.
Step-wise, cross-validated discriminant analyses with subsampling were performed for both datasets, using the principal components that accounted for over 95% of total variance, to assess biological distinctiveness following the methods of Cardini et al. (2009). Specimens which were the only representative of their species were excluded (i.e., Homo naledi, Homo rudolfensis, Australopithecus africanus, and Paranthropus aethiopicus), along with Paranthropus boisei due to the lack of closely related specimens, resulting in a hominin dataset of 48 specimens. Discriminant analyses were repeated using three classification systems: the first classified specimens into genus and was applied to the non-hominin primate dataset; the second classified specimens into genus and species, respectively, and was applied to both the non-hominin primate and hominin datasets; the third classified the non-hominin primate dataset into subspecies where applicable. 1,000 random subsamples were taken from each taxonomic group. For the non-hominin primate dataset, subsample size was set to n = 16 for genus-, n = 8 for species-, and n = 4 for subspecies-level analyses. These values were chosen due to low sample sizes for some of the subspecific groups, and to reflect the hierarchical composition of each increasing taxonomic rank. For the hominin dataset, sample size was set to n = 7 due to the number of Homo erectus sensu lato included, except for the MPH where n = 4, and Homo habilis where n = 2 due to lower sample sizes, resulting in a total subsample of 27 individuals for each repeat. Classification accuracy was taken to reflect biological distinctiveness for each taxonomic rank (Cardini et al., 2009; Cardini & Elton, 2011), and was calculated as a mean percentage across the 1000 repeats. MPH and Homo habilis were excluded from the final calculation of cross-group hominin species-level classification accuracy, due to the uncertainties around the former group's taxonomic status, and the small sample size of the latter.
2.4.3 Intraobserver error
All landmarking was conducted by SW. Twenty six specimens (18 non-hominin primates and eight Pleistocene hominins) were used in the assessment of intraobserver error (see Table S13). Landmarks defined above in Table 3 were placed on these 26 specimens on four occasions. As this study focused on morphological differences between genera, species, and subspecies, this study followed the method of Lockwood, Lynch, and Kimbel (2002). The repeats for the intraobserver specimens were added to the non-hominin primate and hominin datasets, after which both datasets were put through a GPA. Procrustes distances (which should approximate Euclidean distances from principal components) for intraobserver repeats were then compared to intra- and inter-genus, species, and subspecies Procrustes distances using a one-way ANOVA with post hoc Tukey HSD (honestly significant difference) tests in SPSS 25. Results showed that intraobserver error was significantly lower than intra- and intertaxonomic distances for both datasets (see Tables S14 and S15). It was therefore concluded that any intraobserver error should not significantly affect the outcome of later taxonomic analyses.
3 RESULTS
3.1 Non-hominin primates
3.1.1 Group morphology
Differences between the non-hominin primate genera are shown in Figure 2 (figures are scaled to unit centroid size, and therefore show differences in shape, not overall form). In comparison to Pan, Gorilla were found to have more laterally flaring supraorbital tori that were more anteriorly projecting, and wider nasal columns, narrower frontal bones, and deeper supraorbital sulci relative to overall size. The differences between Papio and Macaca appear to be less marked, reflected in the lower pairwise Procrustes distances (0.117 compared to 0.155 for Gorilla-Pan comparisons; see Table S16). Members of Papio were found to have shorter orbits, thicker supraorbital trigones, and less vertical frontal squamae in comparison to Macaca.

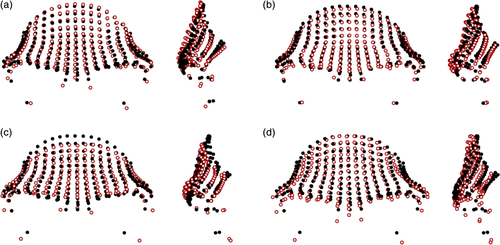
There were slight differences apparent between the Gorilla gorilla subspecies (mean pairwise PrD: 0.112), which were largely focused in the lateral and inferior aspects of the supraorbital tori (Figure 3). Slightly larger differences (mean pairwise PrD: 0.119) were apparent between the Gorilla beringei subspecies, with Gorilla beringei beringei having more superiorly placed dacryon points, more anteriorly projecting frontal squamae and supraorbital tori, and slightly narrower frontal squamae than Gorilla beringei graueri. The differences between the Gorilla species were more marked (mean pairwise PrD: 0.123), with Gorilla gorilla having anteroposteriorly thicker lateral aspects of their supraorbital tori, more anteriorly projecting supraorbital tori in the midsagittal region, and more superiorly placed orbital points than Gorilla beringei.
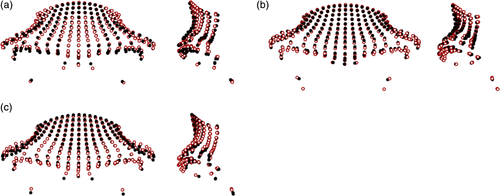
Figure 4 shows minimal differences (mean pairwise PrD: 0.090) between the subspecies of Pan troglodytes. Pan troglodytes troglodytes was found to have more anteroinferiorly placed inferior aspects of the supraorbital tori, deeper post-toral sulci, and more vertical frontal squamae than the average Pan troglodytes schweinfurthii configuration. Pan troglodytes ellioti had slightly more laterally expanded supraorbital trigones, less anteriorly placed supraorbital tori and frontal squamae, and more posteriorly placed orbits than Pan troglodytes troglodytes. Pan troglodytes verus had slightly more anteriorly projecting supraorbital tori, which were taller in the midsagittal region, and less vertical frontal squamae than Pan troglodytes ellioti. In terms of species-level differences within Pan (mean pairwise PrD: 0.096), Pan troglodytes had more projecting supraorbital tori on average, with laterally expanded supraorbital trigones, and more posteriorly placed lower orbital margins, while the uppermost part of the frontal squama of the average Pan paniscus configuration was more anteroinferiorly placed.
