Energetic Priorities Across the Stages of Development: Effects of Age, Sex, and Seasonal Reproduction on Activity Budgets in Verreaux's sifaka (Propithecus verreauxi)
ABSTRACT
The immature period is an essential time of physical and behavioral development in which individuals prepare to navigate their environment as adults. Activity budgets provide valuable insight into the tradeoffs individuals make based on their energetic priorities. We hypothesized that energetic priorities differ across the stages of development based on the distinct social and ecological needs of that stage. We analyzed 31,113.5 h of focal instantaneous sampling data from 2007 to 2024 on 73 Verreaux's sifaka (Propithecus verreauxi) living in Kirindy Mitea National Park, Madagascar to investigate the effects of age class, sex, and seasonality on activity budgets. Juveniles and subadults devoted significantly more time to social activity than adults. Subadults fed less than other age classes, and we detected no differences in resting among age classes. Among all age classes, males devoted more time to social activity than females, and all age classes displayed sex differences in additional activities. All age-sex classes exhibited similar seasonal patterns in activity budgets. Our results indicate that social activity may be especially important in the developmental period to gain experience and establish social relationships before adulthood. Sex differences in social activity appear to emerge earlier than adulthood as a predisposition for the reproductive roles of adulthood. Overall, we found that energetic priorities differ between stages of development, and evidence is mixed regarding whether these differences are primarily due to the onset of reproduction.
Summary
-
Juveniles and subadults spend significantly more time engaged in social activity compared to adults.
-
Sex differences in the amount of time devoted to social activity are present among all age classes, and sex differences in other activities emerge before adulthood.
-
Seasonal variation in activity budgets follow similar trends among all age classes.
1 Introduction
During development, individuals must invest energy into somatic growth (Kramer and Ellison 2010), learning (Pereira and Fairbanks 2002), and behavioral development (Pereira and Leigh 2003). Thus, the immature period leading up to adulthood is a critical time of preparation for the ecological and social challenges of adulthood. Investigating activity in the immature period is important because early life experiences in primates can have cascading effects on physical and social development and, consequently, reproductive success and survival in adulthood (Alberts 2019; Altmann and Alberts 2003; Tung et al. 2023).
The juvenile period is characterized by physical and nutritional independence from caregivers (Pereira and Altmann 1985) and therefore necessitates the acquisition of foraging and social skills (Janson and van Schaik 1993). While some primate studies have shown that individuals acquire adult repertoires of foraging skills and diet by the age of weaning (Schuppli et al. 2016; Schuppli et al. 2012), other studies suggest still nascent foraging skills during this period (Hanya 2003; Eadie 2015; Fragaszy and Boinski 1995). The acquisition of social skills may also require a long period of development (Carvajal and Schuppli 2022; Whiten and van de Waal 2018), particularly for primates whose adult lives are spent in complex social groups. Juveniles must gain experience interacting with individuals beyond their mother in preparation to live successfully in a social group as independent adults (Altmann 2001; Pereira and Altmann 1985). By engaging in social behaviors, individuals begin to establish relationships, acquire advanced motor skills, and learn reciprocity in social interactions (Fagen 1993; Paukner and Suomi 2008). However, exactly how juvenile primates balance the complex needs of social and foraging development during this critical period is unclear.
While some studies posit that individuals transition directly from juvenility to adulthood with the onset of sexual maturity (Kappeler et al. 2009), other researchers have carved out the subadult period as a unique developmental stage (Charpentier et al. 2004; Kraemer et al. 1982; O'connell et al. 2020; Reddy et al. 2021, 2022). Subadulthood, also known as adolescence, is a life stage representing the transition from learning, skill acquisition, and close associations with caregivers to full independence and stepping into adult social roles (Pusey 1990). While definitions differ slightly, we define this stage as beginning with the onset of puberty (Pereira and Altmann 1985; Pusey 1990) and ending with the ability to produce viable offspring (Pereira and Altmann 1985) that survive past the age of weaning (Richard et al. 2002). As subadults reach adult body size and reproductive maturity, other group members begin to view them as social companions or potential mates rather than dependents (Pusey 1990). The subadult period is also a time of dispersal from the natal group (Galezo et al. 2022; Pereira and Altmann 1985), which may include temporary visits to other social groups to gain social information about the individuals that inhabit those groups (Clobert et al. 2009; Leimberger and Lewis 2017). Subadults who stay in their natal groups also experience role shifts during this period as they integrate into adult social structures (Pusey 1990).
