Trabecular variation in the first metacarpal and manipulation in hominids
Funding information: Fondation Fyssen; H2020 European Research Council, Grant/Award Numbers: 336301, 819960; Max-Planck-Gesellschaft
Abstract
Objectives
The dexterity of fossil hominins is often inferred by assessing the comparative manual anatomy and behaviors of extant hominids, with a focus on the thumb. The aim of this study is to test whether trabecular structure is consistent with what is currently known about habitually loaded thumb postures across extant hominids.
Materials and methods
We analyze first metacarpal (Mc1) subarticular trabecular architecture in humans (Homo sapiens, n = 10), bonobos (Pan paniscus, n = 10), chimpanzees (Pan troglodytes, n = 11), as well as for the first time, gorillas (Gorilla gorilla gorilla, n = 10) and orangutans (Pongo sp., n = 1, Pongo abelii, n = 3 and Pongo pygmaeus, n = 5). Using a combination of subarticular and whole-epiphysis approaches, we test for significant differences in relative trabecular bone volume (RBV/TV) and degree of anisotropy (DA) between species.
Results
Humans have significantly greater RBV/TV on the radiopalmar aspects of both the proximal and distal Mc1 subarticular surfaces and greater DA throughout the Mc1 head than other hominids. Nonhuman great apes have greatest RBV/TV on the ulnar aspect of the Mc1 head and the palmar aspect of the Mc1 base. Gorillas possessed significantly lower DA in the Mc1 head than any other taxon in our sample.
Discussion
These results are consistent with abduction of the thumb during forceful “pad-to-pad” precision grips in humans and, in nonhuman great apes, a habitually adducted thumb that is typically used in precision and power grips. This comparative context will help infer habitual manipulative and locomotor grips in fossil hominins.
1 INTRODUCTION
The unique dexterity of the human hand is often linked to two major events in hominin evolution, the development of obligate bipedalism and of complex technology (Lemelin & Schmitt, 2016; Marzke, 2013; Napier, 1993; Richmond, Roach, & Ostrofsky, 2016; Wood-Jones, 1916). The discovery in the late 1950s of stone tools in association with the OH 7 Homo habilis fossil hand, dated to approximately 1.75 million years ago (Ma), was interpreted as potential anatomical and behavioral evidence of human-like dexterity (de la Torre, 2011; Leakey, Tobias, & Napier, 1964; Napier, 1962). Archaeological evidence of hominin tool behaviors has since been found in earlier contexts, dating back to at least 3.3 Ma (Harmand et al., 2015), and is likely a preserved facet of a larger hominin manipulative repertoire that may have older origins (Alba, Moyà-Solà, & Köhler, 2003; Haslam et al., 2009; Kivell, 2015; Panger, Brooks, Richmond, & Wood, 2002). When and how the manipulative capability required for stone tool behaviors evolved, however, is still a key question in human evolution (Panger et al., 2002; Richmond et al., 2016).
As our closest living relatives, the manipulative abilities of hominid hands have been used to functionally interpret fossil hand bones (e.g., Susman, 1994, 1998; Marzke, 1997). Napier's (1956, 1993) broad schema of power grips, usually practiced by apes in locomotion, and precision grips, generally practiced by humans during manipulation, provides an intuitive dichotomy of phylogenetic polarity that has been the basis for this functional inference. Ape-like aspects of fossil hominin hand morphology are often interpreted as useful for arboreal locomotion whereas human-like morphological features are interpreted as advantageous for manipulation (e.g., Kivell et al., 2015; Kivell, Kibii, Churchill, Schmid, & Berger, 2011; Marzke, 2013; Susman, 1994; Tocheri, Orr, Jacofsky, & Marzke, 2008). In particular, compared to other apes, humans possess a relatively long thumb with a robust first metacarpal (Mc1) and broad phalanges that have been interpreted as key to enabling forceful “pad-to-pad” precision grips. Forceful precision grips have been traditionally considered unique to humans (Marzke & Wullstein, 1996), and facilitate stone tool production (Key & Dunmore, 2015; Marzke, 1986; Marzke et al., 1998) as well as use (Key, Merritt, & Kivell, 2018; Williams-Hatala et al., 2018). Here, rather than external shape or size, we analyze another aspect of the Mc1 morphology across great apes, the internal trabecular architecture.
Internal trabecular structure can provide additional evidence of how a bone was loaded during life, rather than the limits of joint movement its external shape permits (Currey, 2002; Ruff & Runestad, 1992), and thus potentially provide novel insight into fossil hominin hand use. Biomechanical loading causes trabeculae to remodel, a process known as bone functional adaptation (Cowin, 1986; Frost, 1987; Ruff, Holt, & Trinkaus, 2006). Though trabecular bone, like all bone, is to some extent heritable (Havill et al., 2010) and is perhaps most responsive early in ontogeny (Lovejoy, McCollum, Reno, & Rosenman, 2003; Wallace, Demes, & Judex, 2017), a variety of nonprimate taxa have demonstrated trabecular bone functional adaptation in controlled experimental conditions (Barak, Lieberman, & Hublin, 2011; Biewener, Fazzalari, Konieczynski, & Baudinette, 1996; Christen & Müller, 2017; Pontzer et al., 2006). This trabecular functional adaptation is most often experimentally demonstrated by increased trabecular bone volume (BV/TV) and increased alignment of trabeculae (degree of anisotropy [DA]) in the direction of a novel load (Barak et al., 2011; Pontzer et al., 2006). The combination of a higher BV/TV and orientation of trabeculae to the principle axis of load, can explain up to 92% of variation in the biomechanical properties of bone (Lambers et al., 2013; Ulrich, Van Rietbergen, Laib, & Ruegsegger, 1999).
Trabecular studies of primate hands have also found that the trabecular architecture is consistent with hand positions thought to be used by different species during locomotion (Barak, Sherratt, & Lieberman, 2017; Chirchir, Zeininger, Nakatsukasa, Ketcham, & Richmond, 2017; Dunmore, Bardo, Skinner, & Kivell, 2019; Tsegai et al., 2013; Zeininger, Richmond, & Hartman, 2011). Many of these studies have focused on the metacarpal heads, likely due to the biaxial movements afforded at the metacarpophalangeal (McP) joints as well as their proximity to prehensile and locomotor substrates, which together allow for relatively straightforward functional interpretation of trabecular morphology. Further trabecular bone architecture has been shown to correlate with grip strength in some human hand bones, including the trapezium (Reina, Cavaignac, Trousdale, Laffosse, & Braga, 2017). Indeed the region of the trapeziometacarpal (TMc) joint in which osteoarthritis first develops, thought to therefore endure the most substantial habitual load (Koff et al., 2003), is also the area in which trabecular bone is densest on the Mc1 (Stephens, Kivell, Pahr, Hublin, & Skinner, 2018). Preserved trabeculae in fossil hominins have been used to infer habitual loading and reconstruct locomotor (e.g., Barak, Lieberman, Raichlen, et al., 2013; DeSilva & Devlin, 2012; Ryan et al., 2018; Su, Wallace, & Nakatsukasa, 2013; Zeininger, Patel, Zipfel, & Carlson, 2016) and manipulative (e.g., Skinner et al., 2015a, 2015b; Stephens et al., 2018) behaviors during human evolution. These functional inferences are based on the comparative context of extant great apes and an association between variation in their trabecular architecture and assumptions about the joint postures they most commonly use (Orr, 2016).
Studies of trabeculae in the thumb have mainly focused on humans. Right human Mc1s have a significantly greater BV/TV than those from left hands (Stephens et al., 2016), consistent with cross-cultural right-hand bias in our species (Faurie, Schiefenhvel, leBomin, Billiard, & Raymond, 2005; Reina et al., 2017) though the trabecular difference was small in absolute terms (Reina et al., 2017; Skinner et al., 2015a, 2015b; Stephens et al., 2016). While BV/TV is significantly greater in the Mc1 head of both humans and chimpanzees relative to the base (Lazenby, Skinner, Hublin, & Boesch, 2011; Stephens et al., 2016), the species differ in the Mc1 base. Specifically, the human Mc1 base has a greater concentration of trabecular bone in its palmar aspect relative to human nonpollical metacarpals (Wong, Meals, & Ruff, 2018) and the Mc1 of Pan (Skinner et al., 2015a, 2015b). Where Skinner et al. (2015a, 2015b) inferred function by qualitatively analyzing three-dimensional (3D) trabecular models, Stephens et al. (2018) quantitatively analyzed an expanded sample of foragers and post-Neolithic humans and found that BV/TV was greatest in the radiopalmar segments of the Mc1 head and base, consistent with a flexed, abducted thumb in precision grips. This study also found that DA was lower in the Mc1 base of foragers relative to a sample of post-Neolithic humans, and related this to more varied loading at this, and other manual, joints in the forager population (Stephens et al., 2018).
We build on this work by analyzing Mc1 trabeculae across extant hominids including modern humans (Homo sapiens), bonobos (Pan paniscus), chimpanzees (Pan troglodytes), as well as for the first time, gorillas (Gorilla gorilla gorilla) and orangutans (Pongo abelii and Pongo pygmaeus). A geometric morphometric (GM), statistical mapping method (Dunmore et al., 2019) is applied to subarticular regions of trabecular models produced by the whole-epiphysis approach. We measure and statistically analyze variation in relative trabecular bone volume (RBV/TV; see below) and DA in the proximal and distal Mc1. The distribution of trabecular volume and level of alignment represented by these variables reflect the ability of the whole bone to resist load in different directions and so should be consistent with habitual thumb loading postures in these species.
2 LOCOMOTION, MANIPULATION, AND THUMB MORPHOLOGY
TMc and McP joint movement and loading is a complex product of both bony and soft tissue morphology (van Leeuwen, Vanhoof, Kerkhof, Stevens, & Vereecke, 2018) and compared to humans little is known of actual loads experienced by the nonhuman great ape thumb, during locomotion or manipulation (Samuel, Nauwelaerts, Stevens, & Kivell, 2018). However, qualitative observations of force, which was judged by how apparently resistant objects were to the grip applied, during food processing do exist for some species (Marzke, Marchant, McGrew, & Reece, 2015; Neufuss, Robbins, Baeumer, Hulme, & Kivell, 2018). Further, combining what is known of this morphology with observed habitual thumb use allows for the broad characterization of habitual thumb postures that are loaded in the species studied.
