In sickness and in death: Assessing frailty in human skeletal remains
Abstract
Stress plays an important role in the etiology of multiple morbid and mortal outcomes among the living. Drawing on health paradigms constructed among the living augments our evolving knowledge of relationships between stress and health. Therefore, elucidating relationships between stress and both chronic and acute skeletal lesions may help clarify our understanding of long-term health trends in the past. In this study, we propose an index of “skeletal frailty,” based on models of frailty used to evaluate the life-long effects of stress on health among living populations. Here, we assess the possible applicability of frailty to archaeological populations. The skeletal frailty index (SFI) is proposed as a methodological liaison between advances made by biological anthropologists studying relationships between stress and health among the living and bioarchaeologists studying stress and health among the dead. In a case study examining skeletal stress in Medieval London, the SFI is applied to nonmonastic (N = 60) and monastic (N = 74) samples. We used analysis of variance/analysis of covariance to compare SFI values between nonmonastic-monastic groups, sexes, and age cohorts. Results indicate higher lifetime morbidity among monastic groups. These results complement previous bioarchaeological findings on the same London populations, wherein lower risks of mortality and longer lifespans were observed for monastic populations. SFI data reflect the morbidity-mortality paradox observed in modern populations and accompany recent findings in bioarchaeology of variation in Medieval monastic and nonmonastic “health.” Ultimately, this study demonstrates the SFI's utility in bioarchaeology, through its application of commonly assessed skeletal biomarkers, its ease of applicability, and its potential usefulness for assessing changes in skeletal health over time and across specific geographies.
1 Introduction
Among humans, stress and physiological health1 are intrinsically linked (Goodman & Leatherman, 1998; Reitsema & McIlvaine, 2014; Temple & Goodman, 2014). Exposures to chronic and acute stressors lead to predictable and measurable hormonal and physiological responses that evolved to protect organisms from immediate damage (McEwen, 2000b; Sterling & Eyer, 1988). Continual stress responses over the lifespan have been shown to weigh heavily on future health, morbidity, senescent decline, and mortality (McEwen & Seeman, 1999; Seeman, Singer, Rowe, Horwitz, & McEwen, 1997). Therefore, being able to assess effects of stress provides significant insight into both current and future health status among living and deceased samples.
To evaluate health among past populations, bioarchaeologists study the last biological vestiges of once living peoples, skeletonized or mummified human remains. Assessing dental and skeletal markers associated with various chronic, degenerative conditions and/or pathological conditions on human remains is the best way to understand stress and physiological health among past populations. For bioarchaeologists, using a biological/physiological approach to measuring skeletal stress is essential for understanding relationships between stress, health, morbidity, and mortality (Goodman, Martin, & Armelagos, 1984; Goodman, Thomas, Swedlun, & Armelagos, 1988).
Washburn's (1951) clarion call for comparative work in biological anthropology instigated decades of methodological research, promoting cross-population studies within the discipline. Among human biologists, this call inspired standardized measures for stress and health to be applied universally across living populations (Ice & James, 2007). These measurements ultimately were adapted to quantifiable measurements like allostatic load (AL) and frailty (Seeman et al., 1997; Seeman, McEwen, Rowe, & Singer, 2001; Rockwood, Mogilner, & Mitnitski, 2004; Rockwood et al., 2005). For human osteologists to conduct inter-population comparative studies, etiological bases for frequently observed skeletal and dental lesions had to be identified and contextualized from living populations. In the decades following the Washburn article, biological anthropologists and skeletal biologists tackled this etiological issue within specific living and archaeological populations, illustrating clear trends in mortality and morbidity associated with skeletal and dental lesions (Armelagos, et al., 2009; Armelagos, Jacobs, & Martin, 1981; Cook & Buikstra, 1979; Goodman & Rose, 1990; Goodman, Martinez, & Chavez, 1991; Goodman, Pelto, Allen, & Chavez, 1992). However, it was not until the conference on and subsequent publication of Paleopathology at the Origins of Agriculture (1984) that regionally diverse osteological studies were collated into the first comprehensive volume addressing how adoption and intensification of agriculture impacted the health of populations throughout North America, Eastern Asia, and the Levant. This seminal work demonstrated the effective application of skeletal biomarkers to questions in human history and prehistory (Cohen & Armelagos, 1984). Within a decade of the conference, standards for skeletal data collection had been established (Buikstra & Ubelaker, 1994; Ortner & Putschar, 1985). While these recording standards have since been universally utilized in bioarchaeology, how these data are analyzed and interpreted relative to health and frailty is not yet universally established. Multiple definitions for frailty and statistical approaches to quantifying frailty currently exist in bioarchaeological literature (DeWitte & Hughes-Morey, 2012; Piperata, Hubbe, & Schmeer, 2014; Wilson, 2014; Wood, Milner, Harpending, & Weiss, 1992).
Due to constraints of preservation and context, the most common approach for analyzing skeletal data involves comparisons of gross and adjusted prevalence values for individual pathological conditions within a sample. Wood et al. (1992) exposed the inherent paradox of these statistical interpretations, which apparently overlooked concerns of demographic non-stationarity, selective mortality, and individual heterogeneity (see Goodman, 1993 for response). Since publication of the osteological paradox, alternative methods for dealing with these inescapable issues in bioarchaeology have been proposed: hazard models (Boldsen, 2007; DeWitte, 2010; DeWitte & Wood, 2008; DeWitte, Boulware, & Redfern, 2013; Wilson, 2014) and life-history studies (Wright & Yoder, 2003). Both approaches enable bioarchaeologists to study health among individuals rather than overall population proportions and ratios, which tend to mute population variability. The hazard model is premised on the fact that all skeletal assemblages are comprised of the frailest individuals (i.e., the dead), so these skeletons reflect the least resilient (i.e., most susceptible to death) among their age and sex cohorts (Usher, 2000). Consequently, the pathological lesions and general demographics from these skeletal collections may be statistically analyzed to determine which variables are associated with a higher risk of mortality. In this way, hazard models mitigate concerns of selective mortality and individual heterogeneity, while defining frailty in terms of highest risk of mortality (DeWitte, 2010; Wilson, 2014).
Life-history approaches to examining health in archaeological populations have not been adapted as yet into a comprehensive, quantitative model considering multiple stressors simultaneously. At an individual level, osteobiographical accounts provide the most comprehensive study of individual health by gathering together and interpreting all skeletal variables in reference to each other within a rich archaeological, historical, and cultural context (Stodder & Palkovich, 2012). However, while these case studies allow researchers to understand the relationships between stress biomarkers in a single person, it remains difficult to compare these extensive individual life-histories on a broader population level. Recent work, using newly adopted techniques and data that facilitate comparisons of longitudinal stress between individuals (e.g., stable isotope analyses and dental microstructural studies), have demonstrated the strength and depth of knowledge to be gained from merging life history and population approaches (Fitzgerald, Saunders, Bondioli, & Macchiarelli, 2006; Sandberg, Sponheimer, Lee-Thorp, & Van Gerven, 2014).
Drawing from research in human biology, which routinely utilizes multiple biomarkers of stress in the models of AL and frailty, we propose a model of stress (skeletal frailty index [SFI]), composed of multiple standardized indicators of stress frequently observed and assessed in bioarchaeological research, to evaluate frailty in past human populations. The SFI is inspired by previously developed (Goodman et al., 1984, 1988; Goodman & Martin, 2002) and widely used (Paine, Vargiu, Signoretti, & Coppa, 2009; Redfern & DeWitte, 2011; Steckel, Rose, Larsen, & Walker, 2002) skeletal health indices in bioarchaeology. Merging the strengths of osteobiographical life-history and populational approaches, this index establishes a quantitative assessment of frailty for each individual in a population, which may then be compared between individuals and across sex, age, and social settings to help establish an overall portrait of population frailty. Unlike the statistical practice of gross prevalence, which evaluates skeletal lesions separately, the SFI model accumulates multiple biomarkers of stress for an individual into a single frailty construct. The frailty score does not reflect susceptibility to death, a definition applied to current bioarchaeological translations and approaches to frailty. Rather, the index is modeled on biological and epidemiological concepts of frailty in living human individuals (Fried et al., 2001, 2009). Through this theoretical lens, frailty is studied as a fluctuating physical state based on exposures to and survival from diseases and other stressors. Those individuals with greater resilience to stressors live longer by surviving multiple stressors over their lifetimes. At the same time, this increased resilience to stressors often comes at the price of increased frailty, that is, a morbidity-mortality paradox similar to those described for men and women in many modern populations (Crimmins, 2001; Kulminski et al., 2008; Oksuzyan et al., 2008). By implementing a similar definition of frailty into skeletal stress research, the SFI is intended to facilitate comparisons and discussions of health and stress as observed in the living by human biologists and the dead by bioarchaeologists.