In adulthood, physical growth and behavioral development plateau, and energetic priorities shift towards investment in reproduction (Emery Thompson 2017; Pontzer 2015; Thompson 2024). Adults can mitigate these additional energetic costs, both of reproduction and larger body size, by increasing food intake and rest at the expense of other activities (Ruivo et al. 2017; Zhou et al. 2007). Alternatively, resting may be a reserve activity that individuals reduce first to increase the amount of time available for other activities, such as feeding and social activity (Dunbar 1992). Social behavior holds importance in adulthood, as evidenced by strong correlations between the strength of social bonds and longevity in primates (Alberts 2019; Silk et al. 2010). Thus, how adults manage these competing demands is still being understood.
Throughout development, sex differences may also drive differential investment of energy into various activities. Studies of juvenile and subadult primates have primarily shown sex differences in investment in specific social behaviors, such as grooming and play (Macaca sp.; Bernstein et al. 1993; Eaton et al. 1986; Cercopithecus mitis stuhlmanni; Cords et al. 2010; Cebus apella; Paukner and Suomi 2008). However, few studies have investigated differences in the overall time devoted to social activity for immature males and females. Sex differences in additional activities appear to become increasingly prominent in adulthood potentially due to the onset of reproduction (Pereira and Leigh 2003). In species with male-biased dispersal, males may invest in activities that maximize access to mates, such as traveling and being social (Sapajus nigritus; Back et al. 2019), whereas females may invest in activities that maximize fecundity and infant survival, such as feeding and resting (Cebus capucinus; Fedigan 1993). Sex differences in adult activity budgets may also be exaggerated in primate species with seasonal reproduction because females incur higher energetic burdens during certain stages of reproduction, such as lactation, compared to males (Koch et al. 2017; Zhou et al. 2007). Therefore, reproduction, and the physical burdens and social roles associated with it, may primarily explain sex and seasonal differences in energetic investments across ontogenetic stages.
Verreaux's sifaka (Propithecus verreauxi) is a sexually monomorphic lemur species characterized by a long, slow life history relative to other strepsirrhines of similar size (Richard et al. 2002). Males and females attain sexual maturity between the ages of 3-5 years, but infant mortality is especially high for females giving birth before reaching 5-6 years of age (Richard et al. 2002). Given this slow life history, debate exists on whether sifaka juveniles transition directly into adulthood at the onset of sexual maturity (Kappeler et al. 2009; Kappeler and Schäffler 2008) or whether subadulthood represents an intermediary stage of physical and social maturity (Lawler 2003; Lewis 2008). Thus, Verreaux's sifaka is a useful model species to investigate the unique energetic priorities of each distinct ontogenetic stage. Sifaka display male-biased dispersal (Leimberger and Lewis 2017; Richard et al. 1993) and seasonal mating (Lewis and Kappeler 2005), which make them useful in understanding how males and females navigate these developmental stages differently across their lifespans.
Activity budgets provide a proxy for energetic priorities because individuals must allocate their limited amount of active time to various activities according to their needs (Mello et al. 2024; Zhou et al. 2022). In this study, we investigated ontogenetic changes in activity budgets in Verreaux's sifaka. We hypothesized that (H1) the juvenile, subadult, and adult stages represent functionally distinct life stages, and energetic priorities differ between these stages (Pereira and Altmann 1985). We thus predicted that (P1.1) juveniles spend more time engaged in foraging and social activities compared to subadults and adults (Janson and van Schaik 1993) to gain more experience in survival and social skills. We further predicted that (P1.2) subadults invest more time in social activity and traveling compared to adults due to dispersal from natal groups and integrating into adult social structures (Pusey 1990). Lastly, we predicted that (P1.3) adults invest more time in resting and feeding compared to immature individuals to maximize energy available for reproduction (Emery Thompson 2017). We additionally hypothesized that this species’ (H2) male-biased dispersal (Leimberger and Lewis 2017) and (H3) seasonal mating patterns (Richard et al. 2000) drive sex differences and seasonal variation in activity budgets. However, we predicted that juveniles and subadults do not display significant (P2.1) sex or (P2.1) seasonal differences because they are unburdened by the energetic and social demands of reproduction. These (P2.2) sex and (P3.2) seasonal differences only become apparent upon adulthood, when individuals are consistently reproducing successfully.