2.1 H. sapiens
Humans are obligate bipeds and so rarely employ grips in locomotion but power grips are used to habitually climb in some populations (Kraft, Venkataraman, & Dominy, 2014). The uniquely human power-squeeze grip is also used in manipulation (Key et al., 2018; Marzke, Wullstein, & Viegas, 1992). This grip flexes fingers around a cylindrical object, which diagonally lies across the palm, while the thumb is adducted with considerable force and controls the direction in which force is applied to the object (Cooney & Chao, 1977; Marzke et al., 1992).
Human precision grips can also be uniquely forceful as demonstrated by the relatively high levels of pressure on the distal thumb of both hands during stone tool production and use (Key & Dunmore, 2015; Williams-Hatala et al., 2018). During stone tool production, a “three-jaw-chuck” grip is commonly used to wield hammerstones, in which the thumb is abducted and rotated to oppose the second and third digits (Marzke, 1997). When using small flake stone tools, humans tend to use “pad-to-side” grips whereas for larger flakes or handaxes they often employ a “cradle” or “five-jaw buttressed pad-to-pad power grip,” which both oppose the thumb to the other fingers with support from the palm (Key et al., 2018; Rolian, Lieberman, & Zermeno, 2011). Biomechanical analysis has also shown large pollical flexion forces are required to stabilize a simulated tool during use (Rolian et al., 2011). While the role of the flexor pollicis longus muscle is debated (Hamrick, Churchill, Schmitt, & Hylander, 1998; Marzke et al., 1998), electromyography (EMG) data has highlighted that flexor pollicis brevis and opponens pollicis are strongly recruited to oppose the thumb to the rest of the fingers in these strong precision grips (Marzke et al., 1998). Clinical EMG studies have also demonstrated that the human opponens pollicis and abductor pollicis brevis muscles are highly recruited in opposition of the thumb during a pad-to-pad grip where they were not as highly recruited in a “pad to side-grip” (Johanson, Valero-Cuevas, & Hentz, 2001).
Humans possess the longest thumb relative to the fingers among hominids (Almécija, Smaers, & Jungers, 2015), which facilitates opposition of thumb to the fingers (Napier, 1956; Marzke, 1997; Feix, Kivell, Pouydebat, & Dollar, 2015; Bardo, Vigouroux, Kivell, & Pouydebat, 2018). Human distal phalanges are capable of passive hyperextension as our deep flexor tendons are long compared to those of other great apes (Preuschoft, 1965; Tuttle, 1967). This movement permits full pad-to-pad precision grips (Napier, 1960) frequently used by humans to forcefully manipulate small objects, especially within the hand (Bardo, Cornette, Borel, & Pouydebat, 2017; Christel, 1993; Key et al., 2018; Marzke & Wullstein, 1996). Humans are unique among hominids in possessing an extensor pollicis brevis muscle, which is well developed and stabilizes the extended McP joint while the first interphalangeal joint is forcefully flexed, as well as abducting the thumb (Diogo, Richmond, & Wood, 2012; Marzke et al., 1999). Indeed, while flexion at the McP joint in great apes appears to be limited to 90° in nonhuman great apes due to their thenar eminence (Tuttle, 1969), clinical data suggests human McP joint flexion is limited to just 70° in humans (Barakat, Field, & Taylor, 2013), possibly due to their larger thenar musculature. Whether in extension or flexion forceful precision grips are achieved through high potential torques of human musculature compared to great apes (Marzke et al., 1999). During finger opposition, large human thenar muscles allow a forceful compound movement of axial rotation, flexion, and abduction of the human thumb (D'Agostino, Dourthe, Kerkhof, Stockmans, & Vereecke, 2017; Feix, Romero, Schmiedmayer, Dollar, & Kragic, 2016; Halilaj, Rainbow, et al., 2014; Napier, 1961). The larger and flatter sellar–facet (Marzke et al., 2010; Tocheri, Razdan, Williams, & Marzke, 2005), as well as a less curved proximal Mc1 and a shorter palmar beak (Marchi, Proctor, Huston, Nicholas, & Fischer, 2017; Niewoehner, 2005), are associated with greater TMc joint mobility in humans compared to other great apes (Cooney, Lucca, Chao, & Linscheid, 1981; Rose, 1992). Although the high radioulnar congruence at the TMc joint may limit abduction, it facilitates resistance to large axial forces generated in human manipulation (Marzke, 2013; Marzke et al., 2010).
2.2 Pongo
Orangutans are primarily arboreal and engage in quadrumanous torso-orthograde locomotion (Manduell, Morrogh-Bernard, & Thorpe, 2011; Thorpe & Crompton, 2006). Hand use during arboreal locomotion is not well studied (Thorpe & Crompton, 2005), but orangutans are thought to habitually use hook grips or power grips that only recruit their fingers (Rose, 1988; Sarmiento, 1988). However, orangutans may oppose the thumb to the fingers when climbing small-diameter substrates (Sarmiento, 1988), and preliminary behavioral evidence shows more frequent recruitment of the thumb than traditionally thought (Mcclure, Phillips, Vogel, & Tocheri, 2012).
In captivity, orangutans do recruit the thumb in pad-to-side precision grips during manipulative tasks (Bardo et al., 2017; Christel, 1993). However, they far more frequently use a power-grip, especially for larger objects (Pouydebat, Gorce, Coppens, & Bels, 2009), or a “V-pocket” grip (Marzke et al., 2015), in which the object is held in the webbing between the full thumb and index finger (Bardo et al., 2017). In both grips, the orangutan thumb may provide support but is unlikely to be strongly recruited, as its relative length would make articulation of the first distal phalanx with all but the largest objects in a power hook grip challenging. Further, this distal phalanx is difficult to articulate with an object already held by the V-pocket at the base of this digit. Orangutans frequently reposition tools with their mouths rather than with their hand (Bardo et al., 2017; Christel, 1993). In the wild, orangutans have not yet been observed using precision grips, even during tool production and use of tools (e.g., Fox, Sitompul, & Van Schaik, 1999; van Schaik, Fox, & Sitompul, 1996).
The lack of thumb recruitment in orangutan grips is likely because the orangutan thumb is the shortest, relative to the fingers, of any great ape (Almécija, Smaers, & Jungers, 2015; Bardo et al., 2018; Tuttle, 1969). As such, the theoretical “work space” for manipulating small objects between the tip of the thumb and the tip of the index finger, a “tip-to-tip” grip, has been shown to be the smallest of all great apes (Feix et al., 2015). The manipulative capability of orangutans is also constrained by a lack of a distinct flexor pollicis longus that inserts on the distal phalanx, as well as the well-developed thenar musculature, found in humans (Strauss, 1942; Tuttle, 1969; Zihlman, Mcfarland, & Underwood, 2011). Orangutans, however, have the largest range of hyperextension (25°) and radioulnar movement at the first McP joint (36°) of all nonhuman great apes, especially ulnarly (Tuttle, 1969). This range of movement may relate to the fact that unlike other nonhuman great apes, the palmar aspect of the orangutan Mc1 head is rotated ulnarly relative to its base, which is argued to be a consequence of the short thumb opposing the rigid palm rather than mobile fingers in this species (Drapeau, 2015). This McP joint mobility may partially offset a TMc joint that has been described as generally more congruent in orangutans than in other great apes, including humans, which presumably limits its range of motion somewhat (Rafferty, 1990). However, the range of movement at this joint has also been described as highly variable (Rafferty, 1990) and a quantitative study found few significant differences in surface congruity at this joint between orangutans and other great apes (Marzke et al., 2010).
2.3 P. troglodytes
Chimpanzees predominantly knuckle-walk, a mode of locomotion that does not recruit the thumb (Doran, 1996; Wunderlich & Jungers, 2009). However, chimpanzees are also arboreal and those of the Taï forest frequently vertically climb or scramble in trees (Doran, 1993), and this species has been described as more arboreal than gorillas (Doran, 1996; Remis, 1995; Thorpe & Crompton, 2006). Depending on branch diameter, chimpanzees use their thumbs in adducted, abducted, and opposed positions during power or hook grips (Hunt, 1991; Marzke & Wullstein, 1996; Neufuss, Robbins, Baeumer, Humle, & Kivell, 2017). Unlike gorillas, chimpanzees only oppose the thumb in-line with, rather than wrapping it around, arboreal substrates during diagonal power grasping (Alexander, 1994; Marzke et al., 1992; Neufuss et al., 2017).
The chimpanzee thumb is frequently involved in tip-to-tip and pad-to-side precision grips during manipulative activities in captivity (Christel, 1993; Jones-Engels & Bard, 1996; Marzke & Wullstein, 1996; Pouydebat, Reghem, Borel, & Gorce, 2011). In the wild, rare pad-to-pad precision grips have been observed in Mahale chimpanzees during feeding but pad-to-side grips are the most frequent, employing an adducted thumb (Marzke et al., 2015). This study also highlighted that the pad-to-side grip was used with considerable force when pulling against slender resistant materials such as small diameter branches, vines, grasses, and meat fibers (Marzke et al., 2015).
These observed grips may be a result of the chimpanzee thumb to finger ratio that is intermediate between gorillas and orangutans (Almécija, Smaers, & Jungers, 2015; Drapeau & Ward, 2007). Chimpanzees generally have smaller thenar muscles than those of humans (Ogihara, Kunai, & Nakatsukasa, 2005) that can generate lower potential torques, due to shorter moment arms (Marzke et al., 1999). Conversely, the transverse head of the adductor pollicis muscle is equivalent to or larger in chimpanzees than in humans and can create larger potential torques (Jacofsky, 2009; Marzke et al., 1999; Tuttle, 1969). As flexor pollicis brevis and opponens pollicis muscles tend to secondarily adduct the TMc joint in chimpanzees, while they abduct the joint in humans, Marzke et al. (1999) have linked this myological morphology to adduction of the thumb in pad-to-side grips in this species. The chimpanzee TMc joint itself is relatively incongruent, especially dorsopalmarly, which may allow for mobility at this joint at the cost of stability (Marzke et al., 2010; Rafferty, 1990).