The proposed SFI follows past models indexing skeletal health and stress using both dental and whole body assessments (e.g., Cohen & Armelagos, 1984; Goodman & Martin, 2002; Larsen, 2003, 2006; Pechenkina & Delgado, 2006; Steckel, Kjellström, Wittwer-Backofen, & Engel, 2009) by directly relating these biomarkers to aspects of frailty in living people and building an index therefrom. The Museum of London (MoL) Centre for Human Bioarchaeology's (CHB) Human Osteology Method Statement and Global History of Health Project (GHHP), for example, have both constructed robust frameworks for such assessments, considering a variety of skeletal indicators associated with health among the living, including cribra orbitalia, porotic hyperostosis, trauma, and non-specific periosteal lesions (Goodman & Martin, 2002; Powers, 2012; Steckel et al., 2005). The GHHP catalogs information about individual skeletons and their relative health in comparison to other individuals from the same population, codifying information such that it may be compared both cross-culturally and through time. Nevertheless, how these data have been presented—mainly as gross prevalences and discrete skeletal lesions—may underscore the importance of a life-history model, which recognizes interactions between pathological lesions and focalizes on individual stress (Stodder & Palkovich, 2012; Williamson & Pfeiffer, 2003). Garnering standards from the MoL Center for Human Bioarchaeology Database and GHHP suites of skeletal stress biomarkers, our model develops a cumulative score from these variables for each individual within a population. Subsequently, we analyze these scores and their distributions in two samples. We present the SFI as a possible tool for bioarchaeologists to compare frailty levels between individuals of different age cohorts, sexes, and socioeconomic classes within and among populations.
2 Stress
This model of skeletal frailty is premised on the idea that studying dental and skeletal markers associated with various degenerative conditions and/or pathologies does not lead us to a direct assessment of illness so much as it results in a tally of skeletal stress markers serving as proxies for health. Health is a construction of biological, social, and cultural factors, so it always is a latent variable within any study of the living or non-living. Under this premise, our model utilizes recent advances made in stress research among the living to help inform studies of past populations (Goodman et al., 1984; Goodman & Martin, 2002). Capitalizing on the model's utility requires a coherent definition of stress and a method for assessing effects of stress on the skeleton comparable to those indices being used among the living.
To date, the most useful definitions identify stress in living individuals as a continuous internal process rather than as an object (e.g., an external or internal pressure or change). Proponents of this viewpoint suggest stress is an ongoing process through which organisms respond to risk factors (stressors) while utilizing somatic resources to resist deleterious outcomes (stress responses) to the best of their abilities (Crews, 2007; Crews et al., 2012; Sterling & Eyer, 1988). Walter Cannon (1932) was among the first researchers to identify stress as a process when he recognized the role of the sympathoadrenal system in coordinating “fight or flight” responses (see Johnson, Kamilaris, Chrousos, & Gold, 1992), a concept later expanded by Hans Selye (1936). Modern viewpoints conceptualize stress as a coordinated process involving integrated responses from the neuroendocrine, central nervous, and other physiological systems coping with psychosocial, environmental, and physical stressors which challenge somatic homeostasis (Johnson et al., 1992; McEwen, 2000b; Schulkin, 2004; Sterling, 2004).
3 Measuring Stress
Among living populations, three primary approaches to measuring stress can be identified: environmental, psychological, and biological/physiological. Based on individuals' identifications of key life events and perceptions of the relative impacts of these events, both environmental and psychological measures of stress require communication with study participants and therefore have little possibility of being operationalized among archaeological populations. However, measuring stress using solely physiological and biomedical measurements such as frailty requires no input from participants. Given that frailty is accessible to and frequently utilized by bioarchaeologists, we argue that a modeled approach to frailty will provide a novel and accurate means for assessing outcomes of life-long cumulative stress in skeletal samples.
Building on definitions of stress emphasizing somatic reactions to stimuli (McEwen & Stellar, 1993; Seeman et al., 1997; Schulkin, 2004), measures using frailty evaluate changes in physiological systems of our somas following repeated responses to stressors over the lifespan. Proposed physiological approaches to measuring stress focus either on associations between stress and an individual biomarker (e.g., cortisol, see Goldman et al., 2005; Glover, 2006; Strahler et al., 2010) or the association between stress and an index measure encompassing several biomarkers (e.g. AL, see Karlamangla, Singer, McEwen, Rowe, & Seeman, 2002; Karlamangla, Singer, & Seeman, 2006; Kusano et al., 2016; Maselko, Kubzansky, Kawachi, Seeman, & Berkman, 2007; Seeman et al., 1997).
3.1 Index biomarker assessments
3.1.1 Frailty in living human populations
Frailty, as studied in living humans, is considered a phenotype reflecting decreased reserve capacity and reduced resistance to stressors, following cumulative declines across multiple physiologic systems (Fried et al., 2001; Walston, 2004, 2005). Today, the most widely accepted definition proposes frailty as a “biologic syndrome of decreased reserve and resistance to stressors, resulting from cumulative declines across multiple physiologic systems, and causing vulnerability to adverse outcomes” (Fried et al., 2001: M146). Higher frailty results from functional declines across multiple physiological systems and their synergistic interactions as altered systems express a variety of frail and non-frail phenotypes (Fried et al., 2001). Frailty is highly individualistic as well as independent of chronic disease morbidity and chronological age (Fried et al., 2009). Additionally, frailty is useful for assessing individuals' current functional capacity and susceptibility to future adverse outcomes (Crews, 2005, 2007).
In living human populations, frailty is operationalized as an index measure that assesses multiple aspects of functional decline at the somatic level. As research into stress has shown, measures that simultaneously assess multiple biomarkers consistently outperform measures of individual biomarkers when assessing stress (Goldman et al., 2005; Glover et al., 2006; Maselko et al., 2007; Seeman et al., 2001; Schulkin, 2004; see also Leahy & Crews, 2012 for a recent review). A “Frailty Index,” comprising 70 deficits observable during clinical examination, has also been proposed to identify frail individuals (Rockwood et al., 2007). These frailty indices have been shown to predict vulnerability to disability (Fried et al., 1998, 2001; Walston et al., 2006; Xue, Walston, Fried, & Beamer, 2011), decreased mobility (Fried et al., 2001), reduced capacity to complete activities of daily living (ADLs) (Fried et al., 2001), falls (Xue et al., 2011), need for long-term care, and mortality (Fried, Ferrucci, Darer, Williamson, & Anderson, 2004) in modern samples. Proposed markers of the “frailty phenotype” include atrophy (sarcopenia, leading to loss of strength, and weight loss), loss of endurance, decreased balance and mobility, slower performance, relative inactivity, and decreases in cognitive function (Fried et al., 2001, 2004, 2005). Other authors have proposed difficulty in completing ADLs/IADLs (ADL/Instrumental ADL), incontinence, mobility, and unintentional weight loss as indicators of frailty (Rockwood et al., 1999; Crews, 2007). Frailty may be calculated, as is AL, by summing the number of components for which an individual's values are in the highest risk quartile. This may be the bottom (e.g., low albumin) or top (e.g., glucose, triglycerides) quartile for respective parameters (Edes, Wolfe, & Crews, in press). Frailty scores also may include as few as five biomarkers (slow walking speed, weakness, weight loss, low activity, and fatigue) (Walston et al., 2006) while other frailty indices include upwards of 70 variables (Rockwood et al., 2005).