2 Methods
2.1 Study site and subjects
Data were collected at the Ankoatsifaka Research Station (20°47′17 S, 44°10′08 E) in Kirindy Mitea National Park (KMNP), western Madagascar. This dry deciduous forest receives approximately 850 mm of rainfall each year with temperatures ranging from 7°C to 40°C and a mean temperature of 24°C (Lewis and Axel 2019; Rasambainarivo et al. 2014). The rainy season spans from December to March with most rain falling in January and February (Axel et al. 2024; Lewis and Axel 2019). The dry season occurs from April to November and is characterized by a lean vegetation period from July to November (Lewis and Axel 2019). The Ankoatsifaka study site is a 1 km2 subsection of the 1400 km2 park and is divided into a grid system by trails every 25 m (Axel et al. 2024).
Verreaux's sifaka are diurnal, folivorous lemurs which inhabit western and southwestern Madagascar (Lewis and Kappeler 2005). They primarily live in small, mixed-sex groups of between 2 and 16 individuals composed of 0-3 females and 0-3 males (Leimberger and Lewis 2017; Lewis and Kappeler 2005; Sussman et al. 2012). Females tend to remain in their natal groups more often than males, who tend to disperse initially between the ages of 3-6 years (Leimberger and Lewis 2017; Richard et al. 1993). Females give birth in the dry season between July and September (Brockman 1994; Leimberger and Lewis 2017; Lewis and Kappeler 2005).
Individuals living within the Ankoatsifaka study area are habituated and marked with nylon collars and identification tags at approximately 1 year old as part of the Sifaka Research Project. We analyzed data from eight distinct social groups from October 2007 (the beginning of behavioral data collection) through September 2024. The number of distinct groups varied from four to eight in a given year. Sex was determined based on identification of external genitalia. We classified individuals as juveniles (1–2 years old), subadults (3–4 years old), or adults (5+ years old; Lewis and van Schaik 2007; Richard et al. 2002). Individuals advance to the next year of age on September 1st because not all exact birth dates are known and births predominantly occur in July and August (Leimberger and Lewis 2017). Thus, an individual was considered a subadult on September 1st of the third year following their birth year. One male and one female were excluded from analysis because of uncertainty of birth year due to immigration into the population.
2.2 Data Collection
Activity data (Table 1) were collected using focal animal sampling with 1-h samples and instantaneous observations recorded at 10-min intervals. Focal animals were randomly chosen in advance and switched every hour. Juveniles were systematically included as focal individuals starting in 2017, but some data on juveniles were available for 2010-2012. Observers followed a group for several consecutive days (exact number of days dependent on season and number of observers available) before switching groups. Individual groups were observed for 8 ± 6 days/month (mean ± SD) for 7 ± 3 h/day (mean ± SD). Data were collected year-round, although fewer data were collected in April, August, and December due to fewer available observers. No data were collected in 2009 due to Cyclone Fanele (Lewis and Rakotondranaivo 2011). Observers generally followed the group from sleep tree to sleep tree. However, sifaka are active beyond the daylight hours in some months (Erkert and Kappeler 2004), hence we were not always able to capture complete active periods. We classified season (mating: January–March; gestation: April–June; birth: July–September; lactation, October–December) based on the reproductive timing of events (Lewis and Kappeler 2005).
| Category | Definition | Activity recorded |
|---|---|---|
| Feed | Food in mouth, food being pulled to mouth, must be chewing or harvesting, includes collecting water from a surface with the tongue | Feed Lick |
| Forage | Actively search for food by close inspection of the flora, prepare food for ingesting, or hold food before ingesting | Forage |
| Rest | Any form of inactivity or self-directed behavior where spatial position does not change | Autogroom Rest Vigilant |
| Social | Any active interaction with another member of the group, does not include close contact | Social Groom Mating Play |
| Travel | Any form of movement from current spatial position | Travel |
| Other | Any activity recorded that does not fit within the above categories | Scent-mark |
| Out of sight | Animal is present but the activity was not observed | Out of sight Unknown No information recorded |
- Note: Ethogram was modified from Brockman (1994).