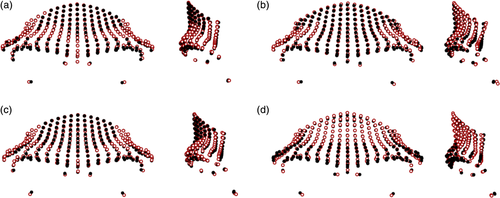
Comparisons between the average shapes of the Papio groups are shown in Figure 5. The differences between Papio anubis and Papio cynocephalus were relatively small (mean pairwise PrD: 0.098), with the latter having slightly narrower supraorbital tori and more anteriorly placed frontal squamae in the medial region, while the lateral aspects were relatively more inferiorly placed. Papio anubis had more anteriorly protruding supraorbital tori in the glabella region in comparison to Papio cynocephalus. Differences between Papio anubis and Papio kindae were more apparent (mean pairwise PrD: 0.106), with the latter having taller, more anteriorly and laterally projecting frontal squamae, and the former having more inferiorly placed supraorbital margins and orbits. The differences between Papio cynocephalus and Papio kindae were less apparent (mean pairwise PrD: 0.094), although the latter again was found to have a more superiorly and laterally projecting frontal squamae on average.
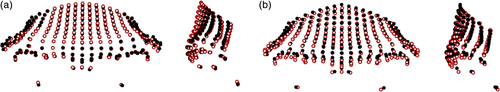
Figure 6 shows the differences between the Macaca taxa. There were slight differences between the Macaca species (mean pairwise PrD: 0.099), which were largely found in the supraorbital trigones and superior frontal squama region. Macaca fascicularis was found to have the most anteriorly projecting supraorbital tori in the medial region, while the lateral aspects of the supraorbital tori in Macaca fuscata were more projecting. The orbits of Macaca mulatta showed a higher degree of orbital frontation than in the other two species, and the frontal squamae in this group were more vertically aligned. Differences between the Macaca fuscata subspecies were relatively low (mean pairwise PrD: 0.087); Macaca fuscata yakui specimens had slightly more laterally thickened supraorbital trigones and mediolaterally narrower frontal squamae, which were more vertically aligned than seen in Macaca fuscata fuscata.
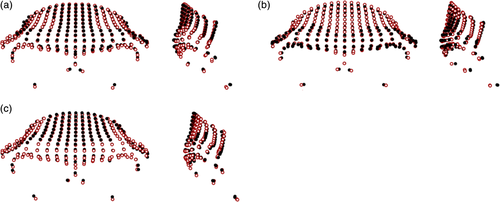
3.1.2 Principal component analysis
Only the results of the tangent space analyses are presented here as they provide the most reliable levels of taxonomic differentiation (see Discriminant analysis). The results of the form and allometry-free shape space analyses are available in Supporting Information (SI-6).
Principal component analysis on the non-hominin primate dataset resulted in 460 principal components (PCs), with the first 32 accounting for >95% of the total sample variation combined, and the first 13 accounting for >1% of variation individually. The first PC accounted for 40.4% of sample variation. More negative values on PC1 were associated with less laterally flaring and anteriorly projecting supraorbital tori, a minimal postorbital sulcus, a higher degree of orbital frontation, a narrower nasal column, and a smaller degree of postorbital constriction (Figure 7). The second PC accounted for 14.9% of sample variation. Negative values on this component were associated with superoinferiorly thicker supraorbital tori, a lack of a post-toral sulcus, and a particularly narrow nasal column.
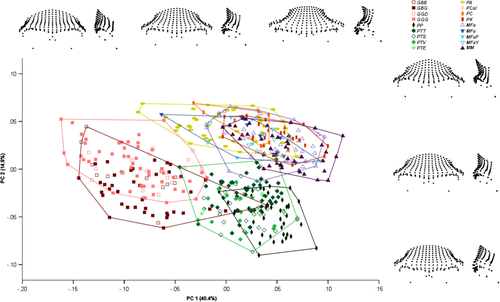
The non-hominin primates were spread along two parallel trajectories in this plot, with one comprising the non-human apes, which had lower values on PC2, and the other the papionins. At genus level, Gorilla and Pan were separated along both PC1 and PC2, although with some overlap, with Gorilla having lower values for PC1 and higher values for PC2 (Figure S3). Papio and Macaca overlapped to a larger extent, with Papio having slightly lower values along PC1 and higher values on PC2.
At species level, Gorilla beringei specimens were found to have lower values along PC2 combined with slightly higher values on PC1 in comparison to Gorilla gorilla (Figure S4). Pan paniscus was differentiated from Pan troglodytes by slightly higher values along PC1 and lower values on PC2. Papio cynocephalus was mostly encompassed by Papio anubis, while Papio kindae was somewhat differentiated by higher values for PC1. The three Macaca species showed considerable overlap for values on both PC1 and PC2.
In terms of subspecific differences, Gorilla beringei beringei had higher values along PC2, while Gorilla beringei graueri had lower values along this component, and a wider range of values along PC1 (Figure S5). Gorilla gorilla diehli was almost fully encompassed by Gorilla gorilla gorilla, although it sat toward the lower end of this taxon's range for PC2. Pan troglodytes verus and Pan troglodytes ellioti were almost fully encompassed by the Pan troglodytes schweinfurthii specimens, while Pan troglodytes troglodytes was somewhat differentiated by lower values along PC1 and higher values along PC2. Macaca fuscata yakui was found to have slightly lower values along PC1 than Macaca fuscata fuscata.