3.1.2 Skeletal frailty in past human populations
Currently, frailty is promoted as an assessment for evaluating influences of lifelong stress on health and disease risks in living populations and measuring loss of somatic function secondary to sarcopenia and somatic wasting. Unfortunately, hormonal, physiological, and functional measures incorporated into frailty indices for the living are not represented in archaeological assemblages, whether dealing with complete or incomplete skeletal materials. Perhaps this difficulty has precluded bioarchaeologists from proposing indices of skeletal health modeled on frailty indices for living samples. However, the model of skeletal frailty presented herein overcomes this pitfall by drawing on previous research linking skeletal markers to their etiologies in the living and focusing on markers which mimic aspects of frailty measured among modern samples (Goodman et al., 1984; Goodman & Martin, 2002). By studying skeletal frailty as a syndrome among the living, and not as a measure of increased susceptibility to death, we acknowledge the dual meaning of skeletal lesions both as representations of survival from past stressor events (resilience) and indicators of ongoing and cumulative somatic stress at the time of death.
Our proposed index builds on previously proposed determinants of skeletal health (Goodman et al., 1984, 1988; Goodman & Martin, 2002), by uniquely analyzing and interpreting these biomarkers within a model for stress/health applied to living human populations: frailty. By studying frailty as a syndrome in archaeological populations (as it is in living populations), and not exclusively as a measure of increased susceptibility to death, we evaluate skeletal lesions in terms of morbidity and establish frailty in archaeological populations as a variable state of cumulative stress. Of primary importance is whether components commonly used to assess frailty in the living leave permanent alterations on the skeleton. While none of the hormonal indicators of somatic dysregulation skeletonize, some key skeletal and dental indicators of stress are useful for our purpose (Steckel et al., 2002, 2009). For example, dental enamel hypoplasias may indicate chronic childhood stress; fractures and other traumatic lesions capture interpersonal as well as accidental stress events; and stature consistently proves to be a robust indicator of overall health during periods of childhood and adolescent growth (Bailey et al., 1984; Bogin & MacVean, 1983; Friedman & McEwen, 2004; Goodman & Rose, 1991; Griffin & Donlon, 2007; Larsen, 1997, 2015; May, Goodman, & Meindl, 1993; McEwen, 2000b; Steckel et al., 2002). Additional skeletal indicators of daily wear-and-tear are observed in archaeological human remains. For example, slow walking speed is often, although not ubiquitously, associated with osteoarthritis and degenerative joint disease (DJD) during life (Ling & Bathon, 1998). Muscle strength, physical activity, and sarcopenia may be reflected by osteoporosis and overall robusticity of long bones (Fried et al., 2001; Siris et al., 2001). When an individual is immunocompromised, the body is more susceptible to chronic inflammation, which may manifest skeletally as periosteal new bone (PNB) growth or periodontal disease (DeWitte & Bekvalac, 2011). Certain morbid conditions, including infectious diseases and some forms of anemia are reflected in the skeleton as non-specific periosteal lesions and cribra orbitalia/porotic hyperostosis, respectively (Eyre-Brook, 1984; Simpson, 1985; Walker et al., 2009).
3.1.3 Operationalizing skeletal frailty
Drawing from frailty models, which consider multiple somatic stress responses, the proposed SFI considers 13 cumulative and active skeletal biomarkers of stress within four broad categories (Table 1): growth disruption, nutritional deficiency and infection, activity, and trauma. Early childhood stressors, which manifest as disrupted skeletal and dental growth, often leave individuals immunologically compromised and susceptible to infection in their adult years (McDade, 2003). Compounded with childhood physiological burdens are the stressors perpetually placed on the body by malnutrition and infection, when the body reallocates energy away from normal maintenance (Scrimshaw, 2003). The stress of physical activity also eventuates in non-reversible wear-and-tear to the soma (specifically joints), which restricts mobility and overall movement (Waldron, 2009). Finally, traumatic episodes result in lesions that add further strain to the body throughout the healing and recovery process (Ortner, 2003). Echoing scoring standards for living humans (e.g., AL), all frailty variables are scored as “0” or “1” according to stress impact. Cut-points for high risk quartiles are determined based on the sample population distribution because patterns of growth, development, and senescence vary substantially within and among populations in an age-independent, individualistic, and multifactorial fashion (Arking, 2006; Crews, 2007). If, relative to the whole population, a datum (e.g., osteoarthritis, stature) falls within the quartile or category associated with highest risk/stress, an individual is scored “1” for this variable, while all other values receive a “0” (see Seeman et al., 1997; Vidovic, Sharron, & Crews, 2015). For each individual, these scores are summed for all 13 variables, resulting in a possible SFI between 0 and 13.
| Stress category | Frailty variables | Scores and measurements | Frailty score “1” |
|---|---|---|---|
| Growth | Femoral Lengtha-d | Lengths in quadrants | Shortest lengths (¼)* |
| Femoral Head Diametere | Diameters in quadrants | Smallest diameters (¼)** | |
| Linear Enamel Hypoplasiaf-i | Present/absent | Present | |
| Nutrition and Infection | Periostitis/Osteomyelitisj-l | Active, healing, absent | Active |
| Periodontal Diseasei, m, n | Present/absent | Present | |
| Porotic hyperostosis (PH)/Cribra Orbitalia (CO)o, p | Present/absent | Present | |
| Rickets/Osteomalaciaq, r | Present/absent | Present | |
| Neoplasms | Present/absent | Present | |
| Osteoporosis | Present/absent | Present | |
| Activity | Osteoarthritiss-u | Present/absent | Present |
| Intervertebral Disc Disease (IVD)n, v | Present/absent | Present | |
| Rotator Cuff Disorder (RCD)n | Present/absent | Present | |
| Trauma | Fracturew-y | Present/absent | Present |
- Sources.
- a Bogin and MacVean (1983).
- b Bogin et al. (1989).
- c Saunders and Hoppa (1993).
- d Steckel (1995).
- e Ruff et al. (1997).
- f Goodman and Armelagos (1984).
- g Goodman et al. (1991).
- h Goodman et al. (1992).
- i Hillson (1996).
- j DeWitte (2014).
- k Ortner (2003).
- l Ortner and Putschar (1985).
- m Hillson (2014).
- n Waldron (2009).
- o Stuart-Macadam (1992).
- p Walker et al. (2009).
- q Mays, Brickley, and Ives (2006).
- r Reginato and Coquia (2003).
- s Anderson and Loeser (2010).
- t Jurmain (1980).
- u Loughlin (2001).
- v Walker (2012).
- w Lambert (1997).
- x Milner and Smith (1990).
- y Walker (1989).
- *Male femoral length quartiles: x ≤ 447 mm, 447 mm ≤ x < 461 mm, 461 mm ≤ x < 475 mm, x ≥ 475 mm; female femoral length quartiles: x ≤ 414 mm, 414 mm ≤ x < 434 mm, 434 mm ≤ x < 443 mm, x ≥ 443 mm (highest frailty measurements underlined).
- **Male femoral head diameter quartiles: x ≤ 46.7 mm, 46.7 mm ≤ x < 48.4 mm, 48.4 mm ≤ x < 49.9 mm, x ≥ 49.9 mm; female femoral head diameter quartiles: x ≤ 41.2 mm, 41.2 mm ≤ x < 42.7 mm, 42.7 mm ≤ x < 45.4 mm, x ≥ 45.4 mm (highest frailty measurements underlined).
3.1.3.1 Femoral length
Stature is directly affected by nutritional and environmental stressors in childhood and is acknowledged to be an indicator of general adulthood well-being (e.g., Bailey et al., 1984; Bogin & MacVean, 1983; Steckel et al., 2002). Undernourishment during childhood results in shorter stature in adulthood as compared to individuals who receive adequate nourishment during growth periods (Bailey et al., 1984). This discrepancy is consistently observed despite undernourished children experiencing adolescent growth periods (catch-up growth) up to a year longer than adequately nourished children (Bailey et al., 1984). Studies among modern samples suggest the long-term consequences of compromised longitudinal growth, with short-statured individuals suffering poorer health and increased morbidity in adult years relative to their taller contemporaries (Cameron & Demerath, 2002; Cameron, 2007). As it remains difficult to reconstruct stature in past populations, due to the disarticulated nature of most skeletons, femoral length provides a single element proxy for longitudinal growth (Ruff, 2003; Steckel et al., 2002). Due to disparities in longitudinal growth between males and females, the confounding variable of sexual dimorphism is avoided by comparing femoral lengths within sexes only. For the SFI, sex must be estimated from pelvic and cranial morphological characteristics (Buikstra & Ubelaker, 1994), and not long bone dimensions, at the risk of confounding sex estimations with long bone longitudinal growth and robusticity components.
