Variation in chewing efficiency of Yakushima Japanese macaque (Macaca fuscata yakui)
Funding information: Sun Yat-sen University; Primate Research Institute, Kyoto University
Abstract
Objectives
Chewing efficiency plays an important role in the survival and distribution of primates. Yet, little is known about the intra-specific variation of chewing efficiency. The purpose of this study is to report the pattern of seasonal and regional variation in chewing efficiency among Yakushima Japanese macaques (Macaca fuscata yakui).
Materials and Methods
Fecal samples of Yakushima Japanese macaques were collected from lowland, highland and summit areas in Yakushima between July 2015 and March 2016 (n = 236). Using sieving analysis, we compared fecal particle size (dMEAN) and proportion of finest particles p(0) between different geographical areas and seasons.
Results
Seasonally, in the lowland zone, there was a non-significant decrease in dMEAN during spring, while p(0) was significantly higher during summer than it was during winter and spring. Regionally, dMEAN was higher in the summit zone than it was in other areas during autumn, while p(0) was also higher in the summit zone.
Conclusions
While seasonal variation in dMEAN can be explained by the reported difference in the proportions of food categories in diet between seasons, its influence is mitigated, possibly by the selective feeding of less mechanically challenging parts in each category. Regional variation in dMEAN and p(0) may be the results of bamboo consumption in this area. Combining our data with studies that focus on seasonal and regional variations of food properties or gut microbes might provide a better understanding of the relation between diet, chewing and digestion in Yakushima macaques.
1 INTRODUCTION
One signature behavior shared by most mammals is mastication or chewing, which is the ability to reduce food size through cyclic occlusion of the teeth (Herring, 1993; Lucas, 2004; Reilly, McBrayer, & White, 2001). Chewing is an intermediate process between food ingestion and the digestive processes. It helps the reduction of food particle size during intake, and thus helps producing a larger relative surface area for the attachment of the gut microbes (Pond, Ellis, & Akin, 1984) and digestive enzymes (Livesey et al., 1995; Wondra, Hancock, Kennedy, Hines, & Behnke, 1995). Effective chewing can therefore increase the proportion of nutrients absorbed during digestion, and mammals have evolved a multitude of physiological and morphological adaptations that enhance their chewing efficiency, which is defined as the degree of comminution during mastication (Schwarm, Ortmann, Wolf, Streich, & Clauss, 2009). Examples of such adaptations include specialized dental morphologies (Evans & Sanson, 1998; Freeman, 1981; Kay, 1975) or rumination (i.e., regurgitation and re-chewing of food previously digested in a forestomach) (Domingue, Dellow, & Barry, 1991; Matsuda et al., 2014). The outcome of the digestion process is also closely related to food comminution before and after mastication (Jaster & Murphy, 1983; Shaver, Nytes, Satter, & Jorgensen, 1988). An excessively low chewing efficiency can lead to reducing in nutritional and energy input, and thus to undernutrition and death (King et al., 2005; Pahl, 1987; Veiberg et al., 2007). Therefore, chewing efficiency is an important aspect of biological anthropology as it provides information on the quality of dietary environment, the effects of diet on primate digestion as well as how primate adapt to their dietary environment.
A primate's chewing efficiency can be affected by several intrinsic and extrinsic factors. Intrinsic factors include chewing behavior (Pérez-Barbería & Gordon, 1998; Ross, Iriarte-Diaz, Reed, Stewart, & Taylor, 2016), dental morphology (Pérez-Barbería, Pérez-Fernández, Robertson, & Alvarez-Enríquez, 2008; Venkataraman et al., 2014) and dental senescence (King et al., 2005; Venkataraman et al., 2014). Extrinsic factors include pre-oral processing (Zink & Lieberman, 2016) and food physical properties such as size, structural properties or mechanical properties (Agrawal, Lucas, Prinz, & Bruce, 1997). Differences in chewing efficiency resulting from these factors can influence animals' survival and distribution. For example, Dunbar and Bose (1991) deduced that a lower chewing efficiency prevented gelada baboons (Theropithecus gelada) from competing with sympatric ruminants. On the other hand, a relatively high chewing efficiency may be the consequence of adaptations interacting with the above-mentioned factors. For example, Matsuda et al. (2014) compared the chewing efficiency of proboscis monkeys (Nasalis larvatus) and 12 other primate species and found that the chewing efficiency of N. larvatus was particularly higher, which supported a “rumination” behavior in proboscis monkeys.