2.4 P. paniscus
Like chimpanzees, bonobos also primarily knuckle-walk, both arboreally and terrestrially, which does not recruit the thumb. However, they are argued to be more arboreal than chimpanzees (Alison & Badrian, 1977; Crompton, Sellers, & Thorpe, 2010; Susman & Badrian, 1980) and engage in arboreal palmigrady more frequently (Doran, 1993). While the thumb is frequently observed in use during vertical climbing and suspension, it may not be meaningfully loaded (Samuel et al., 2018). While the thumb may be recruited in palmigrady, data on this are lacking and so it is possible the thumb is most frequently loaded during manipulative behavior in this species.
Captive bonobos use precision grips and, uniquely among nonhuman great apes, they can independently flex their first distal phalanx while performing them (Christel, 1993; Christel, Kitzel, & Niemitz, 1998). The most frequent grips used by bonobos during manipulative tasks, that employ the thumb, are the V-pocket and pad-to-side grips, in which the thumb is adducted (Bardo, Borel, Meunier, Guéry, & Pouydebat, 2016). In naturalistic environments bonobos use, albeit rarely, tools in social behaviors and to shelter from rain (Furuichia et al., 2015; Hohmann & Fruth, 2003; Ingmanson, 1996). Sanctuary-living bonobos have also been reported to employ a variety of different grips during nut-cracking, including many that involve an adducted thumb that may be flexed or extended (Neufuss, Humle, Cremaschi, & Kivell, 2016).
Bonobos have a similar relative thumb length (Almécija, Smaers, & Jungers, 2015) and a comparable kinematic workspace (Feix et al., 2015) to chimpanzees. This species has well-developed thenar musculature that can exert high pressures at the TMc joint and includes a tendon of the flexor digitorum profundus that flexes the distal phalanx (van Leeuwen, Vanhoof, et al., 2018), a trait that is weakly expressed or absent in chimpanzees (Susman, 1998; Tuttle, 1969). However, the bonobo thumb is not capable of the same level of force as is the human thumb (van Leeuwen, Vanhoof, et al., 2018). Further, this species demonstrates a fusion of thumb and index finger musculature that may limit complex precision grips that require independent movement of these digits (van Leeuwen, Vanhoof, et al., 2018). The shape of the bonobo TMc joint is similar to that of humans; however, unlike humans, strong volar ligaments at this joint in bonobos restrict the extension at the TMc to just 30° (van Leeuwen et al., 2018). The rounded Mc1 base palmar beak is also thought to limit axial rotation and mediolateral movements of the Mc1 on the trapezium and therefore the compound movement involved in pad-to-pad opposition grips (van Leeuwen, Vanneste, et al., 2018).
2.5 Gorilla
The most frequent locomotor mode in gorillas is terrestrial knuckle-walking, which does not recruit the thumb (Inouye, 1994; Matarazzo, 2013; Remis, 1998). Gorillas are also arboreal and when captive lowland gorillas climb large diameter supports, they recruit, but do not oppose, the thumb keeping it in line with the rest of the digits (Sarmiento, 1994). Unfortunately, relatively little is known about wild western lowland gorilla hand use compared to that of mountain gorillas (Byrne, Corp, & Byrne, 2001; Neufuss et al., 2017). Wild mountain gorillas also adduct their thumbs in grips of >50 cm diameter substrates but they oppose the thumb in-line with, or around, 6–10 cm diameter substrates and the thumb is particularly important in counter-stabilizing descent grips on lianas (Neufuss et al., 2017). While arboreal behaviors may have been traditionally underestimated in gorillas (Crompton et al., 2010; Neufuss et al., 2017), this genus most frequently terrestrially knuckle-walks, which does not recruit the thumb, and therefore the thumb may be most often used during manipulation.
Captive gorillas can perform “tip-to-tip” precision grips (Christel, 1993; Pouydebat, Laurin, Gorce, & Bels, 2008), although they also often use power grips and “interdigital brace” grips during manipulative tasks (Bardo et al., 2017). The latter grip threads an object between the adducted thumb and index finger as well as the palmar or dorsal aspects of the ulnar digits (Bardo et al., 2017; Lesnik, Sanz, & Morgan, 2015). Wild mountain gorillas most frequently employ precision grips that adduct the thumb during food processing, including interdigital brace, thumb wrap, and pad-to-side grips (Neufuss et al. (2018)). The latter grip is reported as the most frequent and the authors suggest this may due to the fact that gorilla thumb may not be able to resist the seemingly forceful grips observed, in tip-to-tip position (Neufuss et al., 2018). However, Neufuss et al. (2018) have emphasized the great variety of grips and thumb positions used by mountain gorillas in food processing and while they did not observe precise in-hand manipulation (sensu Landsmeer, 1962) in this community, it has been reported in others (Bardo et al., 2017; Byrne et al., 2001). Gorillas have also been observed engaging in tool-use behaviors (Breuer, Ndoundou-Hockemba, & Fishlock, 2005; Kinani & Zimmerman, 2015).
The use of the gorilla thumb in a variety of grips may be linked to its long thumb, relative to the fingers, which is relatively longer than that of all nonhuman great apes (Almécija, Smaers, & Jungers, 2015; Susman, 1979). These hand proportions provide for the largest theoretical kinematic workspace, between the thumb and index finger, compared with all other nonhuman great apes (Feix et al., 2015). The Mc1 head is of comparable breadth to that of humans, which may facilitate a similar degree of movement at the McP joint in gorillas (Hamrick & Inouye, 1995; Susman, 1998). The distal fibers of abductor pollicis longus muscle do not separate into a distinct muscle belly in gorillas (contra Sarmiento, 1994), which is the extensor pollicis brevis muscle in humans. However, the abductor pollicis longus muscle does insert on the proximal phalanx more frequently than in other great apes (Diogo et al., 2012), which may facilitate increased thumb dexterity. Similarly, gorillas have a less congruent TMc joint than Pongo (Rafferty, 1990) allowing for a greater range of motion at this joint, although this difference is small quantitatively (Marzke et al., 2010) and different approaches used to quantify TMc joint surface congruence are often difficult to compare (Halilaj, Moore, et al., 2014).
3 PREDICTIONS
All nonhuman great apes appear to habitually use pad-to-side or V-pocket grips in which the thumb is adducted, and variably flexed (Bardo et al., 2016, 2017; Jacofsky, 2009; Marzke et al., 2015; Neufuss et al., 2018). While detailed quantitative data on the level of in vivo flexion during these grips in the species is sparse, the pad-to-side grip typically involves contacting the side of the index finger with the distal thumb pad in nonhuman great apes, a movement that intuitively requires little flexion of the first McP (Figure 1b, top), relative to pad-to-pad opposition with the fifth distal phalanx, for example. Conversely, the V-pocket grip requires even less flexion of the McP to maintain a grip on an object between the ulnar aspect of the first proximal phalanx and the radial side of the second metacarpal. Thus while the first McP can flex further than it can adduct in nonhuman great apes, the full range of flexion does not appear to be used in these habitual grips. Therefore, we predict that both subarticular relative trabecular volume (RBV/TV, see Section 5) and DA will be greatest disto-palmarly in the ulnar aspect of Mc1 head of these species, reflecting moderate flexion and adduction of the first McP (H1a; Figure 1b). Gorillas may be an exception to this pattern because while they frequently adduct the thumb in interdigital brace grips (Bardo et al., 2017), they also have a wide Mc1 head (Hamrick & Inouye, 1995; Susman, 1998) and frequently recruit the thumb in abducted positions (Neufuss et al., 2018). As a result, we expect that gorillas may have lower DA across the Mc1 head than other nonhuman great apes (H1b). Conversely, even though, the human thumb is also recruited in many different postures, due to frequent use of forceful precision grips in which the thumb is flexed and abducted (Feix et al., 2016; Marzke, 2013; Napier, 1956), we predict that humans will have greater subarticular DA and RBV/TV in the radiopalmar aspect of the Mc1 head relative to all other great apes studied (H1c; Figure 1a).
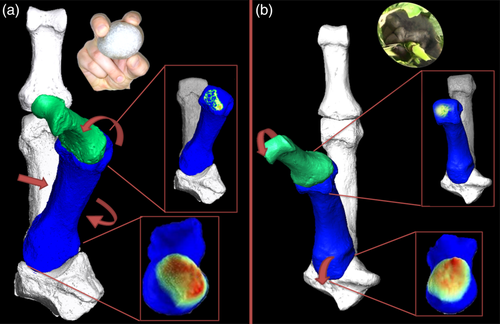
Given gorillas have been shown to use a variety of thumb positions during manipulation we predict they will demonstrate lower DA throughout the Mc1 base than other nonhuman great apes, which themselves, will have a relatively uniform distribution of DA values across the TMc joint due to less frequent thumb use than in humans (H2a). We also predict RBV/TV values will be greatest in the palmar aspect of the Mc1 base in great apes (H2b; Figure 1b) as the TMc joint is primarily flexed in these species, during both arboreal power grasping (Neufuss et al., 2017) and precision grasping (Marzke, 1997; Marzke et al., 2015; Bardo et al., 2016, 2017; Neufuss et al., 2018). While manipulative grips habitually practiced by nonhuman great apes involve adduction of the thumb, this movement may be restricted at the TMc joint by a thicker anterior oblique ligament in nonhuman great apes compared with that of humans (van Leeuwen, Vanneste, et al., 2018). The rounded palmar beak at the Mc1 base in nonhuman great apes, relative to humans, is also thought to restrict axial rotation of the Mc1, and thus adduction (Marzke et al., 1992; van Leeuwen, Vanneste, et al., 2018). When similar pad-to-side grips are experimentally performed in humans, the contact area of the TMc surfaces is larger ulnarly but the closest point between them is at the palmar beak of the Mc1 (D'Agostino, Dourthe, Kerkhof, Stockmans, et al., 2017; Figure 1b). Therefore, we predict flexion will be the dominant habitual movement in these grips, rather than adduction, reflected by the trabecular distribution in the nonhuman primate TMc joint (Figure 1b, bottom). In humans we predict RBV/TV and DA will be greater in the radiopalmar aspect of the Mc1 base (H2c; Figure 1a), due to habitual abduction and flexion during precision grasping (D'Agostino, Dourthe, Kerkhof, Stockmans, et al., 2017; Feix et al., 2016; Napier, 1956) as has been demonstrated before for RBV/TV (Stephens et al., 2018).