3.1.3 Discriminant analysis
Results of the discriminant analyses in the three shape spaces are shown in Table 4. Primate taxa were found to have the highest biological distinctiveness using principal components from analyses in tangent space at the subspecies and species levels, and form space at the genus level (although biological distinctiveness was similar when using PCs from tangent space analysis at the genus level). Results of the discriminant analyses using principal components from PCA in form and allometry-free shape space can be found in Supporting Information SI-6. Using results from the PCA in tangent space, classification accuracy was highest for the genus-level analysis (97.5%), followed by the species-level analysis (75.4%), and the subspecies-level analysis (45.2%). Genus classification accuracy, taken to reflect biological distinctiveness, ranged from 96.6% in Papio to 98.9% in Pan (Table 5). The range of biological distinctiveness for the species groups was broader; from 56.4% in Papio cynocephalus to 83.9% in Gorilla gorilla (Table 6). Biological distinctiveness for the included subspecies showed a similarly broad range, with Pan troglodytes ellioti having the lowest classification accuracy at 30.7%, and Gorilla beringei graueri the highest at 63.3% (Table 7).
| Subspecies | Species | Genus | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Tangent | Form | Allometry-free | Tangent | Form | Allometry-free | Tangent | Form | Allometry-free | |
| GBB | 42.7 | 25.3 | 29.7 | 83.8 | 70.5 | 57.2 | 97.1 | 95.4 | 69.4 |
| GBG | 63.3 | 35.9 | 47.7 | ||||||
| GGD | 57.2 | 35.7 | 40.8 | 83.9 | 70.7 | 52.1 | |||
| GGG | 49.3 | 38.1 | 34.8 | ||||||
| PP | 81.6 | 82.8 | 76.3 | 98.9 | 98.3 | 94.7 | |||
| PTT | 44.6 | 33.1 | 38.0 | 78.3 | 76.4 | 72.2 | |||
| PTS | 38.3 | 19.3 | 36.6 | ||||||
| PTV | 35.5 | 20.4 | 34.7 | ||||||
| PTE | 30.7 | 10.9 | 36.2 | ||||||
| PA | 69.2 | 38.7 | 62.3 | 96.6 | 99.9 | 95.2 | |||
| PC | 56.4 | 35.2 | 56.9 | ||||||
| PK | 85.9 | 80.0 | 86.9 | ||||||
| MFa | 68.3 | 71.8 | 57.2 | 97.4 | 99.7 | 73.5 | |||
| MFuF | 36.1 | 57.9 | 30.5 | 72.1 | 48.5 | 63.6 | |||
| MFuY | 54.6 | 55.6 | 45.4 | ||||||
| MM | 74.8 | 66.4 | 63.9 | ||||||
| 45.2 | 33.2 | 37.4 | 75.4 | 64.1 | 64.9 | 97.5 | 98.3 | 83.2 | |
- Note: Mean percentage classification accuracy across the 1,000 subsamples (n = 16, for Genus; n = 8 for Species; n = 4 for Subspecies) is shown by taxon. See Table 1 for list of abbreviations. See tables in Supporting Information SI-6 for detailed results for form and allometry-free shape space analysis. Significance of Cells in gray show species which do not contain subspecies.
| Gorilla | Pan | Papio | Macaca | Genus | |
|---|---|---|---|---|---|
| Gorilla | 97.1 | 2.5 | 0.4 | 97.1 | |
| Pan | 0.5 | 98.9 | 0.1 | 0.5 | 98.9 |
| Papio | 96.6 | 3.4 | 96.6 | ||
| Macaca | 0.1 | 2.5 | 97.4 | 97.4 |
- Note: Mean percentage classification accuracy across the 1,000 subsamples (n = 16) is shown by taxon. Specimens were classified by genus, and overall genus classification accuracy was 97.5%.
| GB | GG | PP | PT | PA | PC | PK | MFa | MFu | MM | Species | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| GB | 83.8 | 10.8 | 1.2 | 4.1 | 0.1 | 83.8 | |||||
| GG | 12.0 | 83.9 | 0.4 | 2.1 | 1.1 | 0.1 | 0.1 | 0.2 | 0.1 | 83.9 | |
| PP | 0.3 | 0.1 | 81.6 | 17.7 | 0.2 | 81.6 | |||||
| PT | 1.1 | 0.9 | 17.6 | 78.3 | 0.1 | 0.4 | 0.7 | 0.2 | 0.1 | 0.7 | 78.3 |
| PA | 0.5 | 0.1 | 69.2 | 15.5 | 12.2 | 0.6 | 0.8 | 1.2 | 69.2 | ||
| PC | 14.0 | 56.4 | 19.3 | 1.1 | 4.4 | 4.8 | 56.4 | ||||
| PK | 4.8 | 6.9 | 85.9 | 0.8 | 0.3 | 1.2 | 85.9 | ||||
| MFa | 0.1 | 0.1 | 0.2 | 0.8 | 1.2 | 1.4 | 68.3 | 13.8 | 14.1 | 68.3 | |
| MFu | 0.1 | 0.7 | 2.3 | 0.4 | 14.2 | 72.1 | 10.2 | 72.1 | |||
| MM | 0.1 | 0.1 | 0.1 | 1.8 | 4.2 | 2.3 | 8.8 | 7.9 | 74.8 | 74.8 |
- Note: Mean percentage classification accuracy across the 1,000 subsamples (n = 8) is shown by taxon. Specimens were classified into species groups, and overall species classification accuracy was 75.4%. See Table 1 for list of abbreviations. Significance of gray color are Cells shaded in gray show correct species-level classification. Cells bounded in gray show correct genus-level classification.