Interspecific comparison of chewing efficiency has proven its power in clarifying the general differences in digestion among animals with different digestive systems and chewing behaviors (Clauss, Nunn, Fritz, & Hummel, 2009; Fritz et al., 2009). However, since the interspecific-level differences come from the combination of multiple factors, it is limited in clarifying the effects of a single factor. In contrast, documenting the chewing efficiency variation in a single species could limit the effects of digestive system, dental morphology, chewing behavior, etc. to a smaller degree. Recently, intraspecific-level studies showed its importance in revealing the effects of age and food properties (Venkataraman et al., 2014; Weary, Wrangham, & Clauss, 2017). Such studies may relate chewing efficiency with the variation in a species' diet, tooth senescence as well as age-sex related dental morphology, and therefore provide better understandings of the relation between these factors and digestion.
The Japanese macaques in Yakushima (Macaca fuscata yakui) are a dietary generalist (Hill, 1997) and it can feed on mechanically challenging foods (e.g., bark) (Hanya, 2010). They inhabit a wide range of habitats, from the coastal forests to the coniferous sub-montane forests and even bamboo grassland in Yakushima Island, Japan (Yoshihiro et al., 1999). The diet of these macaques shows significant intraspecific variations (Table 1) (Hanya, 2004; Hanya et al., 2003). Seasonally, in all the habitats lower than 1,230 m, these macaques' diet includes young and mature leaves during spring; fruits, fungus, animals or mature leaves during summer; and fruits as well as seeds during autumn (Hanya, 2004; Hanya et al., 2003). They also consume more fibrous food including mature leaves during winter, especially at higher altitudes (Agetsuma, 1995; Hanya, 2004; Hanya et al., 2003). Regionally, Yakushima macaques living in lower coniferous forests (800–1,199 m) consume a majority of fibrous food, while in low altitude areas fruit and seeds compose the major part of their diet (Hanya, 2004; Hanya et al., 2003). This high temporal and spatial heterogeneity in diet makes M. fuscata yakui a suitable model for investigating seasonal and regional chewing efficiency variations within a single subspecies.
| Based on feeding time | ||||||||
|---|---|---|---|---|---|---|---|---|
| Habitat | Season | Fruit | Seed | Leaf | Animal | Fungi | Flower | Other |
| Lowland1 | Summer | **** | *** | * | ** | * | − | * |
| Autumn | **** | **** | * | * | * | * | * | |
| Winter | ** | **** | ** | * | * | * | * | |
| Spring | ** | * | **** | * | * | ** | * | |
| Highland2 | Summer | * | − | ***** | * | ** | − | * |
| Autumn | *** | ** | ** | * | ** | * | ** | |
| Winter | * | − | ***** | * | * | ** | − | |
| Spring | − | − | *** | * | * | **** | * | |
| Summit3 | Bamboo eating was observed but without detailed data | |||||||
| Based on dry weight of fecal content | ||||||
|---|---|---|---|---|---|---|
| Habitat | Season | Fruit /seed | Fiber | Animal | Fungi | Other |
| Lowland4 | Summer | ++++ | + | + | + | − |
| Autumn | ++++ | + | + | + | − | |
| Winter | ++++ | ++ | + | + | + | |
| Spring | ++ | +++ | + | + | + | |
| Highland4 | Summer | ++ | +++ | + | + | − |
| Autumn | +++++ | + | + | + | − | |
| Winter | ++ | +++ | − | + | − | |
| Spring | + | +++++ | − | + | − | |
| Summit3 | Summer | + | +++++ | − | − | − |
| Autumn | ++ | ++++ | − | − | − | |
| Winter | No data | |||||
| Spring | No data | |||||
- Note: Lowland: 0–800 m; Highland: 800–1,600 m; Summit: >1,600 m.
- Note: Propotion of monthly feeding time: *0–12%; **13–24%; ***25–36%; ****37–48%; *****49–60%; − did not detect.