We excluded data for particular months for any individual that had less than 8 h of in-sight observations in that month. We determined this threshold by calculating the average monthly activity budget across all individuals in our data set and determined that we needed approximately 8 h of focal sampling to adequately capture the average percentages of each activity. Thus, we included 73 focal animals in our final analysis (nJuvenile = 23; nSubadult = 32; nAdult = 47) out of the 97 total marked animals based on known age, sex, and adequate number of in-sight observations. Due to the longitudinal nature of our data, some individuals are represented in more than one age class.
Demographic data were collected using monthly censuses, supplemented by information recorded daily during other protocols (e.g., focal animal samples). These demographic data were used to calculate average monthly group size (defined as the mean number of independent individuals recorded in a group across all days in a given month). Adults, subadults, and juveniles were included in group size estimates. Infants were included as independent group members beginning March 1st in the year following their birth as a conservative estimate of locomotor independence in Verreaux's sifaka (Malalaharivony et al. 2021).
2.3 Data analysis
All data were summarized in R version 4.4.1 (R Core team 2024) using the dplyr package, and the analysis code is available in the Supporting Materials. A monthly activity budget was calculated for each individual by dividing the total number of instances of each activity by the total count of instantaneous point samples for the individual in that month. Descriptive statistics were summarized across all individuals and by age class using mean ± standard deviation to provide an average activity budget for individuals in the population.
To investigate (H1) the effect of age class on activity frequency, we ran GLMMs with a Poisson error distribution. For our main model, we included the monthly count of observations of each activity as the outcome variable. The fixed effects included activity, age class, sex, and mean monthly group size, as well as separate interactions of activity with all other fixed effects. We included mean monthly group size because the number of conspecifics for social interaction or competition for resources may influence activity budgets (Pollard and Blumstein 2008). To account for variable numbers of observations for each individual, we included the natural logarithm of total number of instantaneous point samples for that individual in the month as an offset. Lastly, we included focal animal identity as a random intercept to control for individual-specific variation. We chose not to include group identity as a random effect because initial analyses of the effect of group identity explained little to no variation in activity and instead included group size as a fixed effect. For post-hoc analyses, we utilized Tukey's Honestly Significant Difference to conduct pairwise comparisons of activity frequency between age classes controlling for sex using the emmeans package.
To further investigate the effect of (H2) sex and (H3) seasonality on activity for each age class, we ran three identical GLMMs, one for juveniles, one for subadults, and one for adults. For each model, the outcome, fixed effects, random effects, and offset were identical to the previous model, although we excluded age as a fixed effect. We again ran post-hoc tests using Tukey's HSD to conduct pairwise comparisons of (1) activity frequency between sexes for each age class, (2) activity frequency between sexes for each reproductive season, and (3) activity frequency within a sex across the four reproductive seasons.
We ran an additional post-hoc analysis to parse out the effects of group composition from the effects of group size on activity. We fit an additional GLMM with an identical outcome variable, offset variable, and random effect to our previous GLMM testing H1, but with the presence (1) or absence (0) of immatures and average monthly group size as fixed effects. We then used Tukey's HSD to conduct pairwise comparisons of activity frequency between groups with immatures versus groups without immatures controlling for group size.
3 Results
3.1 Overall Activity Budget Averaged Across Age-Sex Classes
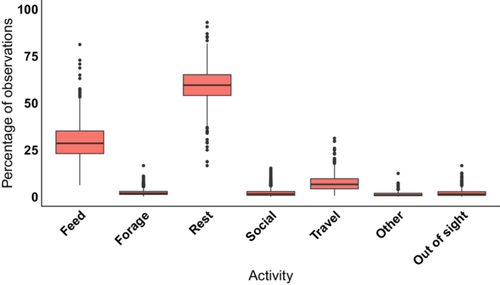
Among all age classes, resting was the most frequent activity followed by feeding (Figure 1, Table S1 for full activity budget). Sifaka spent a mean of 59.5% ± 9.4% of their time resting and 29.4% ± 9.5% of their time feeding. Sifaka devoted much less time to being social, spending only 2.2% ± 2.2% of total observations engaging in social activity. Animals were rarely out of sight (2.2% ± 2.1%).