| GBB | GBG | GGD | GGG | PP | PTT | PTS | PTV | PTE | PA | PC | PK | MFa | MFuF | MFuY | MM | Subspecies | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| GBB | 42.7 | 24.5 | 11.5 | 11.0 | 0.8 | 1.9 | 1.0 | 1.8 | 1.6 | 0.2 | 0.3 | 0.3 | 0.6 | 0.6 | 0.7 | 0.7 | 42.7 |
| GBG | 12.6 | 63.3 | 5.0 | 5.8 | 1.1 | 2.0 | 2.1 | 2.7 | 3.7 | 0.2 | 0.2 | 0.1 | 0.3 | 0.3 | 0.3 | 0.6 | 63.3 |
| GGD | 10.3 | 5.5 | 57.2 | 17.6 | 0.4 | 2.3 | 0.7 | 1.1 | 0.6 | 1.7 | 0.4 | 0.5 | 0.4 | 0.4 | 0.4 | 0.8 | 57.2 |
| GGG | 11.7 | 7.9 | 20.5 | 49.3 | 0.6 | 1.8 | 0.7 | 1.8 | 1.0 | 1.7 | 0.5 | 0.9 | 0.4 | 0.3 | 0.4 | 0.7 | 49.3 |
| PP | 0.5 | 0.8 | 0.3 | 0.3 | 50.3 | 9.6 | 11.7 | 10.8 | 11.7 | 0.4 | 0.9 | 1.1 | 0.6 | 0.2 | 0.4 | 0.6 | |
| PTT | 1.0 | 1.3 | 1.6 | 1.3 | 7.9 | 44.6 | 13.7 | 10.4 | 12.1 | 0.7 | 1.4 | 0.4 | 0.8 | 0.9 | 0.6 | 1.4 | 44.6 |
| PTS | 0.3 | 1.2 | 0.6 | 0.3 | 10.3 | 15.0 | 38.3 | 13.2 | 17.4 | 0.5 | 1.1 | 0.4 | 0.6 | 0.2 | 0.3 | 0.5 | 38.3 |
| PTV | 0.7 | 1.6 | 0.9 | 0.9 | 10.0 | 11.4 | 13.9 | 35.5 | 22.4 | 0.8 | 0.7 | 0.3 | 0.3 | 0.3 | 0.2 | 0.3 | 35.5 |
| PTE | 0.3 | 1.8 | 0.3 | 0.5 | 14.7 | 10.4 | 14.9 | 22.4 | 30.7 | 0.7 | 0.7 | 0.3 | 0.9 | 0.6 | 0.5 | 0.6 | 30.7 |
| PA | 0.3 | 0.3 | 1.1 | 1.0 | 0.5 | 0.7 | 0.4 | 0.5 | 0.6 | 54.8 | 18.3 | 12.5 | 1.8 | 2.1 | 2.7 | 2.5 | |
| PC | 0.1 | 0.2 | 0.3 | 0.3 | 0.8 | 1.3 | 0.6 | 0.5 | 0.6 | 15.6 | 43.7 | 17.3 | 2.6 | 8.5 | 4.1 | 3.7 | |
| PK | 0.2 | 0.1 | 0.2 | 0.3 | 0.6 | 0.2 | 0.4 | 0.5 | 0.2 | 5.7 | 9.7 | 74.7 | 2.3 | 1.7 | 0.9 | 2.6 | |
| MFa | 0.5 | 0.3 | 0.3 | 0.4 | 0.8 | 1.3 | 0.6 | 0.2 | 0.5 | 2.8 | 3.4 | 3.5 | 47.6 | 12.3 | 11.4 | 14.0 | |
| MFuF | 0.9 | 0.1 | 0.2 | 0.5 | 0.2 | 1.3 | 0.2 | 0.1 | 0.3 | 1.8 | 3.2 | 2.3 | 13.7 | 36.1 | 27.6 | 11.5 | 36.1 |
| MFuY | 0.2 | 0.1 | 0.1 | 0.2 | 0.1 | 0.3 | 0.1 | 0.1 | 0.2 | 2.7 | 2.7 | 1.1 | 8.4 | 22.7 | 54.6 | 6.6 | 54.6 |
| MM | 0.7 | 0.4 | 0.7 | 0.7 | 0.6 | 1.2 | 0.4 | 0.2 | 0.4 | 2.5 | 5.1 | 4.6 | 10.5 | 11.9 | 8.1 | 52.2 |
- Note: Mean percentage classification accuracy across the 1,000 subsamples (n = 4) is shown by taxon. Specimens were classified into subspecies groups where possible, and overall subspecies accuracy was 45.2%. See Table 1 for list of abbreviations. Significance of Cells shaded in light gray show correct subspecies-level classification. Cells shaded in dark gray show correct species-level classification. Cells bounded in gray show correct genus-level classification.
3.2 Hominins
3.2.1 Group morphology
Large differences, reflected by a mean pairwise PrD of 0.127 (see Table S23), were found between the average Homo sapiens and Homo neanderthalensis shapes (Figure 8). Homo sapiens had taller frontal squamae which were more vertically aligned, their orbits were more anteriorly placed, and their supraorbital tori were narrower mediolaterally. In addition, Homo sapiens had more superiorly placed nasion landmarks and thinner supraorbital trigones in comparison to Homo neanderthalensis. The shape differences were even larger between Homo erectus sensu lato and Homo sapiens (mean pairwise PrD: 0.172), with the latter having parallel frontal squamae and lateral aspects of the supraorbital tori, thin supraorbital trigones, high, vertical frontal squamae, more superiorly located nasion points, and less protruding supraorbital tori. The differences between the mean Homo neanderthalensis and Homo erectus sensu lato configurations were less visible (mean pairwise PrD: 0.117), with Homo neanderthalensis having slightly taller and more vertical frontal squamae, less anteriorly projecting supraorbital tori, and slightly thinner, less laterally flaring supraorbital trigones.