3.1.3.2 Maximum femoral head diameter
Similar to height, bone robusticity provides information on growth patterns, reportedly indicating body weight, diet, physiological health, work and activity patterns, and mobility (Ruff, Trinkaus, & Holliday, 1997; Steckel et al., 2002). Robusticity, as a proxy for several indicators of frailty among modern populations, approximates walking performance, physical activity, and strength (Fried et al., 2001). These indicators, included in evaluations of frailty among modern populations, have been shown to predict vulnerability to disability (Fried et al., 1998, 2001; Walston et al., 2006; Xue et al., 2011), decreased mobility (Fried et al., 2001), reduced capacity to complete ADLs (Fried et al., 2001), falls (Xue et al., 2011), need for long-term care, and mortality (Fried et al., 2004). This frailty index implements femoral head diameter as a simplified variable for body robusticity. Previous studies (Ruff et al., 1997; Stock & Shaw, 2007) demonstrate the direct correlation between body mass and femoral head diameter. As the femoral head often preserves in human archaeological skeletal populations, the maximum diameter presents a recoverable and measurable variable for the SFI. However, femoral head diameters often represent a sexually dimorphic variable within populations, so individuals should be separated according to sex and scored relative to quartiles established for males and females.
3.1.3.3 Linear enamel hypoplasias
The secretory phase of amelogenesis can be disrupted in such a way as to lead to reductions in enamel thickness, for example, linear enamel hypoplasias (LEH) (Hubbard, Guatelli-Steinberg, & Sciulli, 2009; Guatelli-Steinberg, 2015). Across animals and humans, the function of ameloblasts, cells which secrete a protein matrix into which mineral is embedded, is often reduced or impaired during periods of physiological stress, and, notably, malnutrition (Goodman & Rose, 1991; Hillson, 1996; May et al., 1993). In addition to nutritional stress and malnutrition, studies among modern humans and non-human primates associate formation of enamel hypoplasias with systemic disturbances, metabolic stress, neonatal disruption, and life history events, including birth, parturition, and social stress (Bowman, 1991; Dobney, et al., 2004; Hillson, 1996; Kreshover & Clough, 1953; Lukacs, 2001; Pindborg, 1982; Skinner & Hung, 1989; Suckling, Elliot, & Thurley, 1983, 1986; Suckling & Thurley, 1984). Rarely have LEH been attributed to genetic predisposition (Goodman & Rose, 1991); instead, they reflect general levels of physiological stress experienced by specific populations and levels of systemic poor health (Armelagos, et al., 2009; Griffin & Donlon, 2007; Skinner & Goodman, 1992; Yaussy, DeWitte, & Redfern, 2016).
3.1.3.4 Non-specific periosteal lesions/osteomyelitis
Non-specific PNB manifests as skeletal lesions implicating the periosteum, followed by cortical bone and, in extreme cases, the medullary cavity (Larsen, 1997, 2015; Ortner, 2003). Lesions are characterized as osseous plaques with demarcated margins or irregular elevations of bone surfaces (Aufderheide & Rodriguez-Martin, 1998). When stimulated by infection or injury, osteoblasts lining the subperiosteum quickly create unorganized woven bone along the periosteal surface (Eyre-Brook, 1984; Simpson, 1985). Recent studies have suggested active (woven) rather than healing (lamellar) PNB, in particular, to be associated with higher risk of mortality (DeWitte, 2014). Osteomyelitis is the extension of lesions resulting from infection/injury to endosteal and periosteal bone surfaces, resulting in a restricted in diameter of the medullary cavity (Larsen, 1997, 2015). When not associated with bone trauma (e.g., healing fractures), osteomyelitis is generally the result of localized infection induced by exogenous bacteria, e.g., Staphylococcus aureus (Waldron, 2009). These basic inflammatory responses are also attributed to leprosy, tuberculosis, and treponemal disease (Larsen, 1997, 2015; Ortner, 2003). As with active PNB, osteomyelitis is an indicator of an individuals' physiological stress at the time of death, representing infectious morbidity or the physiological burden of responding to trauma. These direct somatic responses to a stressor parallel the modeled mechanism for allostasis, whereby the body is confronted with an insult to which it must respond quickly to reestablish homeostasis (Beckie, 2012; Edes et al., in press). Active, disorganized periosteal bone reflects a stressful event to the soma in which the individual did not completely recover, likely indicating an immunologically-compromised, dysfunctional state of health.
3.1.3.5 Periodontal disease
Periodontal disease results in horizontal and vertical alveolar resorption and is associated with a chronically compromised immune system (Hillson 1996, 2014). When untreated, periodontal disease leads to significant bone and tooth loss, eventuating in edentulousness. Among living populations, periodontal disease has been shown to correlate significantly with cardiovascular mortality (Ajwani, Mattila, Tilvis, & Ainamo, 2003; DeStefano et al., 1993). DeWitte and Bekvalac (2011) confirmed the severity of periodontal infection among Medieval London populations, demonstrating higher risk of mortality among individuals with diagnosed periodontitis.
3.1.3.6 Porotic hyperostosis/cribra orbitalia
Identified as an area of pitting and porosity on the orbital roofs and external surface of the cranial vault, cribra orbitalia, and porotic hyperostosis, respectively, are among the most commonly reported pathological conditions in archaeological collections (Walker, Bathurst, Richman, Gjerdrum, & Andrushko, 2009). Generally thought to be a result of marrow hyperplasia, cribra orbitalia and porotic hyperostosis are etiologically related to anemias linked to significant and sustained increase in red blood cell production (Walker et al., 2009) as well as metabolic diseases such as scurvy, rickets, syphilis, and cancer. Similar lesions can also result from vitamin D deficiency, chronic scalp infections, and other infectious diseases (Lewis, 2007; Ortner, 2003). Both have been documented in a variety of archaeological contexts, and often are used to assess the health and nutritional status of these past populations (e.g., Cohen & Armelagos, 1984; El-Najjar, Ryan, Turner, & Lozoff, 1976; El-Najjar & Robertson, 1976; Mittler & van Gerven, 1994; Pechenkina, Benfer, & Zhijun, 2002; Steckel, Rose, Larsen, & Walker, 2002; Walker, 1985, 1986). Both are included in the model of skeletal frailty as indicators of physiological stress caused by increased disease burden and disease itself.
3.1.3.7 Rickets/osteomalacia
Rickets (osteomalacia for adults) represents the skeletal manifestation of chronic vitamin D deficiency during formative juvenile and sometimes adult years. Vitamin D is an essential element for the transformation of more amorphous osteoid into more rigid osteon structures (Ortner, 2003). When a deficiency of vitamin D exists, juvenile bones especially are more malleable and bend under body weight, resulting in rickets' characteristic bowing of femora and tibiae (Aufderheide & Rodriguez-Martin, 1998). While this condition may be rectified through an introduction of adequate vitamin D, leading to femoral and tibial shaft lengths within population averages, chronic rickets may cause irreversible changes to the morphological structure of lower limb bones and, consequently, the entire skeleton (Waldron, 2009). These morphological changes to the skeleton often result in retarded growth and muscle weakness, which inevitably affect limb movement and functionality (Hollick, 2006).
3.1.3.8 Neoplasms
Neoplasms, new, uncontrolled growths within the body, present either a long- or short-term stress to the soma. Although most neoplasms affect soft tissue and may not present skeletally, secondary metastases from malignant tumors arise, and may be observed, throughout the skeleton. Sclerotic and lytic lesions throughout the axillary skeleton, for example, may attest to progressive lung, breast, or prostate cancer, conditions which have prevailed throughout populations in history (Aufderheide & Rodriguez-Martin, 1998; Waldron, 2009). While button osteomas may only produce aesthetically-jeopardizing effects, neoplasms, such as metastasized ovarian cancer, seriously compromise an individual's daily movements and activities, consequences which may culminate in social and economic problems.