4 MATERIALS
Subarticular trabecular bone was analyzed in the Mc1 of H. sapiens (n = 10), P. paniscus (n = 10), P. troglodytes (n = 11), G. gorilla gorilla (n = 10), Pongo sp. (n = 1), P. pygmaeus (n = 5), and P. abelii (n = 3, Table 1). All specimens were considered adult based on complete epiphyseal fusion of the Mc1 and other postcranial elements, free from external signs of pathology and all nonhuman specimens were wild-shot. Human specimens were drawn from four populations: Nubians of ~5th century AD Sayala, Egypt (Paoli et al., 1993; Strouhal & Jungwirth, 1979), Yámanas individuals from 19th century Tierra del Fuego (Marangoni et al., 2011), 20th century individuals from Syracuse, Italy and 20th century individuals from a cemetery in Inden, Germany (Großkopf, 2015). The samples were sex balanced for each species, although one P. pygmaeus and two human specimens were of unknown sex. For the nonhuman apes, an effort was made to analyze even numbers of left and right Mc1s, as there are some signs of lateral asymmetry in metacarpal trabecular (Stephens et al., 2016) and cortical bone (Sarringhaus, Stock, Marchant, & McGrew, 2005). However, these differences are slight in absolute terms (Sarringhaus et al., 2005; Skinner et al., 2015a, 2015b) and so Mc1 side unlikely to meaningfully affect the current analysis. Conversely, humans are cross-culturally right-handed (Faurie et al., 2005) and this is reflected in Mc1 trabecular bone (Reina et al., 2017; Stephens et al., 2016). Therefore, the human sample was drawn from right hands to avoid potential bias related to handedness.
| Side | |||
|---|---|---|---|
| Species | Sex | Left | Right |
| Gorilla gorilla gorilla | Female | 3 | 2 |
| Male | 2 | 3 | |
| Homo sapiens | Female | — | 4 |
| Male | — | 5 | |
| Unknown | — | 1 | |
| Pan paniscus | Female | 3 | 2 |
| Male | 2 | 3 | |
| Pan troglodytes | Female | 2 | 3 |
| Male | 3 | 3 | |
| Pongo abelii | Female | 1 | 1 |
| Male | — | 1 | |
| Pongo pygmaeus | Female | 1 | 2 |
| Male | — | 1 | |
| Unknown | — | 1 | |
| Pongo sp. | Male | 1 | — |
5 METHODS
5.1 Micro-computed tomography scanning
Specimens were scanned with a BIR ACTIS 225/300, Diondo D3, or a Skyscan 1172 high-resolution micro-computed tomography (CT) scanner at the Department of Human Evolution, Max Planck Institute for Evolutionary Anthropology, Germany, or with the Nikon 225/XTH scanner at the Cambridge Biotomography Centre, University of Cambridge, UK. Scans were performed at 100–160 kV and 100–140 μA, using a brass or copper filter of 0.25–0.5 mm. The scans were reconstructed to create images with an isometric voxel size of 28–41 μm depending on the size of the specimen.
5.2 Image processing
Avizo 6.3 (Visualization Sciences Group, Germany) was used to isolate and rotate micro-CT scans of each Mc1 into a standardized anatomical position (Figure 2a) and the ray-casting algorithm (Scherf, 2009) was used to segment bone tissue. Trabecular structure was analyzed with the whole-epiphysis method, which has been described and tested in detail (Gross, Kivell, Skinner, Nguyen, & Pahr, 2014). Briefly, medtool 4.2 (Dr Pahr Ingenieurs e.U.) was used to run the image through a series of image filters that separated the inner trabecular structure from the cortical shell (Figure 2b). Specifically, an algorithm casts mathematical rays from the edge of the cortical bone inward in seven directions, the three orthogonal axes as well at the four diagonals of the unit cube. Where at least five of these seven rays met the first “inner-air” voxel, that is part of the image that is not bone and inside the cortical shell, they were marked as part of the inner structure. A smooth kernel, with a diameter equal to the measured average trabecular thickness in that bone, was then used to close the gaps in this inner structure, the trabeculae, to delimit the volume of the inner trabecular structure (Pahr & Zysset, 2009). A 3D grid was then superimposed on the inner structure and overlapping spherical volumes of interest (VOIs) with a 5 mm diameter were positioned at each vertex within the 2.5-mm-spaced grid. BV/TV and DA were then measured for each VOI (Figure 2c) as several studies have demonstrated these properties correlate with bone biomechanics (Barak et al., 2011; Lambers et al., 2013; Odgaard, Kabel, van Rietbergen, Dalstra, & Huiskes, 1997; Pontzer et al., 2006; Uchiyama et al., 1999), and are not strongly affected by allometry (Barak, Lieberman, & Hublin, 2013; Doube, Kłosowski, Wiktorowicz-Conroy, Hutchinson, & Shefelbine, 2011; Ryan & Shaw, 2013). The mean intercept length method was used to calculate the second order fabric tensor and DA as 1—(lowest eigenvalue/greatest eigenvalue). Thus, DA values of 0 represent total isotropy and values of 1 represent total anisotropy. Each trabecular variable was then separately interpolated on 3D tetrahedral mesh created using CGAL (www.cgal.org; Figure 2d). The outer surface of this trabecular mesh was then isolated using Paraview (www.paraview.org), and smoothed to permit landmark sliding (see below) in MeshLab (Cignoni et al., 2008) via a screened Poisson surface reconstruction filter (Kazhdan & Hoppe, 2013; Figure 2e). For left Mc1s, this smoothed mesh was oriented in the same way as right Mc1s by a reflection filter in MeshLab to allow for homologous comparisons.
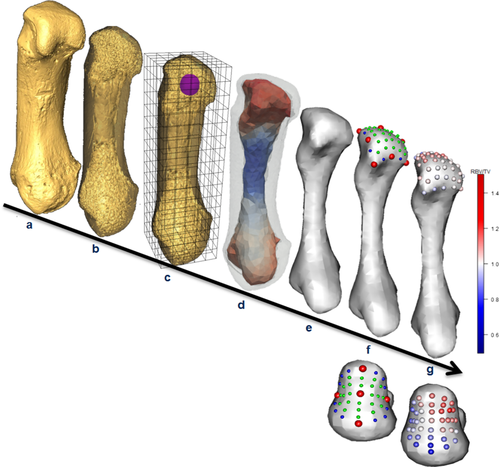
5.3 Geometric morphometric mapping
Only the subarticular trabecular bone of the Mc1 head and base was analyzed rather than the entire volumetric trabecular model created by the whole-epiphysis approach. This subarticular trabecular bone is the first point of transmission for external loads from the cortical shell to the deeper trabecular structure and should contain a functional adaptation signal (Marzke et al., 2010; Sylvester & Terhune, 2017; Zhou et al., 2014). We apply 3D GM techniques (Gunz & Mitteroecker, 2013) to the analysis of trabecular bone (Dunmore et al., 2019) in a similar manner to the method described by Sylvester and Terhune (2017).
5.4 Anatomical landmark definitions
Many landmark sets have been used to analyze the primate Mc1 proximal base (Marchi et al., 2017; Niewoehner, 2005), and recently a set has been used to analyze the shape of the primate Mc1 head (Galletta, Stephens, Bardo, Kivell, & Marchi, 2019). The location and type (Bookstein, 1991) of anatomical landmarks used here for the head and base of Mc1 are given in Tables 2 and 3, respectively. Previously identified cortical landmarks employed in these studies were accurately transposed to the inner trabecular surface as thin cortical bone at the metacarpal head and base in hominids (Tsegai et al., 2017) allows for high correspondence between these surfaces.
| Number | Type | Description | Reference |
|---|---|---|---|
| 1 | Type II | Most proximal point under the ulnar palmar epicondyle (anterior eminence) | Galletta et al. (2019), Rein (2018), and Yeh and Wolf (1977)) |
| 2 | Type III | The point of maximum curvature on the interepicondylar ridge between Points 1 and 3 | Drapeau (2015), Galletta et al. (2019), and Rein (2018) |
| 3 | Type II | Most proximal point under the radial palmar epicondyle (anterior eminence) | Galletta et al. (2019), Yeh and Wolf (1977) |
| 4 | Type III | Point of maximum curvature on the radial ridge separating the articular surface from the radial lateral sulcus | Galletta et al. (2019), Rein (2018), Yeh and Wolf (1977) |
| 5 | Type II | Most radially projecting point on the dorsal aspect of the distal articular surface | Galletta et al. (2019) and Rein (2018) |
| 6 | Type III | The midpoint point on the dorsal limit of the distal articular surface, between Points 5 and 7 | Galletta et al. (2019) and Rein (2018) |
| 7 | Type II | Most ulnarly projecting point on the dorsal aspect of the distal articular surface | Galletta et al. (2019) and Rein (2018) |
| 8 | Type III | Point of maximum curvature on the ulnar ridge separating the articular surface from the ulnar lateral sulcus | Galletta et al. (2019), Rein (2018), and Yeh and Wolf (1977) |
| 9 | Type II | Most distally projecting point on the subarticular surface | Galletta et al. (2019) and Rein (2018) |
| Number | Type | Description | Reference |
|---|---|---|---|
| 1 | Type II | Most palmar aspect of the proximal articular surface, the “tip” of the palmar beak | Marchi et al. (2017) |
| 2 | Type II | The most dorsal aspect of the articular surface on the metacarpal base | Marchi et al. (2017) |
| 3 | Type II | The most ulnar aspect of the articular surface on the metacarpal base | Marchi et al. (2017) |
| 4 | Type II | The most radial aspect of the articular surface on the metacarpal base | Marchi et al. (2017) |
| 5 | Type III | The deepest point of the articular surface, that lies on the intersection of the orthogonal chords formed between Points 1 and 2 and 3 and 4, respectively | — |
5.5 Repeatability
Three random Mc1 specimens from each species were landmarked on their head and base, five times respectively, over several days with Checkpoint (Stratovan Corporation, Davis, CA), following Fernández et al. (2015). The Morpho package in Rv3.3.0 (R Development Core Team, 2016; Schlager, 2017) was then used to generate Procrustes coordinates for the five repeats of three individuals per species and articular surface. These coordinates were then plotted on the first two principal components (PCs) of each of the 10 repeatability comparisons (Figure S1, Supporting Information). Pairwise permutational multivariate analysis of variances (MANOVAs), with Bonferroni correction, conducted on PC1 and PC2 scores demonstrated that repeats of individual configurations were significantly different from the other two specimens in each case, so landmarks were considered repeatable (Figure S1, Supporting Information).