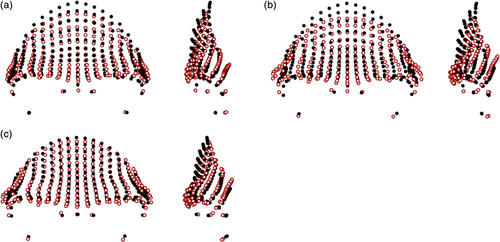
Comparisons between early Homo and related hominins are shown in Figure 9. The Homo rudolfensis specimen (KNM-ER 1470) showed a more superoinferiorly bulging frontal squama in comparison to the Homo habilis specimens (mean pairwise PrD of 0.100), with more inferiorly placed orbits, and a more superiorly located nasion. Homo naledi showed closer affinities to early Homo in the PCA plots (and especially to KNM-ER 3733 and OH 24), and lower mean pairwise PrD to Homo erectus sensu lato and Homo rudolfensis (0.118 and 0.119, respectively) in particular, despite its Middle Pleistocene age, so it is also considered here. DH1 had a slightly taller supraorbital torus than the mean Homo habilis shape, with much more angled orbits which were also wider, and a shorter frontal squama. Homo habilis had a more angled frontal squama in this orientation (i.e., a narrower angle between mid-frontotemporale-nasion-orbitale) than that found in the Australopithecus africanus specimen (Sts 5; mean pairwise PrD of 0.114), as well as more superiorly placed orbits and inferior supraorbital torus, and a mediolaterally wider frontal squama.
There are fairly large visible shape differences between Sts 5 and the Paranthropus aethiopicus specimen (KNM-WT 17000; Figure 10), reflected in the mean pairwise PrD of 0.133. For instance, KNM-WT 17000 had thick, laterally flaring, and posteriorly rotated supraorbital trigones, and a lower frontal squama than Sts 5. Differences between the Paranthropus species were less pronounced (mean pairwise PrD: 0.116): Paranthropus boisei was found to have less posteriorly rotated supraorbital trigones, slightly more vertical frontal squamae, slightly wider orbits and nasal columns, and slightly more anteriorly projecting supraorbital tori than seen in Paranthropus aethiopicus. Caution must be taken when interpreting these results, due to the low sample sizes available for the earlier hominins.
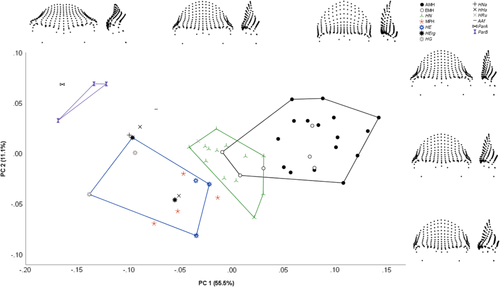
3.2.2 Principal component analysis
PCA in tangent space resulted in 54 principal components, with the first 17 accounting for over 95% of the total sample variance combined, and the first 10 accounting for >1% of variance individually. Figure 11 shows a plot of specimens by PC1 and PC2, accounting for 55.5% and 11.1% of variance, respectively. PC1 broadly corresponded with robusticity of the supraorbital region, with the more robust specimens (e.g., Paranthropus) having more negative values along this axis, and the more gracile Homo sapiens having the most positive values. Positive values corresponded to taller and more curved frontal squamae which occupied a larger area of the recorded morphology, minimal expression of the supraorbital tori in all dimensions, an absence of a supraorbital sulcus, a relatively superiorly positioned nasion, and a higher degree of orbital frontation. PC2 separated the early Homo sapiens, Homo neanderthalensis, MPH, and Homo erectus sensu lato, which had lower values along this axis, from the later Homo sapiens and earlier hominins. Specimens with higher values were associated with more laterally flaring supraorbital trigones, less projecting supraorbital tori with minimal post-toral sulci, more vertical frontal squamae, lower positions of dacryon points, and more vertically oriented orbits. Homo sapiens had the highest values along PC1 and PC2, with some earlier members (Omo 1, Jebel Irhoud 1, and Skhūl V) falling within the Homo neanderthalensis convex hull due to their lower values on PC1. The Homo erectus sensu lato specimens were separated from Homo neanderthalensis due to their relatively lower values along PC1. The MPH largely overlapped with the Homo erectus sensu lato, although they fell more toward the Homo erectus sensu stricto end of the convex hull, due to their higher values along PC1.
3.2.3 Discriminant analysis
The results of the hominin tangent space discriminant analysis are shown in Table 8. Species classification accuracy was 87.3% across the 1,000 subsamples. Classification accuracy was highest for Homo neanderthalensis (92.2%) and Homo sapiens (87.6%), followed by Homo erectus sensu lato (82.3%). Twenty of the 22 Homo sapiens were classified correctly in the majority of repeats (Table S24), although Skhūl V was only correctly classified in 47.2% of subsamples in which it was randomly selected (and was classified as Homo neanderthalensis in 39.3% of cases). Jebel Irhoud 1 and Omo 1 were most frequently classified as Homo neanderthalensis (in 73.6% and 48.7% of cases, respectively). All of the Homo neanderthalensis and Homo erectus sensu lato were most frequently correctly classified when included in subsample repeats. OH 24 was classified as Homo habilis in 49.0% of subsamples, while KNM-ER 1813 was classified as Homo erectus sensu lato in 64.1% of cases, reflecting its placement in the PC plot.