3.1.3.9 Osteoporosis
Osteoporosis is a metabolic condition defined by the loss of bone density and characteristically is associated with menopause, extreme exercise, and general deteriorative aging. Individuals suffering from osteoporosis, and its incipient condition osteopenia, suffer from skeletal frailty, which heightens risks of non-traumatic and minimally traumatic fractures (Brickley & Ives, 2009). As increased vulnerability to fractures is associated with falls and decreased mobility, osteoporosis correlates with high frailty and mortality in modern populations (Siris et al., 2001). Albeit considered a condition of modern populations, bioarchaeological studies have demonstrated the timelessness of osteoporosis throughout human history, discrediting osteoporosis as a disease of modernity (Agarwal, 2008). Consequently, osteoporosis, functioning as a proxy for strength and physical activity, serves as a direct marker for frailty in both modern and archaeological samples (Fried et al., 2001).
3.1.3.10 Osteoarthritis
Bone modifications indicative of osteoarthritis/DJD are associated with repetitive motions and/or old age. While we recognize that some individuals with osteoarthritis are asymptomatic, other individuals with this affliction suffer from pain and stiffness in the joints, limited mobility, and decreased flexibility (Ling & Bathon, 1998). This marker is included in the model of skeletal frailty because these symptoms suggest individuals with decreased mobility (Fried et al., 2001) and reduced capacity to complete ADLs (Fried et al., 2001), both of which are associated significantly with higher levels of frailty.
3.1.3.11 Intervertebral disc disease
Intervertebral disc disease (IVD) is diagnosed by the gradual deterioration of vertebral centra, ultimately bringing about ankylosis, or fusion, of vertebrae. Continuous movement on the back initially irritates and inflames the outer portion of the vertebral disc, the annulus pulposus (Waldron, 2009). Over time, severe inflammation and degeneration (normal or pathological) of the vertebral discs results in macroporosity of the centra and bony growth (marginal osteophytes) along the central rims (Roberts, Evans, Trivedi, & Menage, 2006). This process eventuates in compression of vertebral bodies and related neural and vascular symptoms (Gilchrist, Slipman, & Bhagia, 2002; Waldron, 2009). As with osteoarthritis, although presenting similar effects from a differing etiology, IVD directly impacts an individual's ability to engage in daily activities.
3.1.3.12 Rotator cuff disorder
The rotator cuff is composed of four muscles—subscapularis, supraspinatous, infraspinatous, and teres minor muscles—stabilizing the humeral head within the shoulder joint. Through decades of use, the tendons of these muscles become strained and weakened. Among modern populations, by the seventh decade of the life, twenty-five percent of individuals suffer from rotator cuff disorder (RCD), and this percentage increases to fifty percent by the ninth decade (Tashjian, 2012). In archaeological populations, RCD is a common pathological lesion observed among even younger age cohorts, due to the continuous stress placed on joints during daily labor (Waldron, 2009). RCD manifests as active porosity and marginal osteophytes on the humeral and scapular muscle insertions of these muscles, contrasting with osteoarthritis, whose inflammatory condition affects the joint capsule and surface independent of RCD. As an irreversible condition, the presence of this disease would only exacerbate with years and result in decreased movement or immobilization of the shoulder joint and arm.
3.1.3.13 Fractures/trauma
Individuals who have experienced psychosocial trauma, including veterans (Groer & Burns, 2009), mothers of pediatric cancer patients (Glover et al., 2006), and individuals with PTSD (Friedman & McEwen, 2004), are more likely to have higher stress than individuals who have not experienced a comparable form of trauma (Friedman & McEwen, 2004; McEwen, 2000b). As it is not possible to observe the effects of psychosocial stressors on the skeleton, we incorporate physical trauma (e.g., fractures, projectile injuries, puncture wounds, and surgical operations such as trepanations) into the skeletal frailty model. This is based upon the hypothesis that physical trauma instigates as much, or more, physiological stress response as do psychosocial stressors (Schulkin, 2004; Sterling, 2004).
3.1.4 SFI applicability and limitations
The SFI allows us to compare individual frailty scores, thereby providing an estimated distribution of prior morbid events by sex and age cohorts. By contrast, gross prevalence values exclusively compare percentages of affected individuals relative to a total sample. Individuals within a sample are not treated discretely using a gross prevalence analysis, as there is no comprehensive evaluation of health for each individual skeleton. Rather, conditions are tallied for overall sample prevalences. Such approaches may miss subtle and demonstrative variation in frailty across a population.
As with previous methodological, statistical, and theoretical approaches to studying health and stress in human skeletal remains, the SFI poses inherent shortcomings. An immediate dilemma recognized by any bioarchaeologist is the inescapable issue of skeletal preservation and context. Most bioarchaeologists are presented with highly decomposed or decontextualized skeletal remains either in the field or among previously excavated material. Commingled remains, for example, would be precluded from SFI analysis. Even with well-preserved populations, a large portion of the population may be excluded based on the absence of skeletal elements. In these cases, it may be advisable for bioarchaeologists to establish site-specific SFI based on observed preservation. Consequently, with this new method, interpopulation comparisons would only be possible when SFI include identical frailty biomarkers.
Selecting and quantifying skeletal frailty biomarkers raises additional issues requiring further bioarchaeological discussion. While we have presented all biomarkers as proxies for physiological decline or dysregulation, some biomarkers, for example LEH, have been argued to be evidence of relatively lower frailty. Among the Medieval Danish populations of Æbelholt and Næstved, higher prevalences of LEH were observed among the older subadult (6.6–20.0 years; 0.45) age than younger age (0–6.5 years; 0.024) cohorts, suggesting that individuals with LEH lesions maintained sufficient immunomodulatory capabilities to overcome the related period of chronic childhood stress; thus, higher LEH prevalence within this specific population was theorized to be predictive of decreased frailty (Bennike, Lewis, Schutkowki, & Valentin, 2005; Wood et al., 1992). These data fit the frailty model, followed here, whereby LEH (and other skeletal lesions) may suggest greater resilience, and this is confirmed by our SFI findings among monastic and nonmonastic skeletal series.
Of concern to bioarchaeologists may be how we quantify pathological lesions into a relatively high risk (1) and other (0) categories, as well as our decision not to weigh biomarkers within the individual SFI composite. For example, a high risk “1” score for trauma will be used to describe one individual's fractured hip that may have followed an accidental fall, but also it is used to describe another individual's multiple concussive fractures to the cranium. Both individuals must have diverted resources from general body functions toward survival recovery mechanisms. The relative degree of physiological and psychological stress sustained by one fracture is very different from the stress experienced by another individual enduring multiple, repetitive strikes to the head. The latter may have been living in an environment where interpersonal violence was endemic, and, unfortunately, this stress can neither be evaluated in the skeleton nor discriminated from accidental traumas in the SFI. As with criticisms of AL biomarkers, that no current scales exist for accurately assigning weights, bioarchaeologists may debate our equal weighings of frailty biomarkers (Goldman, Turra, Glei, Lin, & Weinstein, 2006). Among the nutrition- and infection-related biomarkers, for instance, non-specific active PNB and malignant neoplasms receive equal weight in the total SFI score. Active periosteal bone often is associated with systemic or localized infection, but even simple insults to the body (e.g., bump on the leg or strain to the ankle) may incite a periosteal reaction. Relative to the impacts malignant tumors have on the soma, these periosteal flare-ups arguably have a smaller effect on overall somatic health. For this reason, it may be proposed that malignant tumors or bacterial infections like tuberculosis make a greater contribution to the SFI. However, the individual died while these lesions were active, so it is wrong to completely rule out the effects of PNB on an individual's death. At present, we offer an equally-weighted comparative SFI as an option and springboard for others to conceptually approach and develop a multifactorial evaluation of skeletal health and frailty.
4 Case Study: Frailty in Monastic and Nonmonastic Medieval (1000–1500 CE) London Populations
In a recent article by DeWitte et al. (2013), monastic and nonmonastic populations in Medieval London were evaluated through hazard models to determine risk of mortality associated with these differing social environments. Results demonstrated a significant correlation between monastic lifestyles and decreased risk of mortality, a correlation researchers (2013) attributed to the living conditions and resources available to the religious community. Applying the frailty index to these populations provides complementary information about the relative morbidity of these discrete communities.