5.6 Geometric morphometric procedure
Both landmark templates (Figure 2f) were created by defining sliding semilandmarks on curves at the subarticular surface margins of a random specimen in Checkpoint. These curves were each bordered by anatomical landmarks following Gunz, Mitteroecker, and Bookstein (2005). For the Mc1 head template, single sliding semilandmarks were defined on each of the eight curves. For the Mc1 base template, three sliding semilandmarks were defined for each of the four curves between anatomical landmarks. Where subarticular margins were smoothed, a translucent model of the cortical surface was overlaid in Paraview to ensure correct placement of the template landmarks. Additional sliding semilandmarks were then distributed over each subarticular surface in Avizo 6.3 (Visualization Sciences Group) to produce a 49 landmark template for the Mc1 head, comprising nine anatomical landmarks, eight sliding semilandmarks on curves and 32 surface sliding semilandmarks. The 40 landmark template for the base contained five anatomical landmarks, 12 sliding semilandmarks on curves, and 23 surface sliding semilandmarks (Figure 2f). Subsequently, anatomical landmarks were placed on every specimen and then each landmark template was projected onto each of the other 49 Mc1 heads and 48 bases, respectively, using the Morpho package in R (Schlager, 2017). A single P. pygmaeus specimen did not have a fully-preserved base and was excluded from the base analyses. Each template was relaxed onto the surface of each Mc1 by minimizing bending energy and then semilandmarks were slid along their respective curves or surfaces by minimizing Procrustes distances, using the Morpho package in R (Schlager, 2017).
5.7 Data mapping
A custom Python script was run using Paraview to allow the nonsmoothed surface mesh triangles to inherit trabecular values (BV/TV and DA) from their originating tetrahedra. The Python module SciPy (Jones, Oliphant, & Peterson, 2001) was then used in medtool 4.2 (Dr Pahr Ingenieurs e.U.) to interpolate the trabecular values to the closest landmark (Figure 1g). This procedure is analogous to measuring trabecular structure with 1 mm diameter spherical VOIs, centered 0.5 mm deep to the normal of the inner trabecular surface, at the location of a landmark. A Procrustes procedure was then performed using the geomorph package (Adams, Collyer, Kaliontzopoulou, & Sherratt, 2017) in R to produce two sets of homologous landmarks each with the trabecular parameters mapped to them (Figure 2g).
5.8 Relative trabecular volume
An RBV/TV measure was calculated for interspecific comparisons, in which the raw BV/TV values were divided by the mean of all landmark BV/TV values on that subarticular surface. If a landmark value is close to the average of that Mc1 surface it will have a value of ~1, whereas a landmark with a lower than the average BV/TV will have an RBV/TV <1 and with a higher value will have RBV/TV >1. This standardization of BV/TV values was performed for several reasons. BV/TV can vary systemically across species (Tsegai, Skinner, Pahr, Hublin, & Kivell, 2018) and thus may diminish the potential functional signal under investigation here. Further, while BV/TV yields functional information relating to the response of trabecular bone to both magnitude and direction of load, it conflates these signals. The present study is focused on the latter response, as it is more directly related to thumb joint postures in extant hominids. RBV/TV landmark values reflect the distribution of trabecular bone beneath an articular surface, in any given bone, irrespective of the average, or global, subarticular BV/TV present. Where this RBV/TV distribution is uneven, higher landmark values are consistent with habitual loading of articular surface in that region and, in turn, consistent with joint postures that load the surface in this manner. Comparisons of these values between groups therefore test for differences in the distribution of bone volume and thus for differences in habitual loading postures at a joint. If these values were not standardized, the region of highest landmark values on a given subarticular surface may not be differentiated from the lowest values on another subarticular surface with higher average, or global, BV/TV. That is, despite different distributions of BV/TV across these two subarticular surfaces, reflecting different habitually loaded joint postures, these values appear the same. Standardizing these BV/TV values is therefore necessary to assess if their subarticular distribution, rather than magnitude, varies between the species studied (Sukhdeo, Parsons, Niu, & Ryan, 2018; Sylvester & Terhune, 2017). Further, intraspecific variation in BV/TV has been shown to be considerable in a large sample of humans, yet the relative differences in BV/TV at several VOIs appear to show a consistent functional signal across populations (Saers, Cazorla-Bak, Shaw, Stock, & Ryan, 2016). Therefore, here, we opt to use a relative measure to somewhat control for nonfunctional trabecular signals and analyze which areas of the subarticular trabecular bone have most adapted to habitual loads.
5.9 Statistical analysis
In order to test for significant differences in the distribution of subarticular RBV/TV and DA values between species a dual statistical approach was employed. In order to investigate where subarticular regions were significantly different between species, trabecular values at each landmark were compared using univariate statistics. These regional comparisons, however, do not assess if the distribution of values over an entire subarticular surface are significantly different between species. Small areas of significantly different trabecular structure, while functionally interesting, may not sum to a significantly different distribution of trabecular values over a subarticular surface and so must be interpreted with caution. Therefore, as a corollary, variation in trabecular variable distribution over the subarticular surface was compared between species with multivariate analysis. Together this dual, multivariate and univariate, approach tests if the distribution of trabecular values differs over the entire subarticular surface and in which regions these differences occur.
5.10 Univariate regional analysis
For the univariate analysis, significant species differences in trabecular parameters were independently tested for at each landmark using “mass-univariate” statistics, following Friston et al. (1995). Shapiro–Wilk tests identified significantly non-normal data (p < .05) for both trabecular parameters at some landmarks. Therefore, nonparametric Kruskal–Wallis tests were run at each homologous landmark for consistency. If these omnibus tests were significant at a given landmark post hoc Dunn's tests (Dunn, 1964) were used to identify significant pairwise species differences at p < .05 after a Bonferroni correction (Dunn, 1961). Trabecular values were compared at homologous landmarks between species rather than with spatially correlated neighboring landmarks. Polarity and the effect size of pairwise comparisons were determined via Z-scores. Significant univariate species differences at each landmark could then be mapped to an average Mc1 model, to show regional differences for functional interpretation. Though Dunn's test is conservative (Dunn, 1964) this univariate approach may still be subject to Type I error. Therefore, significant trabecular value differences were only considered functionally meaningful if they occurred at a minimum of four spatially contiguous landmarks (as this was 8–10% of each template) to further ameliorate Type I error.
5.11 Multivariate whole-surface analysis
To investigate whether distribution of RBV/TV and DA were different between species over the whole subarticular surfaces multivariate analyses were performed. For Mc1 head and base subarticular surfaces, separately, a PC analysis (PCA) was performed using variation in RBV/TV or DA at each landmark as a variable, resulting in four PCAs. In each PCA individual, Mc1s are represented by a number of PC scores. Since three first three PCs cumulatively explain the majority, in this case more than 65%, of the variation, the first three PC scores of each individual were combined into a multivariate response variable. An omnibus one-way permutational MANOVA was then used to test for significant differences in this multivariate response variable across species. Where this omnibus test was significant, subsequent pair-wise MANOVA's indicated that the distribution of either RBV/TV or DA values was significantly different between these species. These tests were run using the Vegan package (Oksanen et al., 2018) in Rv3.3.0 (R Core Development Team, 2016) and the pairwise tests were run with a Bonferroni correction. To visualize interspecific differences, and intraspecific variation, in subarticular value distribution across Mc1 subarticular surfaces each PCA is plotted. The landmarks at which trabecular values provided the largest negative or positive loadings for a particular PC are visualized on the subarticular surface at the negative and positive end of the PC, respectively, similar to the approach of Sylvester and Terhune (2017). This aids in the post hoc qualitative interpretation of exactly where trabecular value distributions are different between species, but does not specifically test for this as the univariate regional analysis does.
6 RESULTS
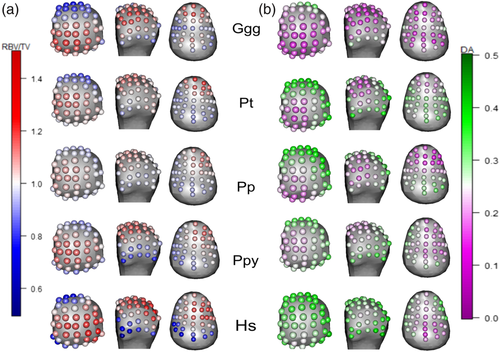
6.1 Average species values and univariate landmark comparisons
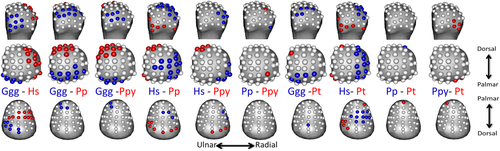
Average values at each landmark, per species are depicted in for RBV/TV (Figure 3a) and DA (Figure 3b), respectively. Significantly different values at each landmark, in each pairwise species comparison are depicted for RBV/TV (Figure 4) and DA (Figure 5) for both subarticular surfaces of the Mc1. As post hoc Dunn's test pairwise comparisons were too numerous to be easily interpreted in table format, the effects size of each test, the Z-test statistics, are summarized in Table 4. These Z-scores were transformed into unsigned, absolute values to demonstrate the size of differences in trabecular values between species at each subarticular surface.
| RBV/TV base | DA base | RBV/TV head | DA head | |
|---|---|---|---|---|
| Min | 2.582 | 2.598 | 2.588 | 2.577 |
| Max | 4.416 | 5.133 | 5.093 | 5.093 |
| SD | 0.557 | 0.551 | 0.625 | 0.549 |
| Average | 3.359 | 3.229 | 3.413 | 3.293 |
- Abbreviations: DA, degree of anisotropy; RBV/TV, relative trabecular bone volume.