| HS | HN | MPH | HEsl | HHa | Species | |
|---|---|---|---|---|---|---|
| HS | 87.6 | 9.1 | 2.1 | 0.9 | 0.3 | 87.6 |
| HN | 3.3 | 92.2 | 1.0 | 3.6 | 92.2 | |
| MPH | 0.2 | 15.8 | 67.3 | 12.0 | 4.8 | 67.3 |
| HEsl | 7.3 | 5.2 | 82.3 | 5.2 | ||
| HHa | 3.3 | 11.3 | 47.4 | 38.1 | 38.1 |
- Note: Mean percentage classification accuracy across the 1,000 subsamples (n = 7 except for the MPH where n = 4 and Homo habilis where n = 2) is shown. Specimens were classified into species groups, and overall species classification accuracy was 87.3%. MPH and Homo habilis were excluded from this figure due to uncertain taxonomic status and small sample size, respectively. See Table 2 for list of abbreviations. Significance of Cells shaded in gray show correct species-level classification.
4 DISCUSSION
A proper understanding of biological variation is fundamental to the study of evolutionary processes, however the accurate documentation of phenotypic variation in the fossil record is particularly challenging due to its fragmentary nature. Studies have found that different elements of the primate craniofacial complex have varying levels of effectiveness in differentiating groups at different taxonomic levels (Bjarnason, Chamberlain, & Lockwood, 2011; Bjarnason, Soligo, & Elton, 2015, 2017; Cardini & Elton, 2008a; Lockwood, Kimbel, & Lynch, 2004; von Cramon-Taubadel & Smith, 2012). As such, it is important to establish the extent to which aspects of the craniofacial morphology that are relatively well documented in the fossil record reflect taxonomic differentiation in extant primates. The supraorbital torus is one such region, being particularly well-preserved in the hominin fossil record, potentially due to the robusticity of this cranial superstructure.
This study identified notable differences between accepted non-hominin primate taxa in the recorded morphology, indicating that the primate supraorbital region can be used in taxonomic differentiation. Larger differences were noted at the genus level, although this varied between families and analyses; Pan and Gorilla were more separated in the principal component plots than Papio and Macaca, although all genera showed relatively high biological distinctiveness (97.5% in tangent space). Differences between species were less substantial (75.4% in tangent space), especially in the case of Papio cynocephalus, Papio anubis, and Macaca fascicularis. Differences between non-hominin primate subspecies were even more subtle, with several subspecies groups being fully encompassed within other closely related subspecies in terms of key principal components, and with subspecies groups having lower biological distinctiveness (45.2% in tangent space).
This pattern of increasing biological distinctiveness with increasing taxonomic rank is not unexpected. Subspecies are considered by some to be incipient species, populations which have begun to diverge from their conspecifics but which have not achieved full speciation and are genetically reticulate (Boggs, 2001; Groves, 2004; Mayr, 1982; Simpson, 1961). As such, the morphological divergence between subspecies groups is predicted to be lower than that between fully diverged species. Species, in contrast, are considered by many researchers to reflect biological entities (Balakrishnan, 2005; Cracraft, 1983; Eldredge & Cracraft, 1980; Ghiselin, 1974; Tattersall, 1992), and should therefore be expected to have more distinct boundaries than those between subspecies (Simpson, 1961). Genera are classified among the higher taxa, and in turn are expected to show a greater degree of biological divergence (Tattersall, 2017).
It is important to put the biological distinctiveness of the catarrhine supraorbital region into its wider context. Genus-level results were very similar to those found for hairy armadillos (weighted average of 98.5% classification accuracy; Abba et al., 2015), although no comparable studies could be found within the primates. The values for species-level analyses were comparable to those found for marmots (87.4%; Cardini et al., 2009) and Cercopithecus (88.3%; Cardini & Elton, 2008b), at least for the non-hominin apes, but lower than those for red colobus monkeys (97.0%; Cardini & Elton, 2011) and hairy armadillos (94.1%; Abba et al., 2015). For subspecies-level analyses, the values of the present study lay between those for marmots (36.6%; Cardini et al., 2009) and red colobus monkeys (80.1%; Cardini & Elton, 2011). Nevertheless, comparisons to other studies are imperfect as some used anatomical landmarks across the cranium and mandible (Cardini & Elton, 2008b, 2011), rather than focusing on specific areas of the craniofacial complex. As studies indicate that different craniofacial regions may differentially preserve phylogenetic histories (Bjarnason et al., 2011; Bjarnason et al., 2015; von Cramon-Taubadel, 2009), it could be predicted that they may also preserve different taxonomic signals. Future research could investigate the taxonomic utility of other well-represented regions, such as the temporal bone and basicranium, to further contextualize the results of the current study.
As shown by the group morphologies, differences between non-hominin primate and hominin taxa in supraorbital morphology are frequently subtle, especially in the case of the lower taxa (i.e., species and subspecies). Despite this, the above discriminant analyses resulted in relatively high classification accuracy in some groups. This contrasting result highlights the efficacy of geometric methods, which allow quantitative analysis of morphology, especially when differences between taxa may be difficult to detect through qualitative assessment of craniofacial morphology. Regardless of whether primate subspecies are sufficiently biological distinct, at least in the supraorbital region, and the ability of geometric morphometric methods to distinguish between these groups, researchers have suggested that the likelihood of having sufficient samples of primate fossil specimens to identify subspecific distinctions is low (Kimbel, 1991; Simpson, 1943, 1961; Tattersall, 1986, 1992).