- Note: Relative dry weight in feces: + 0–20%; ++ 21%–40%; +++ 41%–60%; ++++ 61%–80%; +++++ 81%–100%; − did not detect.
- Note: 1, Agetsuma, 1995; 2, Hanya, 2004; 3, Honda, unpublished data; 4, Hanya, Noma, & Agetsuma, 2003.
As a non-invasive way to assess chewing efficiency, fecal particle size has been widely used in previous studies (Dunbar & Bose, 1991; Fritz et al., 2009; Matsuda et al., 2014; Venkataraman et al., 2014; Weary et al., 2017). Many studies confirmed the relation between fecal particle size and chewing and therefore confirm the validity of this method (Freudenberger, 1992; Fritz et al., 2009; McLeod & Minson, 1988; Murphy & Nicoletti, 1984; Poppi, Norton, Minson, & Hendricksen, 1980). While discrete mean particle size (dMEAN) is the standard measurement of chewing efficiency (Fritz, Streich, Schwarm, & Clauss, 2012), it may conceal the variation in very fine particles and soluble matters in feces. In the standard sieving experiment for fecal particle size, these matters cannot be caught by the system. In cattle, however, in terms of dry weight, more than 20% of fecal matter consists of the finest particles and soluble matters (Jaster & Murphy, 1983; Shaver et al., 1988). Furthermore, Jaster and Murphy (1983) reported a larger proportion of such matters when animals were fed with chopped food. However, the existence of such associations depends on the fiber content of foods. Shaver et al. (1988) found forage condition only produce little influence on particle size reduction when cattle fed on low-fiber forage. These results suggest that in the condition of fiber-rich diet, better food comminution, resulting either from an efficient chewing or other factors, should result in a larger proportion of finest particles p(0), which makes it a useful complement for the interpretation of dMEAN.
The objectives of this study were to determine whether the variation of chewing efficiency occurs in Japanese macaques in Yakushima, and to describe its fluctuation pattern within this subspecies. We collected fecal samples of wild Japanese macaque from regions of different altitudes in Yakushima Island, from July 2015 to March 2016. We then measured fecal particle sizes to evaluate chewing efficiency. In accordance with fallback food theory (Marshall & Wrangham, 2007), we expected a decrease in chewing efficiency (i.e., larger fecal particle size) and consequently a lower proportion of the finest particles when foods with less fiber content (high-quality food, for example, seeds and fruits) were less available, either regionally in the highland and the summit zones, or seasonally during winter and spring.
2 MATERIALS AND METHODS
2.1 Study site
Yakushima island is located in the south-western part of Japan (30°N, 131°E). The island covers an area of 503 km2. The highest peak of the island is 1936 m a.s.l. The annual precipitation was between 2,019 and 4,389 mm in the lowland zone (from 1980 to 2018) (Japan Meteorological Agency, 2019), but can exceed 8,600 mm in the higher area according to a previous study (Eguchi, 1984). In the coastal area, the mean annual temperature was 21°C and the mean temperature of the coldest month (February) is 11°C during the study period from July 2015 to March 2016 (Japan Meteorological Agency, 2019). At the altitude of 1,050 m, the two measurements are 12 and 3.4°C, respectively (Hanya et al., 2004; Tagawa, 1980).
- The lowland zone (ca. 0–800 m), which is mainly characterized by warm-temperate evergreen broad-leaved trees, locally associated with subtropical plants below 100 m;
- The highland zone (ca. 800–1,600 m), characterized by conifers, which progressively outnumber evergreen broad-leaved trees, and become the dominant plant form above 1,200 m;
- The summit zone (ca. >1,600 m), where no tall tree grows while a bamboo species, Pseudosasa owatarii Makino, covers the whole area.
As each vegetation is representative of each zone but heterogeneous within each altitude range, we also relied on field observation of vegetation when associating a sample to a zone.
We defined seasons based on previous studies, with spring spanning from March to May, summer from June to August, autumn from September to November and winter from December to February (Hanya et al., 2003; Hanya et al., 2006).