6.2 H. sapiens
H. sapiens had the largest range of average RBV/TV values among the sample, with higher values at disto-palmar landmarks on the radial aspect of the Mc1 head (Figure 3a). This region had significantly higher RBV/TV values compared with all other great apes (Figure 4). In the Mc1 base, average RBV/TV values were highest radiopalmarly, although not at the most palmar landmarks (Figure 3a). H. sapiens had significantly higher RBV/TV at landmarks in the central and radial aspects of the Mc1 base compared to with Gorilla and P. troglodytes (Figure 4). H. sapiens displayed the highest average DA values throughout the head compared to all other species (Figure 3b), which resulted in significantly higher values than Gorilla at almost every landmark (Figure 5). Further, H. sapiens had significantly higher DA than P. troglodytes, as well as Pongo, at disto-ulnar landmarks and P. paniscus at palmar landmarks. The average H. sapiens DA values were highest in the radiopalmar and ulnar aspects of the Mc1 base. H. sapiens Mc1 base landmarks had significantly higher DA values than P. paniscus in the radiopalmar region and than Gorilla in the central palmar region.
6.3 Pongo
In the Mc1 head, the average RBV/TV in Pongo was highest in the ulno-distal region (Figure 3a). Pongo had significantly greater RBV/TV at landmarks situated ulno-dorsally than Gorilla and H. sapiens (Figure 4). In the average Mc1 base, Pongo displayed a slightly higher RBV/TV in the central palmar region, though the range of values throughout the base was small (Figure 3a). Pongo did not have a region of significantly different RBV/TV from other species in the Mc1 base. DA in the Pongo average Mc1 head was lowest ulno-distally (Figure 3b) and significantly higher than Gorilla in the palmar region (Figure 5). In the base, Pongo had a slightly higher average DA ulnarly but did not show a contiguous patch of landmarks significantly different from any other species, expect P. troglodytes where Pongo had significantly higher DA at radio-central landmarks.
6.4 P. troglodytes
P. troglodytes had the highest RBV/TV values at ulnar landmarks on the disto-palmar aspect of the Mc1 head (Figure 3a). RBV/TV in this species was significantly higher at radiopalmar landmarks compared to Pongo and at ulno-palmar landmarks relative to H. sapiens (Figure 4). The Mc1 base in P. troglodytes had the highest range of average RBV/TV values among nonhuman great apeMc1 bases (Figure 3a). Average RBV/TV was highest in the central palmar base but values were only significantly greater than H. sapiens, at dorso-ulnar landmarks. The Mc1 head of P. troglodytes had lower DA at disto-palmar landmarks on its ulnar side (Figure 3b). P. troglodytes DA was only significantly greater than Gorilla, across radial and dorsal landmarks (Figure 5). The highest average DA values were in the dorsal Mc1 base of P. troglodytes and these DA values were significantly higher than Gorilla, H. sapiens and, to a lesser extent, Pongo at radio-dorsal landmarks. DA values were also significantly higher than P. paniscus in this species at radiopalmar Mc1 base landmarks (Figure 5).
6.5 P. paniscus
P. paniscus possessed the lowest range of average RBV/TV values in the Mc1 head across the sample. The homogenous distribution of RBV/TV values in this species resulted in significantly higher RBV/TV than in Gorilla at dorso-ulnar landmarks and H. sapiens at both dorso-ulnar as well as palmo-ulnar landmarks (Figure 4). In the Mc1 base, P. paniscus had slightly higher average RBV/TV values in its central palmar landmarks, although like Pongo the range of values was low throughout the base. This species had significantly higher RBV/TV relative to H. sapiens at the most extreme dorsally positioned landmarks in the Mc1 base. For DA, the P. paniscus Mc1 head had a similar average pattern to P. troglodytes, although lower average DA values were found at more palmar landmarks (Figure 3b). DA values of the Mc1 head were significantly higher than in Gorilla at dorsal and radial landmarks (Figure 5). In the average Mc1 base, P. paniscus showed higher DA values at dorsal landmarks (Figure 3b) that were significantly greater, especially radially, than in H. sapiens and Gorilla (Figure 5).
6.6 Gorilla
Gorilla had the highest range of average RBV/TV values across Mc1 head landmarks in nonhuman great apes. The highest RBV/TV values were located ulnarly on the disto-palmar aspect of the Mc1 head (Figure 3a). Gorilla was significantly higher in RBV/TV than H. sapiens ulno-palmarly and significantly higher than all other great apes disto-palmarly (Figure 4). The average Gorilla Mc1 base had higher RBV/TV values centered at its most palmar extent. RBV/TV was only significantly higher than that of H. sapiens, at dorso-ulnar landmarks (Figure 4). Gorilla had the lowest average DA of all species throughout both the Mc1 head and base subarticular surfaces (Figure 3b). This species showed significantly lower DA than H. sapiens at most landmarks on the Mc1 head as well as at radial and dorsal landmarks in comparison with P. troglodytes (Figure 5). Relative to P. paniscus, Gorilla again had significantly lower DA at dorsal and radial landmarks, but these were more distally and centrally located than in the comparison with P. troglodytes. Pongo had significantly higher DA relative to Gorilla at centrally located disto-palmar Mc1 head landmarks (Figure 5). Gorilla had low average DA throughout the Mc1 base and significantly lower DA than both Pan species at central and more dorsally located landmarks (Figure 5).
6.7 Multivariate whole-surface comparisons
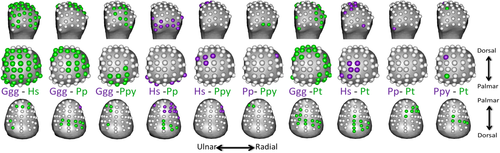
PCA results for RBV/TV and DA value distributions in the Mc1 head and base are depicted in Figures 6 and 7, respectively. Permutational MANOVAs were run using the first three PCs of each subarticular surface, as further PCs each explained less than 10% of the variance in each PCA. These omnibus tests were significant for both RBV/TV and DA for in the head and base (Table 5) indicating there were significant differences between species in overall subarticular trabecular value distribution. The pairwise permutational MANOVA results generally matched those of the univariate comparisons, that is significant subarticular surface value distribution differences were found between species with significantly different trabecular values at numerous subarticular landmarks.
| RBV/TV head | RBV/TV base | DA head | DA base | ||
|---|---|---|---|---|---|
| Omnibus | <.001 | <.001 | Omnibus | <.001 | <.001 |
| Ggg–Hs | 0.003 | 0.002 | Ggg–Hs | 0.002 | 0.068 |
| Ggg–Pp | 0.001 | 1.000 | Ggg–Pp | 0.001 | 0.001 |
| Hs–Pp | 0.001 | 0.002 | Hs–Pp | 0.198 | 0.002 |
| Ggg–Ppy | 0.078 | 1.000 | Ggg–Ppy | 0.017 | 0.459 |
| Hs–Ppy | 0.005 | 0.043 | Hs–Ppy | 0.303 | 0.789 |
| Pp–Ppy | 0.077 | 1.000 | Pp–Ppy | 0.437 | 0.119 |
| Ggg–Pt | 0.038 | 1.000 | Ggg–Pt | 0.001 | 0.003 |
| Hs–Pt | 0.001 | 0.001 | Hs–Pt | 0.241 | 0.007 |
| Pp–Pt | 1.000 | 0.293 | Pp–Pt | 1.000 | 0.039 |
| Ppy–Pt | 0.764 | 1.000 | Ppy–Pt | 0.403 | 0.316 |
- Abbreviations: MANOVA, multivariate analysis of variance; PC, principal component; RBV/TV, relative trabecular bone volume.
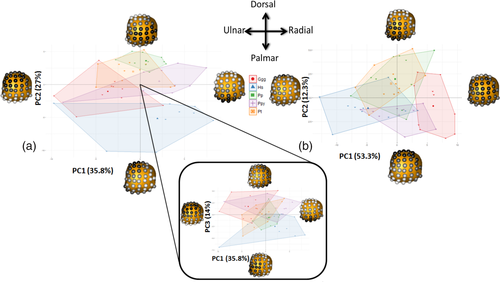
6.8 Mc1 heads
The first PC1 of the RBV/TV data explained 36% of the variation and reflected variation in RBV/TV values at dorsal and palmar Mc1 head landmarks. PC2 represented 27% of the variation in RBV/TV values at radiopalmar and disto-ulnar landmarks, whereas PC3 explained 14% of the variation and reflected radioulnar landmark variation (Figure 6a). Pairwise permutational MANOVAS demonstrated that H. sapiens was significantly different in RBV/TV distribution compared with all other hominids (Table 5). H. sapiens appears to be distinguished from other species studied primarily by radiopalmarly higher RBV/TV in the Mc1 head, on PC2 and PC3 (Figure 6a). Gorilla was also significantly different from both Pan species (Table 5) with apparently higher RBV/TV values ulno-palmarly (Figure 6a).
For DA, PC1 described 53% of the variation and reflected lower or higher values at most landmarks across the Mc1 head (Figure 6b). PC2 explained 12% of the variation and reflected variation in DA values at disto-palmar and dorsal landmarks. Pairwise tests revealed that Gorilla was significantly different from all other species (Table 5) likely due its overall lower DA (Figures 3 and 6b). Conversely, H. sapiens was not significantly different in its subarticular DA distribution relative to any other species, except Gorilla, despite being seemingly distinguished by higher DA values at most landmarks on PC1 (Figures 3 and 6b). Neither PC2 nor PC3, which only explained 7.3% of the variation in DA, differentiated the studied taxa to a notable extent.