Analyses were performed in tangent, form (with size added as a variable), and allometry-free (with the effect of allometric scaling removed) shape space. Taxonomic differentiation was found to be highest in tangent space, as shown by the higher rates of biological distinctiveness of known taxa, indicating that tangent space may be more useful for primate taxonomic differentiation when considering the supraorbital region. This was somewhat unexpected, as form space has been hypothesized to be preferable when size is integral to the morphology under assessment, including classification studies such as this one which include organisms that vary in both size and shape (Mitteroecker et al., 2013; Mitteroecker & Gunz, 2009). The results of the present study did note comparable levels of taxonomic differentiation between form and tangent space analysis at the genus level, which could support this argument as size differences are less pronounced within the genera studied. However, several of the taxa analyzed here are characterized by pronounced levels of sexual dimorphism and, hence, intraspecific size variation, which is likely to blur taxonomic distinctiveness based on size. As such, future work should explore the possibility that form space might more accurately distinguish between taxa that lack pronounced levels of sexual dimorphism.
The Papio kindae specimens were found to be more clearly separated from Papio anubis than Papio cynocephalus in principal component plots, and had higher biological distinctiveness. This may be partially affected by the relatively small size of the Papio cynocephalus sample, as research indicates that Papio kindae and Papio cynocephalus are more closely related to each other than they are to Papio anubis (Jolly, Burrell, Phillips-Conroy, Bergey, & Rogers, 2011; Zinner et al., 2009; Zinner et al., 2013), although this does not adequately explain the difference in biological distinctiveness as subsampling would have mitigated against differences in sample size. An alternative hypothesis is that this distinction is affected by the smaller physical size of Papio kindae, which are suggested to be paedomorphic in comparison to Papio cynocephalus (Dunn, Cardini, & Elton, 2013; Frost et al., 2003; Singleton, Seitelman, Krecioch, & Frost, 2017). Tangent space, while unaffected by isometric scaling, can be influenced by allometry. Papio kindae specimens were distinguished in terms of the key components of morphology in both form and allometry-free shape space (see Supporting Information), indicating that their biological distinctiveness in the supraorbital region may be unrelated to any allometric scaling.
Macaca fuscata yakui was found to have higher biological distinctiveness than Macaca fuscata fuscata overall (although not in form space), which is contrary to the fact that genetic data indicate low differentiation of this group relative to other populations of Macaca fuscata not currently given subspecific status (Marmi, Bertranpetit, Terradas, Takenaka, & Domingo-Roura, 2004). Biological distinctiveness was higher for the Macaca fuscata subspecies in form space, unlike all other non-hominin primate subspecies studied here. Research has shown that Macaca fuscata yakui is the smaller of the two subspecies (Napier, 1981; Yano, Egi, Takano, & Ogihara, 2018), indicating that further research into allometric differences between these taxa is required.
Of the Pan troglodytes subspecies, Pan troglodytes troglodytes was found to have the highest biological distinctiveness in supraorbital morphology, while Pan troglodytes ellioti had the lowest. Genetic analysis has indicated that the first phylogenetic split within Pan troglodytes was between Pan troglodytes schweinfurthii and Pan troglodytes troglodytes on the one hand, and Pan troglodytes ellioti and Pan troglodytes verus on the other (Prado-Martinez et al., 2013). This phylogenetic pattern was not reflected in the supraorbital morphology, with the largest distinction being found between Pan troglodytes schweinfurthii and Pan troglodytes troglodytes. These subspecies appear to have diverged later on than Pan troglodytes ellioti and Pan troglodytes verus, although there is more substantial genetic evidence to support their subspecific status, while the separation of Pan troglodytes ellioti is more debated (de Manuel et al., 2016; Lobon et al., 2016; Stone et al., 2010).
The largest differences between closely related species and subspecies, as measured by pairwise-Procrustes distances, were found within Gorilla. While genetic evidence indicates considerable differentiation between the two Gorilla species, it also shows that hybridization between Gorilla taxa occurred until fairly recently (Ackermann & Bishop, 2010; Thalmann et al., 2011; Thalmann, Fischer, Lankester, Pääbo, & Vigilant, 2007). Lower levels of biological distinctiveness were found for Gorilla beringei beringei in comparison to Gorilla beringei graueri in all shape space analyses. While both subspecies are known to have complicated phylogenetic histories, including periods of hybridization, previous analysis indicates that only the latter group show evidence of this in their craniodental morphology (Ackermann & Bishop, 2010). In addition, Gorilla beringei beringei have relatively small habitats with few remaining individuals, and show strong evidence of inbreeding (Fossey, 1983; Xue et al., 2015), which would be expected to lead to higher homogeneity in craniofacial morphology. Further analysis is required to confirm whether this pattern of intersubspecific biological distinctiveness is consistent across the craniofacial complex.
The biological distinctiveness of the supraorbital morphology of Homo sapiens and Homo neanderthalensis was higher than that of all of the non-hominin primate species, and the value for Homo erectus sensu lato was comparable to the highest of those for the non-hominin primate species. This is somewhat unexpected, as the inclusion of individuals from across the lifespan and geographical range of a species may blur the boundaries between species, although not in all cases (Baab, 2016). In addition, the inclusion of the Middle Pleistocene hominins, which may include transitional and early members of these middle and later Homo species, could have been predicted to reduce the biological distinctiveness of these taxa. The relatively high biological distinctiveness for Homo erectus sensu lato is also surprising due to the considerably wider time range from which these specimens were sampled, and the ongoing taxonomic debate around this group (Antón, 2003; Baab, 2008; Baab, 2016; Bilsborough, 2005; Etler, 2004; Lordkipanidze et al., 2013; Rightmire, Lordkipanidze, & Vekua, 2006). The effect of low sample sizes for early Homo limits interpretation of the frequent misclassification of the KNM-ER 1813 specimen.