2.2 Fecal sampling
We collected fecal samples of wild Japanese macaques in Yakushima between July 2015 and March 2016. Samples were collected in the coastal forest or on and around a forest path from the coast to the summit zone in the western area of Yakushima (Figure 1). Only fresh feces were sampled. The distance between sampling localities in the different zones was no less than 1.5 km (1.54 km between the sampling localities in the lowland zone and the highland zone, 1.81 km between the highland zone and the summit zone, Figure 1), which was larger than the diameter of any known home range of Japanese macaques in Yakushima (Hanya et al., 2004; Hanya et al., 2006; Maruhashi, Saito, & Agetsuma, 1998; Takasaki, 1981; Yoshihiro et al., 1999). According to previous studies in the coastal groups and one group at approximately 1,100 m elevation, no large-scale migration along the elevation has been detected (Hanya et al., 2004). In other words, such an interval ensured that our samples collected in a zone represented the monkeys who lived in that zone. We should note that the monkeys lived in the summit zone may have different migration pattern along the elevation. Previous studies reported that Japanese macaques in Yakushima could be found as high as 1886 m during summer (Yoshihiro, 1995), but during winter their highest range is 1,450 m (Yoshihiro, 1995; Yoshihiro et al., 1999). But according to our preliminary survey, during the sampling period, there were monkeys actively foraging in the summit zone (Honda, unpublished data). Therefore, we matched the fecal samples to the zone of feeding based on the location of sampling.
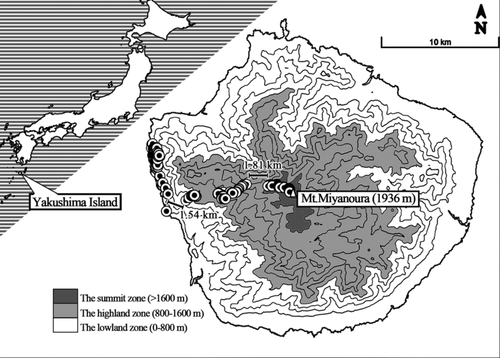
Instead of associating each sample with an individual, we employed opportunistic sampling while moving through the home range of monkeys in all the three zones, because (a) the effects of age and sex fell out of the scope of this study; (b) continuously following and observing monkeys in the highland zone and especially in the summit zone was difficult as monkeys were not habituated; (c) there was no evidence of demographic differences between different habitats and different seasons in Yakushima Japanese macaques living below 1,400 m (Hanya, 2004; Yoshihiro et al., 1999). As a result, we do not expect demographic differences in other Yakushima macaques. Therefore, in this study, the impact of demographic variation was expected to be negligible, comparing to the influence of habitats and seasons.
Every sample was preserved in a sealed tube with 70% alcohol to prevent drying and agglutination of fecal particles. Global Positioning System (GPS) waypoints were set for every sample during collection (Garmin GPSMAP 60CSx and Garmin GPSMAP 62 s, Garmin, Olathe, Kansas, USA). During summer and autumn, at least eight samples were collected in the lowland zone, the highland zone and the summit zone, respectively. However, the highland zone and the summit zone were not accessible during winter and spring because of heavy snowfalls. Therefore, it was only possible to collect samples from the lowland zone in these two seasons. The exact number of fecal samples is shown in Table 2.