4.1 Materials
Skeletal data were obtained from the open-access MoL Wellcome Osteological Research Database, WORD (http://archive.museumoflondon.org.uk/Centre-for-Human-Bioarchaeology/Database/). Therefore, the authors did not diagnose and assign growth, nutritional/infectious, activity-related, and traumatic pathological lesions for this study, but assembled data observed and published by researchers and specialists at the MoL's CHB. This laboratory uses the Human Osteology Method Statement (Powers, 2012) recording criteria, which utilize established skeletal and dental standards (Bass, 1987; Brothwell, 1981; Buikstra & Ubelaker, 1994; Hillson, 1996) to estimate age and sex and evaluate pathological conditions. For the current study, all published adult age categories, sex estimations, and pathological traits from the CHB's WORD database were maintained throughout the analysis and discussion of variables.
In accordance with DeWitte et al.' (2013) study, the same monastic and nonmonastic populations were included in this study. However, only well-preserved skeletons—skeletons where all 13 SFI biomarkers could be observed—were selected for this case study, substantially reducing the sample sizes analyzed by DeWitte et al. From the 528 individuals in monastic contexts, 70 skeletons met the SFI preservation criteria: St. Merton's Priory (N = 40) and Bermondsey Abbey (N = 34). Sixty-four of the 368 individuals in nonmonastic collections were selected for examination: Guildhall Yard (N = 12), Spital Square (N = 13), St. Mary Graces (N = 33), and St. Benet Sherehog (N = 2).
4.2 Methods
For every individual, the thirteen skeletal and dental frailty biomarkers were scored according to SFI criteria. All variables were combined into a total SFI score (0–13). Subsequent analysis of variance (ANOVA) and analysis of covariance (ANCOVA) tests were conducted on frailty scores with monastic/lay lifestyle, sex, and age as independent variables and covariates in SPSS 20.
4.2.1 Scoring variables in the SFI
4.2.1.1 Growth
Skeletal growth perturbations capture infant and juvenile periods of stress (Bogin, Sullivan, Hauspie, & MacVean, 1989). Within the skeleton, these growth disruptions present as short stature, skeletal robusticity, and LEH, three variables inversely correlated with mortality risk (Goodman & Armelagos, 1985; Goodman et al., 1991; Pelletier, 1994; Ruff et al., 1997). As short stature and diminutive body size often reflect compromised growth in childhood years, maximum femoral length (proxy for stature) and femoral head diameter (proxy for body mass/skeletal robusticity) in the lowest data quartile were assigned “1” frailty scores, and all others “0.” As adulthood antemortem tooth loss and caries may limit the observed number of teeth within an individual, LEH were scored for frailty as present (1)—regardless of number per individual—or absent (0).
4.2.1.2 Nutrition and infection
Nutritional deprivation inherently affects an individual's susceptibility to disease, weakening immunological response to non-specific infection and disease (McDade, 2003; Scrimshaw et al., 1968). In the skeleton, the body's response to infection or disease stressors may include such bony changes as non-specific periosteal lesions (i.e., PNB), osteomyelitis, and periodontal disease (DeWitte & Bekvalac, 2011; Waldron, 2009). Recent studies have suggested active (woven) rather than healing (lamellar) PNB is associated with higher risk of mortality, so only active PNB was scored “1” while healing and no PBN were assigned “0” values. By contrast, periodontal disease and other nutritional (porotic hyperostosis/cribra orbitalia, and rickets/osteomalacia) and neoplastic (tumors) conditions were recorded as present (1) or absent (0). As the CHB records osteoporosis as present or absent, based upon standards from Aufderheide and Rodríguez-Martín (1998), this condition was also scored for presence (1) or absence (0).
4.2.1.3 Activity
While the etiologies of various DJD maintain significant genetic components, strenuous and repetitive labor further stresses synovial joints (Anderson & Loeser, 2010; Flores & Hochberg, 2003; Loughlin, 2001). Stress on joints may exacerbate to debilitating conditions such as osteoarthritis, IVD, and RCD. Continuous movement of these affected joints is often painful and leads to partial or complete immobilization of the joint (Waldron, 2009). Although these conditions vary in their skeletal manifestations, it is difficult to assess, for example, the severity of each respective joint disease. Therefore, frailty scores were assigned in terms of presence (1) or absence (0). While osteoarthritis may occur at joints affected by IVD and RCD, identification of osteoarthritis will not preclude these articulations (i.e., shoulders and vertebrae) from evaluation of IVD or RCD, as the etiologies of all three conditions are distinct (Adams & Roughley, 2006; Frieman, Albert, & Fenlin, 1994; Waldron, 2009). For example, the etiologies of RCD and shoulder OA are distinct, although cases of shoulders with RCD and OA have been reported in medical literature (e.g., Edwards et al., 2002), albeit uncommonly. For this reason, we have excluded neither the shoulder nor vertebrae from the OA count.
4.2.1.4 Trauma
Episodes of intentional or accidental trauma present as unhealed and healing fractures in skeletal series. Type and location of fracture may attest to instances of interpersonal violence, which suggest possible psychosocial stressors within an individual's daily environment (Knüsel & Smith, 2014; Walker, 2001). However, due to the dearth of cases of interpersonal violence within the Medieval London population under study, any fracture—regardless of location, type, or extent of healing—likely was associated with increased frailty and therefore scored as “1,” with absence of traumatic lesions scored as “0.”
5 Results
Figures 1-4 and Table 2 show frailty score distributions between monastic-nonmonastic populations, sexes, and adult age cohorts. The average SFI for the total sample (N = 134) is 3.14 (standard deviation 1.47), with individual SFI ranging from 0 to 7. ANOVA indicates a significant difference between the frailty indices of monastic (SFI = 3.42) and nonmonastic (SFI = 2.80) assemblages, a difference for which lifestyle explains nearly half of the variation (R2 = 0.44, p = .015). While no disparities were observed between sexes (SFIfemale = 2.74, SFImale = 2.99), frailty indices between age cohorts were significantly different (p = .041; Table 2). However, the relationship between age and frailty does not reflect a direct, positive correlation. Despite SFI increasing incrementally from age category 2 (26–35 years) to age category 4 (over 46 years), 2.62 to 3.71, the youngest adult cohort (18–25 years) exhibits a relatively higher SFI mean (3.00) than the subsequent age cohort. Regardless, this high SFI average within the youngest age cohort is not significant when compared with any later age cohort (Table 3). Despite the statistically insignificant decrease in frailty between first and second age cohorts, regression equations show that age accounts for 61% of observed SFI variance. Although age distributions were relatively similar in monastic and nonmonastic populations, an ANCOVA test nevertheless was performed with lifestyle as the independent variable and age as the covariate. This analysis was necessary for assessing the confounding variable of age, which almost invariably impacts the emergence and progression of frailty (Fried et al., 2001). Results from this covariate analysis still yielded a significant correlation between SFI and lifestyle (p = .058), indicating that the lifestyle model explained 4.9% of between-population frailty differences, and differential frailty between populations was not entirely the result of population age distributions.