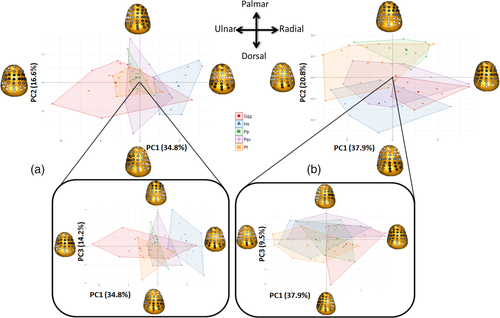
6.9 Mc1 bases
In the Mc1 base RBV/TV values, 35% of the variation was explained by values at radiopalmar and a combination of extremely palmar and dorsal landmarks in PC1 (Figure 7a). PC2 explained 17% of the variation in RBV/TV values and was driven by radioulnar landmark RBV/TV values, while PC3 explained 14% of the variation and was driven by dorso-palmar landmark values. H. sapiens was significantly different to all other species (Table 5) and appeared to be distinguished by higher RBV/TV in the palmar and radial aspects of the Mc1 base on PC1 (Figure 7a). Gorilla tended to separate from the other taxa displaying higher RBV/TV at extremely palmar and dorsal landmarks and plotting at the opposite end of PC1, though this species displayed a large range of DA distributions (Figure 7a), while all other taxa were intermediate between H. sapiens and Gorilla. However, there were no significant differences in RBV/TV of the Mc1 base across nonhuman great apes (Table 5). While PC2 and PC3 were driven by contiguous patches of landmark values they did not distinguish any taxa (Figure 7a).
In subarticular DA of the Mc1 base, PC1 explained 38% of the variation and mainly differences in central and dorsal landmark DA (Figure 7b). PC2 explained 21% of the variation reflecting radiopalmar and dorsal landmark DA values. PC3 explained 10% of the variation and was driven by DA values at radioulnar landmarks. The distribution of Mc1 base DA in both P. troglodytes and P. paniscus was significantly different from H. sapiens and Gorilla (Table 5). This different distribution appeared to be driven by PC2 (Figure 7b). Both Pan species had highest DA at dorsal landmarks, while the latter two species had their highest DA values at radiopalmar landmarks (Figures 2 and 7b). Higher DA values extended more palmarly in P. troglodytes relative to P. paniscus (Figures 2 and 7b) which probably explains their significantly different overall DA distributions (Table 5). Pongo Mc1 base DA distribution appeared to be intermediate between all species studied on PCs 1 and 2 (Figure 7b) and was not significantly different from any other species (Table 5).
7 DISCUSSION
We investigated variation in the subarticular trabecular bone structure of the Mc1 across extant hominids to test whether it is consistent with what is known about their habitual thumb postures. In the Mc1 head, we predicted that subarticular RBV/TV and DA would be greatest at ulnar disto-palmar landmarks in nonhuman hominids (H1a), with the exception of gorillas that were predicted to have lower DA throughout the head (H1b). Conversely, in humans, we predicted DA and RBV/TV would be highest at radiopalmar landmarks on the Mc1 head (H1c). We also predicted that gorillas would have the lowest DA within the nonhuman hominids, which would have relatively uniform DA values across the Mc1 base (H2a). Further, nonhuman hominids were predicted to have the highest RBV/TV values in the central palmar aspect of the Mc1 base (H2b) while humans would have highest RBV/TV and DA values radiopalmarly (H2c).
7.1 Mc1 heads
The data support our predictions concerning subarticular RBV/TV across hominid Mc1 heads (H1a and c) but only partially support those relating to DA (H1 b and c). Average RBV/TV was higher at ulno-distal landmarks in the Mc1 head across all nonhuman great apes, consistent with a habitually adducted thumb used in pad-to-side or V-pocket grips (Bardo et al., 2016, 2017; Marzke et al., 2015; Neufuss et al., 2016, 2018) and some power grasps used in African ape locomotion (H1a; Neufuss et al., 2017, Samuel et al., 2018). Contrary to our hypothesis, however, DA was lowest at the ulno-distal landmarks, where RBV/TV was highest, in chimpanzees, bonobos, and orangutans (H1a). This prediction was based on the concept that stereotypical loading of the first McP joint would cause the realignment of trabeculae, via remodeling, in the direction of this load, resulting in higher DA. Interpreting the results in this way would imply orangutans do not load their Mc1 head in a flexed McP joint posture, the disto-palmar Mc1 articular surface, which does not agree with overwhelming behavioral and anatomical evidence nor the present RBV/TV results. This coincidence of highest RBV/TV and lowest DA values at ulno-distal landmarks instead reflects more trabecular bone with less alignment in this subarticular region that may be better able to withstand load from multiple directions. Indeed, while RBV/TV may be the result of thicker or more trabeculae, or some combination of both, it is notable that this average lower ulno-distal DA pattern was present in the three species with the smallest Mc1s (i.e., P. troglodytes, P. paniscus and Pongo sp.; Figure 3b) and smaller bones tend to have thicker and fewer trabeculae (Barak, Lieberman, & Hublin, 2013). This DA pattern, therefore, is likely the result of the number of trabeculae and is consistent with high ulnar loading of the McP joint in the chimpanzee, bonobo and orangutan Mc1 head, despite displaying the opposite trend to that predicted. However, further work that accounts for variation in trabecular number is needed to substantiate this interpretation of the present DA results.
For both DA and RBV/TV, the current sample consisting predominantly of Taï chimpanzees (P. troglodytes verus), known to use tools, displayed almost no significant differences from bonobos or orangutans, though the former have been observed using very few tools in the wild (Kano, 1982; Koops, Furuichi and Hashimoto, 2015), and neither are known to engage in percussive tool use (Meulman & van Schaik, 2013; van Schaik et al., 1996). It may be that nut-cracking (Boesch & Boesch, 1993) and the use of precision forceful grips during food processing (Marzke et al., 2015) are simply not frequent or forceful enough to stimulate subarticular trabecular remodeling in the Mc1 head. A similar sample of Taï chimpanzee Mc1s have previously been shown to have less robust trabecular architecture than another group of chimpanzees that do not habitually nut-crack (Lazenby et al., 2011). Therefore, either a strong osteogenic signal does not exist for this behavior or it is constrained by more habitual or greater loading of the Mc1 during Taï chimpanzee locomotion (Lazenby et al., 2011; Neufuss et al., 2017).
Gorillas had the highest average RBV/TV at ulnar landmarks situated on the disto-palmar aspect of Mc1 head, consistent with pad-to-side, interdigital brace and “thumb wrap” grips frequently used in wild manipulation (Neufuss et al., 2018). This subarticular pattern, however, is statistically distinguished from bonobos, orangutans and, to a lesser extent, chimpanzees by the greater range of RBV/TV values across the gorilla Mc1 head, suggestive of more habitual or greater loading of the pollical McP joint in adduction in this species. DA values in the gorilla Mc1 were significantly lower than all other species studied and displayed a low range throughout the head (H1b). Combined, this trabecular pattern is consistent with the habitual use of varied thumb positions in gorillas, but also more frequent or forceful loading in thumb adduction, relative to the other nonhuman great apes (Neufuss et al., 2018). However, while recent work has found that Mc1 head is similarly shaped in both subspecies of gorilla (Galletta et al., 2019), it must be noted that this prediction was based on the greater volume of behavioral evidence from mountain gorillas (G. gorilla berengei) rather than the western lowland (G. gorilla gorilla) subspecies studied here. Furthermore, there are limited detailed studies of grip and hand use in other nonhuman hominids in the wild (Marzke et al., 2015) and thus this interpretation must be treated with some caution.
Humans displayed both significantly higher RBV/TV at radial landmarks and higher DA throughout the subarticular Mc1 head than in other great apes (H1c). A radiopalmar concentration has also been found in a similar sample of humans, using a method that analyzed absolute BV/TV in the whole distal epiphysis of the Mc1 rather than just the subarticular region (Stephens et al., 2018). Further, a significantly larger radial palmar epicondyle in the human Mc1 head, relative to other great apes, may also indicate the importance of this region of the Mc1 head to human manipulation (Galletta et al., 2019). This RBV/TV distribution is consistent with a habitually and forcefully opposed thumb, a movement which entails flexion, and importantly, abduction at the McP joint (Napier, 1956). An opposed thumb is used in forceful precision grips during the production (Marzke, 1997; Marzke et al., 1998) and use of stone tools (Key et al., 2018; Rolian et al., 2011) among other manipulative activities (Bardo et al., 2017; Napier, 1993).
In contrast to these RBV/TV results, high DA throughout the human Mc1 head, while not overall significantly different from any species except gorillas, does not match our prediction of the highest DA at radiopalmar landmarks (H1c). Stephens et al. (2018) found slightly lower average DA values in a similar human Mc1 sample, likely due to their sampling of the whole distal epiphysis, but also found little regional differentiation across the Mc1 head in agreement with the results here. The present result may reflect the higher frequency of forceful prehensile thumb use in humans than in other extant hominids. Though the highest or most habitual forces may be resisted by the radiopalmar McP joint during thumb abduction, the frequently used, powerful and mobile human thumb likely engenders a stronger osteogenic signal throughout the head than in other apes and therefore more aligned trabeculae (i.e., higher DA). For example, humans are unique among extant hominids in their ability to forcefully extend and stabilize the first proximal phalanx on the Mc1 while flexing the distal phalanx via distinct flexor pollics longus and extensor pollicis brevis muscles (Diogo et al., 2012; Hamrick et al., 1998; Marzke et al., 1998). The thumb held in this position probably induces considerable loads on the distal McP joint, even if these are not as large as those loads resisted in forceful abduction (Cooney & Chao, 1977; Toft & Berme, 1980). The fact that humans appear to be more restricted in McP joint flexion than in other great apes (Barakat et al., 2013; Tuttle, 1969) also supports this interpretation of the human McP joint as a joint restricted by large thenar musculature that can resist higher loads. The insertion of extensor pollicis brevis on the first proximal phalanx (Diogo et al., 2012) may also explain why this high DA was found throughout the subarticular the Mc1 head but not the Mc1 base in humans.