| Year | Month | Lowland | Highland | Summit | Total | |
|---|---|---|---|---|---|---|
| 2015 | July | Middle | — | — | 8 | 8 |
| Late | — | — | 2 | 2 | ||
| August | Early | 8 | 1 | 13 | 22 | |
| Middle | — | 12 | — | 12 | ||
| Late | — | — | 5 | 5 | ||
| September | Middle | — | 1 | 36 | 37 | |
| Late | — | 4 | 4 | 8 | ||
| October | Early | 2 | — | — | 2 | |
| Middle | 28 | 6 | 3 | 37 | ||
| Late | 8 | 4 | 1 | 13 | ||
| November | Early | 3 | 4 | — | 7 | |
| Late | 8 | 1 | — | 9 | ||
| December | Early | 11 | — | — | 11 | |
| Middle | 5 | 1 | — | 6 | ||
| 2016 | January | Late | 6 | — | — | 6 |
| February | Early | 2 | — | — | 2 | |
| Middle | 1 | — | — | 1 | ||
| Late | 16 | — | — | 16 | ||
| March | Early | 19 | 13 | — | 32 | |
| Total | 117 | 47 | 72 | 236 |
2.3 Sieve analysis
The wet sieving of feces followed a standard protocol (Fritz et al., 2012). We separated each sample into two subsamples. The first subsample was weighed and then desiccated in a vacuum oven until its weight stops changing. With the weight before and after desiccation, we calculated dry matter concentration C. We weighed the second subsample and suspended it in a beaker with water until it was fully dispersed. The suspension was then poured over a cascade consisting of nine sieves with square holes (from bottom to top, the hole size of sieves 1–9 is shown in Table 3). We rinsed the beaker with 1 L of water. Sieves were testing sieves from Tokyo Sanpo, Japan. We used a vibratory sieve shaker to shake the sieve cascade (Retsch AS200 Digit, Germany). The shaking process was 10 min long, while the vibration amplitude was set at 40% (approximately 2 mm). We adjusted water throughput to approximately 2 L/min.
| Sieve number i | Hole size S(i)/mm |
|---|---|
| 1 | 0.063 |
| 2 | 0.125 |
| 3 | 0.250 |
| 4 | 0.500 |
| 5 | 1.000 |
| 6 | 2.000 |
| 7 | 4.000 |
| 8 | 8.000 |
| 9 | 16.000 |
The particles of each fraction were transferred onto pre-weighed tinfoil dishes and desiccated at 103°C for 24 h. After cooling to room temperature in a desiccator, we weighed samples by using an analytical balance with measuring accuracy of 0.0001 g (Shimadzu Libror AEG-220, Shimadzu Scientific Instruments, Japan).
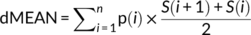 (1)
(1)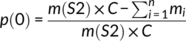 (2)
(2)2.4 Data analysis
A total of 236 samples were included in the analysis. We collected samples from the lowland zone in all four seasons. The data in winter and spring was drastically departed from the normal distribution even after ln transformation. Therefore, to determine seasonal variation in dMEAN and p(0) in the lowland zone, we performed a Kruskal–Wallis test with Dunn's test for post-hoc comparison. We collected samples from all the three zones in summer and autumn. We ln transformed dMEAN and p(0) data from these two seasons to reach normality for statistical analyses (Shapiro–Wilk normality test for transformed data, all group, p > .05). To determine seasonal and regional variation in dMEAN and p(0) in summer and autumn in all the three zones, we performed a two-way analysis of variance (ANOVA) with seasons and zones as factors. We used the Tukey HSD method with Bonferroni correction for post-hoc comparisons (Curtin & Schulz, 1998). We must note that the p(0) in autumn departed from the normal distribution even after ln transformation, so we also applied Kruskal–Wallis test with Dunn's test for post-hoc comparison for the p(0) in autumn to examine the results of the ANOVA and the Tukey HSD test. This result consisted with the result of the ANOVA and the Tukey HSD test. We reported the results of both tests in the results section. For the whole data analysis, the significance level was set to α = 0.05 (for multiple comparisons, adjusted α levels were determined according to specific tests). All the statistical analyses were performed in Microsoft Excel for Office 365 and R 3.4.4 (R Core Team, 2018).
This research was noninvasive and adhered to the legal requirements of Japan. During the research, we adhered to the guideline for field research of non-human primates of Primate Research Institute, Kyoto University (KUPRI). The protocol and procedures employed were ethically reviewed and approved by KUPRI.
3 RESULTS
3.1 Seasonal variations in chewing efficiency and fine particles
We observed a difference in dMEAN between seasons in the lowland zone, but it was not statistically significant (Kruskal–Wallis test, p = .0429, Dunn's test, summer vs spring: p = .0232, which is above the Bonferroni adjusted α, 0.05/6, Figure 2a). Data from the highland and the summit zones only covered summer and autumn. We did not find statistically significant differences between summer and autumn in either of the two zones (two-way ANOVA, p = .3917, Figure 3a).