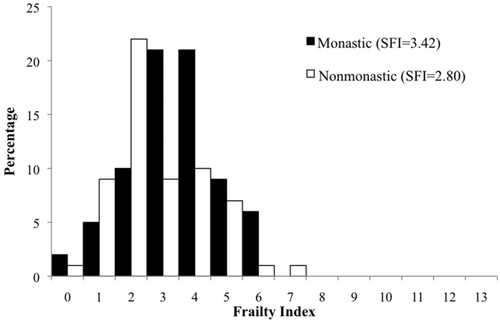
Distribution and mean values of frailty scores between monastic and nonmonastic Medieval (12th–16th centuries CE) London populations (St. Merton's Priory, Bermondsey Abbey, Guildhall Yard, Spital Square, St. Mary Graces, and St. Benet Sherehog)
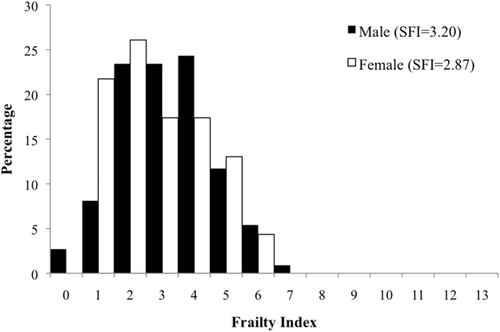
Distribution and mean values of frailty scores between sexes from Medieval (12th–16th centuries CE) London populations (St. Merton's Priory, Bermondsey Abbey, Guildhall Yard, Spital Square, St. Mary Graces, and St. Benet Sherehog)
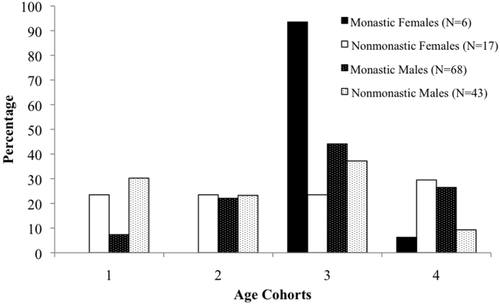
Distribution of age cohorts according to sex and cemetery from Medieval (12th–16th centuries CE) London populations (St. Merton's Priory, Bermondsey Abbey, Guildhall Yard, Spital Square, St. Mary Graces, and St. Benet Sherehog)
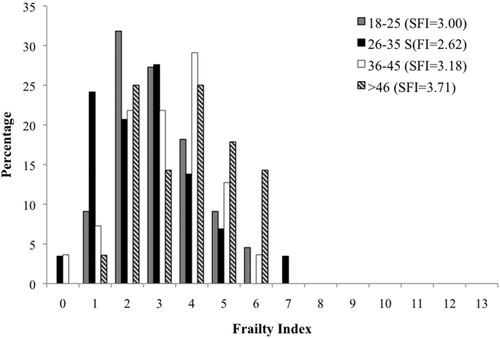
Distribution and mean values of frailty scores between adult age cohorts from Medieval (12th–16th centuries CE) London populations (St. Merton's Priory, Bermondsey Abbey, Guildhall Yard, Spital Square, St. Mary Graces, and St. Benet Sherehog)
| Independent variable | Mean frailty index (Std. deviation) | R2 | p-Value |
|---|---|---|---|
| Lifestyle | 0.44 | .015a | |
| Monastic (N = 74) | 3.42 (1.41) | ||
| Nonmonastic (N = 60) | 2.80 (1.47) | ||
| Sex | 0.007 | .33 | |
| Male (N = 111) | 3.20 (1.46) | ||
| Female (N = 23) | 2.87 (1.52) | ||
| Age (years) | 3.14 (1.47) | 0.61 | .041b |
| 18–25 (N = 22) | 3.00 (1.31) | ||
| 26–35 (N = 29) | 2.62 (1.55) | ||
| 36–45 (N = 55) | 3.18 (1.40) | ||
| >46 (N = 28) | 3.71 (1.47) | ||
| Females (N = 23) | 2.87 (1.52) | −0.132 | .853 |
| 18–25 (N = 4) | 2.75 (1.71) | ||
| 26–35 (N = 4) | 2.00 (1.41) | ||
| 36–45 (N = 9) | 3.00 (1.52) | ||
| >46 (N = 6) | 3.33 (1.29) | ||
| Males (N = 111) | 3.20 (1.46) | 0.042 | .70 |
| 18–25 (N = 18) | 3.06 (1.30) | ||
| 26–35 (N = 25) | 2.72 (1.57) | ||
| 36–45 (N = 46) | 3.22 (1.41) | ||
| >46 (N = 22) | 3.82 (1.44) |
- a Results significant at p = .05 level.
- b Results significant at p = .10 level.
| (a) | ||||
|---|---|---|---|---|
| 18–25 years | 26–35 years | 36–45 years | Over 45 years | |
| 18–25 years | 0.359 | 0.602 | 0.082b | |
| 26–35 years | 0.096b | 0.009a | ||
| 36–45 years | 0.113 | |||
| Over 45 years | ||||
| (b) | ||||
|---|---|---|---|---|
| 18–25 years | 26–35 years | 36–45 years | Over 45 years | |
| 18–25 years | 0.524 | 0.801 | 0.617 | |
| 26–35 years | 0.458 | 0.264 | 0.242 | |
| 36–45 years | 0.673 | 0.177 | 0.690 | |
| Over 45 years | 0.086b | 0.016a | 0.107 | |
- a Results significant at p = .05 level.
- b Results significant at p = .10 level.
- p-Values indicate significant differences between oldest (over 45 years) and two youngest (18–25 years and 26–35 years) age groups for both populations and male subsamples.
6 Discussion
6.1 Frailty differences between monastic and nonmonastic populations
Comparison of frailty indices for Medieval London populations demonstrates significant differences between monastic and nonmonastic populations (Figure 1). Despite increased access to higher quality foods and living amenities (e.g., sanitation and medical care) (Hatcher et al., 2006), these conditions did not buffer the monastic community from cumulative stress, although increased resources seem to have extended the years of life for individuals residing in a monastic setting relative to a nonmonastic setting (DeWitte et al., 2013). In fact, the individuals associated with monastic environments apparently exhibited higher morbidity with lower mortality. When interpreted with DeWitte et al.' evaluation of mortality risks among monastic and nonmonastic populations, these frailty indices demonstrate the paradoxical relationship between morbidity and mortality documented elsewhere for men and women in modern settings (Carey et al., 1995; Crimmins, 2001; Kulminski et al., 2008; Oksuzyan et al., 2008). Although individuals within a monastic context experienced increased longevity relative to their nonmonastic counterparts, during their longer lives they also incurred higher morbidity, that is, cumulative stress to their somas, which resulted in higher frailty scores. However, while age distributions between populations are significantly different (χ2 = 12.92, p < .01), ANCOVA results indicate that age is not the primary driving force of these SFI differences, although monastic populations are represented by higher ages-at-death. When comparing the social and ecological contexts in which monastic persons lived with those conditions of the urban (or urbanizing) lay communities in London, this observed morbidity-mortality paradox between the two samples is better contextualized. Throughout the Medieval period, and prior to the dissolution of the monasteries, monastic communities were sustainable economic, as well as religious, institutions, with the financial and landed means to support labored workers in addition to resident monks (Dickinson, 1961; Threlfall-Holmes, 2005). Concomitant with the concern placed on cleanliness of the soul, care was taken to ensure the salubriousness and health of the monastic residents. Fresh water supplies were critical considerations for the establishment and maintenance of the monasteries. If the community could not be situated near streams or springs, for example, pipe systems often were built to deliver fresh water to the community (Dickinson, 1961). Physical health for monks was promoted through mandatory manual labor—for example, carpentry, farming, or domestic duties—as stipulated by the specific religious order (Lawrence, 1984). These laborious daily activities within hygienically-engineered living and working quarters demonstrate the morbidity-mortality paradox (Crimmins, 2001) in practice: while monastic residents avoided earlier ages-at-death, resulting from fewer infections engendered by unsanitary conditions and poor water supply, these individuals accumulated greater chronic degenerative diseases, like osteoarthritis, with additional years of life and labor. The SFI distributions from our two samples suggest that monastic members better survived early episodes of stress and morbidity than did their nonmonastic counterparts, but at the price of higher, later cumulative frailty.
Among U.S. samples, this morbidity-mortality paradox has been observed among women, who live longer than men but exhibit, on average, higher AL than do similar aged men (Crimmins et al., 1997; Crimmins & Saito, 2001). This differential in male-female mortality patterns has been explained primarily by behavioral, social, genetic, and environmental differences between the sexes (Verbrugge & Wingard, 1987; Waldron 1983a, 1983b, 1985). However, unlike patterns observed in Western populations, this Medieval London sample indicates higher frailty and longer average lifespan for males than females within monastic and nonmonastic populations (Figure 2). This disparity with mortality and morbidity trends observed in living populations suggests that the sociopolitical, economic, and cultural environment of Medieval London enabled males to better buffer life's stressors and proceed to a frail phenotype than could contemporaneous females who, like nonmonastic individuals, may have died earlier with less frailty. Such patterns have been reported among modern communities in South Asia, where men receive preferential food and healthcare resources over women, enabling men to buffer any genetic or biological predispositions to infectious disease or mortality risks (Waldron, 1983a). This difference in SFI between males and females is not significant, nor is the difference between average age at death among sexes. However, it should be noted that these samples, especially the monastic cemeteries (Nfemales = 6), are disproportionately represented by male skeletons. Further comprehensive comparisons between sexes with the SFI in Medieval London will require a more equal representation of males and females. To ensure that the disproportion of male skeletons was not confounding the monastic-nonmonastic covariate, we examined SFI according to sex and lifestyle. When male and female SFI distributions for monastic and nonmonastic samples are observed separately, monastic affiliations still indicate higher morbidity and lower mortality. For lay females, who died at earlier years than their monastic counterparts, the average SFI was 2.53, relative to 3.83 for monastic females; monastic males also showed a higher age-related distribution (SFI = 3.38), relative to nonmonastic males (SFI = 2.91) (Figure 3). These values suggest both males and females living under nonmonastic conditions were less likely to survive morbid events and suffered higher mortality. In this regard, the Medieval monastic environment was conducive to longer lifespans among both men and women, as they better survived both acute and chronic collateral damage to their skeletons, thereby increasing their SFI.