7.2 Mc1 bases
The subarticular trabeculae of the Mc1 base did support our predictions for RBV/TV but not entirely for DA. In concordance with our predictions, average DA throughout the Mc1 base of gorillas was lower than other species studied (Figure 3b) but only significantly different from chimpanzees and bonobos, as these species had significantly higher DA at more dorsal landmarks. Contrary to our predictions, the other nonhuman great apes did not have a uniform distribution of DA values in the Mc1 base (H2a). Rather our results indicate a regional pattern, in which chimpanzees and bonobos had the highest DA values in the dorsal Mc1 base and were both significantly different from gorillas. The functional significance of this pattern is not immediately apparent though it may relate to more varied habitual thumb positions in gorillas relative to the other nonhuman great apes. All nonhuman hominids displayed the highest RBV/TV at extremely palmar and central landmarks consistent with habitual flexion at the TMc joint (H2b), though the range of values throughout the base was lower than in humans. This more homogenous distribution of subarticular RBV/TV is consistent with previous studies of absolute BV/TV throughout a similar sample of Pan Mc1 proximal epiphyses (Skinner et al., 2015a, 2015b; Stephens et al., 2016).
In partial support of our predictions, humans had higher palmar DA values on the radioulnar edges of the Mc1 base (H2c). While values average DA values for this species were highest in the radiopalmar region (Figure 3b) consistent with opposition of the thumb these were only significantly higher than those of bonobos (Figure 5) and DA were also higher at some ulnar landmarks (Figure 3b). Conversely, humans displayed the highest RBV/TV values at the radial and less extreme palmar region of Mc1 base landmarks, consistent with a habitually and forcefully abducted thumb, flexed in opposition (H2c; Napier, 1956; Halilaj, Rainbow, et al., 2014; Feix et al., 2016; D'Agostino, Dourthe, Kerkhof, Stockmans, et al., 2017, Marchi et al., 2017). These results agree with other studies that have found a palmar concentration of Mc1 base BV/TV in comparison to other apes (Skinner et al., 2015a, 2015b) and other metacarpals (Wong et al., 2018). The radiopalmar concentration found here agrees with that found using the whole-epiphysis method, on a similar sample (Stephens et al., 2018), as well as the fact that osteoarthritis first develops in this region of the Mc1 base (Koff et al., 2003). The present results further refine this radiopalmar RBV/TV distribution to a less markedly palmar subarticular area than that found in other great apes. This pattern may be interpreted as habitual loading of the human TMc joint in a less flexed position than in other extant hominids. This may be a result of the “screw-home” mechanism of the TMc joint during human opposition where axial rotation of the Mc1 causes the tip of the palmar beak to enter a recess on the trapezium (D'Agostino, Dourthe, Kerkhof, Stockmans, et al., 2017; Edmunds, 2011). In this position, the majority of the Mc1 base is in compression and stabilizes the joint without the need of a taut palmar peak ligament but the tip of the beak no longer articulates with the trapezium as it moves ulnarly into this recess (Edmunds, 2011). The more rounded volar beak of P. paniscus, for example, would not facilitate this movement as well as the triangular shaped palmar beak of humans (van Leeuwen, Vanneste, et al., 2018). As a result, the palmar tip of the human Mc1 base may undergo less loading during habitual forceful opposition of the Mc1 base and so not engender higher RBV/TV values in this region. Conversely, nonhuman great apes that do not habitually perform forceful pad-to-pad opposition load the most palmar aspect of the Mc1 base during flexion and so have higher RBV/TV values in this region. Proximity patterns of the TMc joint in humans support this interpretation as they demonstrate that the tip of the palmar beak is closer to the trapezium during adduction and during a pad-to-side grip than in abduction and during an abducted power grip (Figure 1; D'Agostino, Dourthe, Kerkhof, Van Lenthe, & Stockmans, 2017).
7.3 Functional interpretation
Taken together, the current results are consistent with habitual loading of the TMc joint in flexion, and the McP joint in adduction, during frequently used precision and power grips in all nonhuman great apes studied. The results also suggest that Gorilla uses more varied thumb postures than either Pan or Pongo as they have lower DA at both joints. While locomotion is not the focus of this paper it is of note that the most terrestrial nonhuman great ape, Gorilla, has a trabecular pattern consistent with loading of the thumb in many positions during manipulation. Not only is this consistent with behavioral observations of mountain gorillas in the wild (Byrne et al., 2001; Neufuss et al., 2018) but also hypotheses that link complex manipulation and tool use with terrestrial behavior in general (Heldstab et al., 2016; Meulman, Sanz, Visalberghi, & van Schaik, 2012). Humans, the most terrestrial species studied exhibit a distinct trabecular morphology that suggests a habitually less flexed more abducted TMc joint and a more strongly, or frequently, recruited flexed abducted McP joint than other great apes. This human pattern is consistent with habitual forceful precision grips uniquely practiced by humans during manipulation.
While these results appear consistent with what is known of hominid behavior and anatomy, the function of trabecular bone is not only biomechanical but also physiological, as it is important for mineral homeostasis (Clarke, 2008). Furthermore, trabecular structure may be affected by systemic factors, including the gut biomes of an animal (Tsegai et al., 2018) and is determined genetically to some extent (Almécija, Wallace, Judex, Alba, & Moyà-Solà, 2015; Havill et al., 2010; Judex, Zhang, Donahue, & Ozcivici, 2013; Lovejoy et al., 2003). Some researchers have also argued that functional adaptation of bone is largely limited to mechanical strains experienced during growth (Bertram & Swartz, 1991; Lovejoy et al., 2003; Wallace et al., 2017). While developmental signals are certainly present in bone, bone functional adaptation also occurs in the mature skeleton (Ruff et al., 2006) and to some extent during senescence (Homminga et al., 2004). However, it should be noted that the Mc1 head develops from a pseudoepiphysis whereas the base arises from a true secondary ossification center (Haines, 1974) reflecting a different evolutionary history from the other metacarpals (Pazzaglia et al., 2018). This developmental difference may also potentially affect trabecular architecture (Lazenby et al., 2011). The functional signal found here, however, appears to be relatively strong, given these potentially confounding variables.
The interpretation of this functional trabecular signal must also consider the shape of the cortical bone which surrounds it as well as soft tissue morphology. It could be argued that the radiopalmar concentration of RBV/TV in humans is due to ulnar deviation of the Mc1 head relative to the base (Drapeau, 2015) in this species rather than an interspecific difference in manipulation. That is, the trabecular pattern is caused by the same vector of manipulative load in all species but in humans, the McP joint load angle differs due to the shape of the Mc1. However, an ulnar trabecular signal is found in the orangutan Mc1 head that is also ulnarly deviated relative to the Mc1 base (Drapeau, 2015) and therefore this signal is likely due to species differences in manipulation. Similarly, the size of subarticular Mc1 surfaces (Tocheri et al., 2005) may also affect the current results, since a constant number of landmarks will necessarily sample less subarticular trabeculae of a larger surface or redundantly oversample a smaller subarticular surface. The use of interpolated trabecular values, however, should ameliorate this sampling effect and it appears to not have affected the relatively large regional patterns found here. Nevertheless, as discussed for the Mc1 head, it would be advantageous to scale DA by trabecular number in future studies to ensure more direct and intuitive comparison of species with differently sized bones. Use of a principle trabecular orientation approach (Barak et al., 2017) in future studies may also obviate this complex interpretation.
8 CONCLUSION
In conclusion, while several predictions regarding DA were only partially supported by the data, interspecific variation found in the subarticular trabecular architecture of the hominid Mc1 head and base indicates four main findings. First, nonhuman great apes have an ulnar concentration of RBV/TV values in the subarticular Mc1 head and a palmar concentration in the Mc1 base, consistent with an adducted thumb in pad-to-side grips frequently practiced by these species. Second, humans have a radial concentration of RBV/TV values in the subarticular Mc1 head and a radiopalmar concentration in the Mc1 base. This statistically distinct pattern in humans is consistent with a habitually abducted thumb used during forceful precision grips, which are employed in many manipulative behaviors, including stone tool production. Thirdly, the low DA found in gorilla Mc1 trabeculae is consistent with more frequent and varied habitual manipulation than has yet been observed in other wild great apes. Finally, this signal is distinct from that of humans, which have significantly higher DA throughout the subarticular Mc1 head, consistent with relatively high loading during flexion and extension of the McP and facilitated by the unique thenar musculature of humans. The use of this comparative sample could aid in the identification of human-like manual behaviors in the hominin fossil record.
ACKNOWLEDGMENTS
The authors are grateful to Inbal Livne (Powell-Cotton Museum), Anneke van Heteren, Michael Hiermeier (Zoologische Staatssammlung München), Christophe Boesch, Uta Schwarz (MPI-EVA), Ana Ragni (NMNH); Maria Teschler-Nicola and Ronald Muehl (Natural History Museum, Vienna), Jacopo Moggi-Cecchi and Silvia Bortoluzzi (University of Florence), Frieder Mayer and Christiane Funk (Museum für Naturkunde - Leibniz Institute for Evolution and Biodiversity Science, Berlin), and Birgit Großkopf (Johann-Friedrich-Blumenbach-Institut für Zoologie und Anthropologie der Georg-August-Universität Göttingen) for access to specimens in their care. The authors would like to thank David Plotzki (MPI-EVA) and Keturah Smithson (Cambridge Biotomography Centre) for assistance in scanning these specimens, as well as Matthew Tocheri for assistance with landmarking software and Leoni Georgiou for discussions that enhanced this manuscript. Finally, the authors would also like to thank the three anonymous reviewers for their comments that improved this manuscript. This research was supported by European Research Council Starting Grant #336301, Consolidator Grant #819960 (C.J.D., M.M.S., T.L.K.), the Max Planck Society (M.M.S., T.L.K.), and the Fondation Fyssen (A.B.).
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.