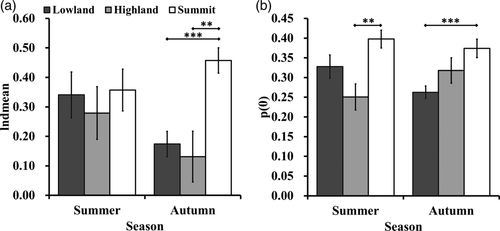
We found significant differences in p(0) between different seasons in the lowland zone (Kruskal–Wallis test, p = .0019, Figure 2b). Data from the lowland zone showed p(0) was significantly higher during summer than it was during winter and spring (Dunn's test: summer vs spring, p = .0054; summer vs winter, p = .0071, Figure 2b). Again, we did not find any statistically significant difference between summer and autumn in the data from the highland and the summit zones (two-way ANOVA, p = 0.0917, Figure 3b).
3.2 Regional variations in chewing efficiency and fine particles
We found a significant difference in dMEAN between the different zones (two-way ANOVA, p = .0002, Figure 3a). During autumn, dMEAN in the summit zone was extremely high. It was significantly higher than it was in the other zones (Tukey HSD: summit vs lowland, p = .0015, summit vs highland, p = .0021). However, no significant difference was observed during summer (Figure 3a).
We found significant differences in p(0) between the zones (two-way ANOVA, p < .001; Kruskal–Wallis test for autumn, p = .0013, Figure 3b). During summer, p(0) was significantly higher in the summit zone than it was in the highland zone (Tukey HSD, p = .0023, Figure 3b), but not higher than it was in the lowland zone (Tukey HSD, p = .8812). During autumn p(0) was significantly higher in the summit zone than it was in the lowland zone (Tukey HSD, p = .0009; Dunn's test, p = .0004, Figure 3b), but not higher than it was in the highland zone (Tukey HSD, p = .5625; Dunn's test, p = .3486, Figure 3b).
4 DISCUSSION
4.1 Seasonal variation in chewing efficiency of Yakushima macaques
We observed a small non-significant difference in dMEAN between seasons in the lowland zone. As food mechanical properties are one of the most prominent extrinsic factors that limit chewing efficiency in primates, especially food toughness and elastic modulus (Agrawal et al., 1997; Fritz et al., 2009; Teaford, Lucas, Ungar, & Glander, 2006; Venkataraman et al., 2014), this difference may indicate a seasonal variation in mechanical properties of the plants consumed by Yakushima Japanese macaques. Specifically, dMEAN was lower during spring than it was during summer (Figure 2a), which is consistent with previous studies reporting a higher proportion of potentially less mechanically challenging plant food, such as young leaves and buds, in the diet macaques from lowland zone during spring (Agetsuma, 1995; Hill, 1997; Maruhashi, 1980). In January, the high dMEAN (Figure 2a) is also consistent with previous studies reporting a higher proportion of potentially mechanically challenging food, such as mature leaves, in the diet of macaques from the lowland zone during the coldest month (Agetsuma, 1995; Hanya et al., 2003; Maruhashi, 1980). However, we must note that the reported difference was not statistically significant. There are several possible explanations for this result. First, macaques reduce the impact of food variation in chewing efficiency through selecting less mechanically challenging plant parts. Such detailed food selection was not reflected in the previous diet composition studies (Agetsuma, 1995; Hanya, 2004; Maruhashi, 1980). A previous study in the Yakushima lowland zone reported the avoidance of tough food plant parts in macaques (Hill & Lucas, 1996). Among the fiber-rich leaves, macaques tended to discard midrib and petiole with high toughness (Hill & Lucas, 1996). Selective feeding was reported in mountain gorillas from the Virunga (Gorilla beringei beringei) and showed its effects in mitigating dietary toughness variations. Even though the leaf toughness would increase with altitude, gorillas consumed plants of similar toughness (Glowacka et al., 2017). Second, the seasonal comparison may mask variations at a finer time scale. Particularly in winter, we did observe that dMEAN was higher in January than it was in the two other months in winter (Figure 2a). However, the sample size in January was not sufficient to support this difference. Further studies with a larger sample size for every month may reveal a more detailed variation pattern in chewing efficiency.