6.2 Frailty patterns between ages in medieval populations
Another enlightening result from this study is the observed relationship between age and frailty (Figure 4). SFI averages for monastic and nonmonastic, and subsequently sex-divided samples, show the youngest (18–25 years) cohort to have a consistently higher SFI average than the succeeding (26–35 years) cohort. Thereafter, SFI increases incrementally from the second to fourth age cohort. This information confirms two precedents for frailty in bioarchaeology. First, this seemingly anomalous value for the youngest adult age group demonstrates how frailty is not a function of senescence, although frailty and age often are highly correlated (Fried et al., 2001). Frailty is a process synergistically interacting with disability, comorbidity, and life's stressors (e.g., infection, injuries, nutrition) (Fried et al., 2004, 2009); nonetheless, it cannot be simplified to or conflated with aging. Additionally, frailty and mortality are discrete concepts in health, which vary in relation to one another based upon biological, genetic, ecological, and sociocultural factors. As the data from Medieval London reveal, heightened frailty may be associated with mortality among young as well as older age classes. The initial decline in SFI from the first to second age cohorts and subsequent SFI increase to the fourth age cohort speak to the broader theoretical conundrum of the osteological paradox. One of the tenets of the osteological paradox is the assumption that skeletal samples represent the frailest individuals from the initial population. Working from this base, the decrease in SFI from youngest to early-middle (26–35 years) adulthood reflects a logical progression of frailty with age: presumably, the frailest individuals within a population will be the first to die, leading to lower frailty values in the next older cohort. However, these data indicate that although the frailest may die the earliest, individuals living into older ages continue to amass frailty, exhibiting the highest SFI, not despite their longevity but because they have survived more stressors. These results run counter to Wood et al.' (1992) interpretation of frailty, which would predict SFI to decrease with age. Instead, among monastic and nonmonastic samples, the highest mean SFI is exhibited by individuals over 45 years of age. As this result may also be a consequence of the Medieval London biocultural environment, it is logical to test this age-frailty relationship within additional skeletal series.
6.3 Evaluating the application of SFI
Our results demonstrate an advantage to applying a SFI to archaeological samples. The approach used by the SFI model seeks to quantify individual, cumulative stress that can be compared between individuals and populations. While gross prevalence values provide an immediate and informative overview of the population, these values cannot account for the individual. Bioarchaeologists must determine from a suite of gross prevalences and average skeletal metrics whether one subsample/sample is “more healthy” than another subsample/sample. This task may be difficult, as different variables may yield contradictory information. To highlight this point, mean femoral length, mean femoral head diameter, and gross prevalence values of conditions incorporated into the SFI were tabulated for monastic and nonmonastic samples (Table 4). Comparison of femoral lengths exclusively between samples suggests the nonmonastic community experienced higher frailty. However, when other variables (LEH, osteoarthritis, IVD, periostitis, periodontal disease, and trauma) are considered, the monastic community projects a more skeletally frail subpopulation. According to which skeletal frailty variable is selected for comparison, relative frailty between monastic and nonmonastic samples may readily fluctuate using only gross prevalences of traits. Nevertheless, it is constructive to compare SFI with gross prevalences to establish which variables are contributing the greatest to frailty differences. For example, osteoarthritis and IVD characterize DJD of synovial articulations and the vertebral column, respectively. These pathological lesions are observed within the monastic communities twice as frequently as within the nonmonastic sample. Since DJD was not found to correlate with age in this study, daily activity and mobility may explain the variation in DJD between individuals. Differences in daily workload and physical movement between monastic and nonmonastic populations in Medieval London may explain prevalences within observed samples. To this end, we advocate the comparison of gross prevalence data with SFI for the most thorough assessment of health in skeletal samples.
| Monastic | Nonmonastic | |||
|---|---|---|---|---|
| Male (N = 68) | Female (N = 6) | Male (N = 43) | Female (N = 17) | |
| Femoral length (mm) | 464 | 433 | 457 | 430 |
| Femoral head diameter (mm) | 48.5 | 43.5 | 47.5 | 43.2 |
| LEH | 0.76 | 0.83 | 0.58 | 0.87 |
| Osteoarthritis | 0.34 | 0.50 | 0.11 | 0.20 |
| IVD | 0.57 | 0.67 | 0.27 | 0.27 |
| RCD | 0.01 | 0.00 | 0.00 | 0.00 |
| Osteoporosis | 0.00 | 0.00 | 0.02 | 0.00 |
| Periostitis | 0.38 | 0.33 | 0.22 | 0.27 |
| Periodontal disease | 0.82 | 1.00 | 0.76 | 0.53 |
| Cribra orbitalia/porotic hyperostosis | 0.09 | 0.33 | 0.13 | 0.07 |
| Rickets/osteomalacia | 0.00 | 0.00 | 0.00 | 0.00 |
| Neoplasm | 0.03 | 0.00 | 0.00 | 0.00 |
| Trauma | 0.28 | 0.17 | 0.20 | 0.13 |
Another variable that must be accounted for when applying the SFI is age. As studies of modern human populations indicate, frailty often significantly correlates with age (Bergman, Wolfson, & Sourial, 2006; Fried et al., 2001; Mitnitski, Graham, Mogilner, & Rockwood, 2002). If a bioarchaeologist chooses to apply the SFI, a cumulative index for frailty, to test interpopulation differences in cultural, sociopolitical, or socioeconomic conditions, age must be considered as a confounding variable through covariate analyses. Otherwise, especially in populations, such as the present study, where significantly different distributions in age exist, the difference in SFI will be representative of age distributions and not frailty.
7 Conclusion
Biological anthropologists studying both modern and past populations benefit from a clear definition of stress as commonly used by human biologists: namely, a process that promotes multiple individual phenotypes in response to variable external and internal stimuli. Adopting a SFI that is based upon results from living humans may aid in providing today's bioarchaeologists with an unambiguous definition of skeletal stress (frailty). The SFI may contribute to measuring stress and developing predictive models mapping relationships between stress and health applicable across populations, geographic zones, and time periods.
Analysis of skeletal remains is the most promising means of estimating and conceptualizing stress among past populations. For bioarchaeologists interested in the physiological health of past populations, adopting a cumulative biological measure for stress, such as frailty, contributes to improved understanding of relationships among stress, morbidity, and mortality within and between archaeological populations. To this end, our proposed frailty index draws from previous palaeopathological research linking skeletal markers to their etiologies in living populations. While certain methodological problems must be addressed, we propose the SFI as modeled here as a measurement technique with potential for addressing stress among archaeological populations.
Implementation and application of the SFI to two Medieval London samples demonstrates the usefulness of the SFI, yielding results about population morbidity that closely reflect observations and patterns of morbidity and mortality among living human populations. Unlike gross prevalence statistics, the frailty index condenses multiple biomarkers of stress into a singular score for each individual. Theoretically, this score quantifies individualized cumulative lifetime stress and enables us to compare individual stressor life histories within and between populations. The SFI, therefore, addresses and alleviates problems when comparing gross prevalences, which always assess neither individuals nor the synergistic and antagonistic interactions of skeletal stress biomarkers and health within individuals.
Based upon previous health models and indices established in bioarchaeological research, we have developed a stress index that uniquely accumulates biomarkers of life-time stressors into a single quantifiable, measurable unit for each individual. By assigning points to individuals using the presence of a condition or highest-risk percentiles, this proposed SFI score enhances interpretations of relationships between stress and health in skeletal populations. Operationally, this model's utility benefits from ease of applicability and its ability to assess changes in patterns of skeletal health over time within a population and within specific geographic areas and zones over time.