The results of the present study would indicate that the hominin supraorbital region is particularly taxonomically informative relative to the wider catarrhine primates. This could be in part due to the higher variability, particularly in the supraorbital torus, in hominins, as well as the later changes in the frontal squama seen in modern Homo sapiens. The supraorbital torus has been acquired, lost, and modified in various populations of hominins (Lahr & Wright, 1996), and has been shown to document distinctive morphologies between species (Athreya, 2006, 2012; Fiscella & Smith, 2006; Gonzalez, Perez, & Bernal, 2010; Lahr & Wright, 1996; Lieberman, 2000; Moss & Young, 1960; Russell, 1985; Schwartz & Tattersall, 2010; Smith & Ranyard, 1980; Weidenreich, 1947). At present, few studies have assessed the evolutionary significance of the hominin brow ridge. The fossils included in the present study indicate that there may have been a transition from a more general hominid form (protruding, bar-like supraorbital tori that are short superoinferiorly) in earlier hominins such as Australopithecus, to a more variable form in Homo (e.g., the swollen, rounded tori in Homo neanderthalensis and MPH), potentially linked to the latter groups increased orthognathy, relatively high levels of craniofacial robusticity, and associated large cranial superstructures (Gonzalez et al., 2010; Lieberman, 2011; Weidenreich, 1941).
This study supported the suggestion of previous studies that Homo sapiens are particularly distinct in their frontal bone morphology (Athreya, 2009; Bruner, Athreya, de la Cuétara, & Marks, 2013; Godinho, Spikins, & O'Higgins, 2018; Kurten, 1979; Lieberman, 2000; Russell, 1985; Schwartz & Tattersall, 2010; Smith & Ranyard, 1980). This distinction appears to be due to the minimally expressed supraorbital torus in our species, along with our tall, bulging frontal squamae. Nevertheless, earlier members of Homo sapiens were found to overlap with Homo neanderthalensis, and Jebel Irhoud 1 and Omo 1 were more frequently misclassified as Homo neanderthalensis in the discriminant analysis. This is likely due to the presence of more plesiomorphic browridges in earlier Homo sapiens (Hublin et al., 2017; Lahr & Wright, 1996; Lieberman, 2000; Russell, 1985; Tattersall & Schwartz, 2008), although the possibility of interbreeding has also been raised in the case of the Jebel Irhoud assemblage (Mounier & Mirazón Lahr, 2019).
Homo neanderthalensis has been described as one of the most clearly defined and delineated extinct hominin species (Tattersall, 1992; Tattersall & Schwartz, 2006; White, Gowlett, & Grove, 2014), and the results of this study would seem to support this conclusion. While DNA analyses have shown that Homo neanderthalensis and Homo sapiens interbred on a number of occasions (Green et al., 2010; Prüfer et al., 2014; Racimo, Sankararaman, Nielsen, & Huerta-Sanchez, 2015; Sankararaman et al., 2014; Sankararaman, Patterson, Li, Pääbo, & Reich, 2012), this interbreeding does not seem to have led to increased similarity in the supraorbital morphology of these taxa, at least in the specimens studied here. Indeed, the results of the current study would support the specific status of Homo neanderthalensis (White et al., 2014). This group had a biological distinctiveness comparable to, and even somewhat higher than, that of Homo sapiens, along with a higher proportion of the sample being most frequently correctly classified across the subsamples. These groups were also clearly separated in terms of key morphology.
5 CONCLUSION
This study found that supraorbital morphology can be used to differentiate between closely related, extant non-hominin primate genera, species, and subspecies, although with a reduced accuracy in the latter taxon. Hypothesized late Middle-to-Late Pleistocene hominin species were found to have relatively higher biological distinctiveness in this region than the extant catarrhine non-hominin primate species, while the Homo erectus sensu lato specimens had biological distinctiveness which was comparable to the higher range of the non-hominin catarrhine species. Overall, the results support the use of supraorbital morphology to assess the taxonomic affiliation of fossil hominins and catarrhines of unknown or debated taxonomy, and suggest that hominin taxa may be more readily distinguished by their morphology in this region. Future studies should explore the different aspects of the supraorbital morphology recorded here to determine which are the most useful for taxonomic differentiation, and compare the efficacy of supraorbital morphology to that of other regions suggested to reflect phylogeny, such as the temporal bone and basicranium.
ACKNOWLEDGMENTS
The authors would like to thank the following curators for allowing access to the specimens in their collections: C. Stringer, R. Kruszynski, and R. Ives (Natural History Museum, London); M. Mirazon-Lahr and M. Belatti (Duckworth Laboratory, University of Cambridge); G. Garcia and E. Hoeger (American Museum of Natural History, New York); E. Gilissen (Royal Museum for Central Africa, Tervuren); K. Hussey and C. Phillips (Royal College of Surgeons, London); I. Livne (Powell Cotton Museum, Kent); H. Hashimoto (Kyoto University Museum, Kyoto); D. Shimizu (Kyoto University Primate Research Institute, Kyoto); P. Semal (Royal Belgian Institute of Natural Sciences, Brussels); S. Bond (Institute of Archaeology, UCL, London); G. Price (Biological Anthropology Collection, UCL, London); K. Helgen (Division of Mammals, Smithsonian, Washington); M. Tocheri (Human Origins Program, Smithsonian, Washington). The authors are grateful to A. Gleeson for his assistance with the coding of statistical analyses, A. Gomez-Robles for her constructive advice on drafts of this manuscript, and to two anonymous reviewers for their insightful comments. This study was supported by an AHRC/LAHP-funded studentship, grant reference number AH/L503873/1.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.