Concurrently, there was a significant decrease in the proportion of the finest particles p(0) during winter and subsequent spring compare to summer (Figure 2b). In January, high dMEAN and a relatively low p(0) appeared synchronously. In contrast, in March, dMEAN and p(0) were both low. This contrasts with the result in cattle which suggested better comminution should lead to a larger proportion of finest particles (Jaster & Murphy, 1983). The pattern showed in our result suggests that the relation between p(0) and dMEAN was also affected by other factors. One possible explanation is that p(0) is more sensitive to the fiber content in food compared to dMEAN, while dMEAN is more sensitive to the mechanical properties of food. The decrease of p(0) indeed occurred in winter and spring, when non-fibrous food is scarce compare to summer, when macaques have to fall back no matter on mechanically challenging mature leaves or relatively less challenging young leaves and buds. Testing this hypothesis requires synchronous measurement of diet composition, dietary fiber content, food mechanical properties as well as p(0) and dMEAN. If this hypothesis was verified, a decrease in p(0) would rather be an indicator of a lower consumption of fruit and other non-fibrous foods than dMEAN.
4.2 Regional variation in chewing efficiency of Yakushima macaques
We also detected a higher dMEAN in the summit zone. However, the extent of this variation was not constant in summer and autumn. This temporary increase in fecal particle size might reflect the special diet of monkeys living in the summit zone. A bamboo species, P. owatarii makino, is the dominant plant species in this zone (Kimura, 1984). Preliminary surveys in the summit zone support the idea that this bamboo species is the most important food source for monkeys in the summit zone (Honda, personal observations). During summer, monkeys who live in this zone almost exclusively fed on shoots and buds of bamboo. In autumn, they also consumed some seeds and other plants, while a large proportion of their diet consisted of bamboo shoots and buds (Honda, personal observations). Preliminary analysis of the composition of fecal content also detected more long fiber in the samples from the summit zone than samples from the other two zones, which also supported that monkeys living in this zone relied heavily on bamboos (Honda, unpublished data). Although the detailed mechanical and chemical properties of this diet remain unknown, the high fiber content in feces was likely to be the direct cause of larger fecal particle size. Especially, during autumn, when the proportion of fruits and seeds in diet increases in the lowland and the highland zones, the effects of a bamboo-based diet in the summit zone are likely to become more distinguishable, which could explain the observed regional variation in dMEAN.
Our current results cannot lead to a solid conclusion on how bamboo consumption caused high dMEAN and p(0) due to the lack of knowledge of the summit zone in Yakushima. The concurrent increase of the two measurements also contrasts with a previous study in cattle (Jaster & Murphy, 1983). One possible explanation is that the diet of monkeys who lived in the summit zone includes both fiber-rich part and easy-digested matters. The fiber-rich part limited the comminution of food, and results in high fiber content in feces and a high dMEAN, while relatively easy-digested matter caused the high p(0). Consider the lack of detailed data on diet, food mechanical properties and nutritional properties; it is possible that some of the food in the summit zone possess unexpected properties. In order to test this idea, future studies focused on the mechanical and nutritional properties of bamboo, its digestibility, as well as the gut microbes of Yakushima macaques will be necessary. Combined with our results, such studies may provide important insights into the effects of food properties in extreme habitat on Japanese macaques' digestion.
In contrast, there was no difference in dMEAN or p(0) between the highland zone and the lowland zone during summer and autumn (Figure 3a). This is unexpected, as the literature reported that the vegetation of Yakushima highland is composed of many fiber-rich plants (Hanya, 2004; Tsuji, 2010) and the diet of macaques included more fibrous foods such as leaves and less non-fibrous foods such as fruit in the highland zone than in the lowland zone (Agetsuma, 1995; Hanya, 2004; Hill, 1997). This unexpected result could be a reflection of two properties of Yakushima macaques' diet which were reported in a previous study (Hanya et al., 2003): (a) in summer and autumn, although the proportion of fruits and seeds tend to decrease along the altitude, fruits and seeds are still a major part of the diet, (2) in summer, macaques increased their consumption of animal matter more drastically in the highland zone than in the lowland zone. This might have affected the ratio of fibrous/non-fibrous material ingested by macaques, mitigating differences in chewing efficiency. Besides this regional comparison did not cover winter and spring. Previous studies reported more drastic diet differences between the highland zone and the lowland zone in these two seasons comparing to summer and autumn. Future studies that take all the seasons into consideration may provide a clearer vision of regional variation in chewing efficiency.
5 CONCLUSIONS
This work shows evidence of variations in the chewing efficiency of Japanese macaques in Yakushima. These variations were limited to some regions and seasons. Seasonally, in the lowland zone, fecal particle size was lower in spring than it was in summer. During winter, fecal particle size was higher in January. However, neither observation was supported by a statistically significant difference. The lack of statistical significance in seasonal comparison likely reflects that some of the seasonal variation in diet and chewing efficiency were mitigated by other factors, such as selective feeding of specific plant parts. Regionally, during autumn, the samples from the summit zone showed larger fecal particle size than samples from either the lowland or the highland zones, which has been interpreted as a local decrease in chewing efficiency. This decrease likely stems from the local consumption of P. owatarii Makino bamboo. The concurrently high p(0) suggested macaques living in this zone are able to compensate for this decrease in chewing efficiency. Due to the lack of detailed data on diet and food properties, we cannot make any solid conclusion about the effects of bamboo consumption on chewing efficiency in Yakushima macaques. Future studies that include data of diet, food mechanical properties and nutritional properties, and chewing behaviors in Yakushima macaques could provide a better vision on the variation pattern of chewing efficiency and clarify the effects of extreme environments in regard to feeding ecology and digestion.
There are several limitations in this study. First, due to the sampling methods and conditions in the field site, our data only covered limited regions and months. As a result, although this study indeed provides evidence for the existence of variation, it is difficult to produce larger scale regional and seasonal conclusions on the variation patterns. A more comprehensive data set in either regional or seasonal level will lead to a clearer vision on these variations. Second, this study did not include detailed data about the factors that may influence chewing efficiency, such as diet composition, chewing behavior, food mechanical properties, food chemical properties, etc. As a result, we cannot point out the reasons behind the variations we detected. Especially, food mechanical properties have been considered as a potentially important factor that limits primate chewing efficiency (Agrawal et al., 1997; Fritz et al., 2009; Teaford et al., 2006; Venkataraman et al., 2014). The relation between chewing efficiency and toughness (Venkataraman et al., 2014) or the combination of toughness and elastic modulus (Agrawal et al., 1997) have been reported. However, only a few studies focused on this association. Exist studies only covered limited taxa among all the primates. As the FMPs are related with fiber content (Choong et al., 1992; Hill & Lucas, 1996) and food selection (Dominy, Lucas, Osorio, & Yamashita, 2001; Glowacka et al., 2017; Lucas et al., 2012; Matsuda et al., 2017; Teaford et al., 2006), we believe they worth further investigation in order to reveal the relation between food, food selection, chewing and digestion.
While the variation of diet is the most plausible explanation for our results, other factors such as variation in tooth wear, chewing behavior and gut microbial composition should not be completely ruled out. Nevertheless, this work provides strong evidence that fecal particle size can be used to infer the impact of diet on chewing efficiency in Japanese macaques in Yakushima and other Old World monkeys. Combining fecal particle analysis with other traditional approaches used in dietary ecology can give us major insights into the reasons underlying spatial or seasonal changes in diet, which includes episodes of fallback food consumption.
ACKNOWLEDGEMENTS
We thank our friends and colleagues at the Primate Research Institute, Kyoto University (KUPRI) and Sun Yat-sen University for their support and help. We thank Prof. Hanya Goro who allowed us to collect data in his lab. He also provided comments and suggestions on this manuscript. Prof. Matsuda Ikki provided patient instruction and suggestions for fecal particle size analysis. We would also like to thank Dr. Vivek Venkataraman, who kindly let us access the data set from their previous work. The Yakushima Forest Environment Conservation Center and Kirishima-Yaku National Park gave us permission to study in the area. We are grateful to these people and organizations. He Tianmeng earned the Sumitomo Corporation Scholarship for Exchange Students during conducting the research. We are grateful for the financial support in living expenses from the Sumitomo Corporation. We also thank Prof. Zhang Peng for promoting the scholarship and exchange program.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.