3.2 Effects of Age Class on Activity Budget (H1)
Age class significantly predicted variation in the amount of time devoted to feeding and social activity (Figure 2; Table S2). (P1.1) Juveniles and (P1.2) subadults were significantly more likely to devote time to social activity than adults. Specifically, juveniles were 35% more likely to be social than adults (z = 4.658; SE = 0.088; p < 0.001), and subadults were 41% more likely to be social than adults (z = 6.787; SE = 0.072; p < 0.001) on average. We detected no difference in time devoted to social activity between juveniles and subadults (z = −0.608; SE = 0.070; p = 0.816). Juveniles were 6% more likely to be observed feeding than subadults (z = 3.305; SE = 0.018; p = 0.003; Table S3) and (P1.3) adults were 3% more likely to be feeding than subadults (z = −2.702; SE = 0.011; p = 0.019). We detected no difference in the likelihood of feeding between juveniles and adults (z = 1.740; SE = 0.015, p = 0.190). We did not find differences in the time spent (P1.1) foraging (Juvenile vs. subadult: z = 0.900; SE = 0.072, p = 0.641; Juvenile vs. adult: z = 0.551; SE = 0.059, p = 0.846), (P1.2) traveling (Juvenile vs. subadult: z = −0.852; SE = 0.033, p = 0.671; Subadult vs. adult: z = 1.663; SE = 0.024, p = 0.220), or (P1.3) resting (Juvenile vs. adult: z = −1.606; SE = 0.010, p = 0.243; Subadult vs. adult: z = 0.380; SE = 0.008; p = 0.924) across age classes. Focal animal identity explained < 0.001 of the variation in overall activity budgets.
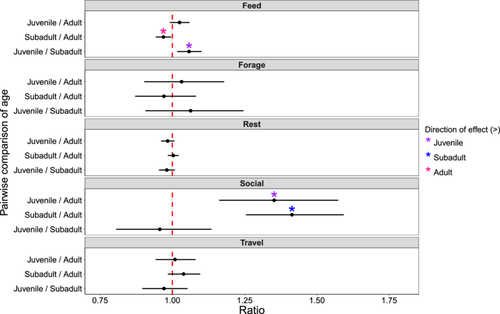
Post-hoc analyses showed that the presence of immature individuals in a group significantly increased the likelihood of engaging in social activity (z = −6.672; SE = 0.037; p < 0.001; Table S4). In contrast, the presence of immature individuals significantly decreased the likelihood of feeding (z = 3.139; SE = 0.011; p = 0.002), resting (z = 13.270; SE = 0.009; p < 0.001) and traveling (z = 4.036; SE = 0.021; p < 0.001). Presence of immatures did not significantly affect the likelihood of foraging (z = 0.042; SE = 0.037; p = 0.966).
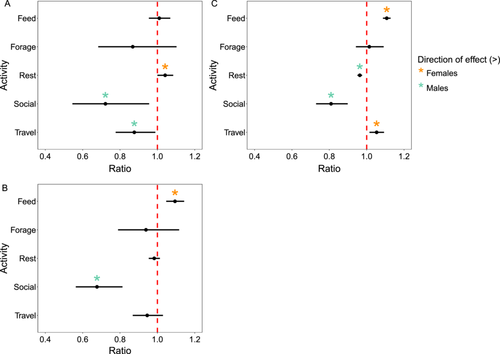
3.3 Effects of Sex on Activity Budgets Within Age Classes (H2)
Sex influenced sifaka activity budgets within all age classes (Figure 3; Table S5). Sex significantly predicted the likelihood of engaging in social activity among (P2.1) juveniles and subadults, with social activity being 28% more likely in juvenile males than females (z = −2.274; SE; = 0.103; p = 0.023; Figure 3A) and 32% more likely in subadult males than females (z = −4.167; SE;= 0.063; p < 0.001; Figure 3B). Among juveniles, females were 4% more likely to rest than males (z = 1.965; SE = 0.022; p = 0.049) and males were 12% more likely to travel than females (z = −2.141; SE = 0.054; p = 0.032). For subadults, females were 9% more likely to feed than males (z = 4.033; SE = 0.024; p < 0.001). In (P2.2) adults, sex significantly predicted variation in all but one activity. Females were 11% more likely to feed (z = 10.862; SE = 0.010; p < 0.001) and 5% more likely to travel (z = 2.761; SE = 0.020; p = 0.006) than males (Figure 3C). Males were 4% more likely to rest (z = −5.748; SE = 0.006; p < 0.001) and 19% more likely to engage in social activity (z = −3.973; SE = 0.043; p < 0.001) than females on average. Focal animal identity explained < 0.001 of the variation in activity for juveniles, subadults and adults.
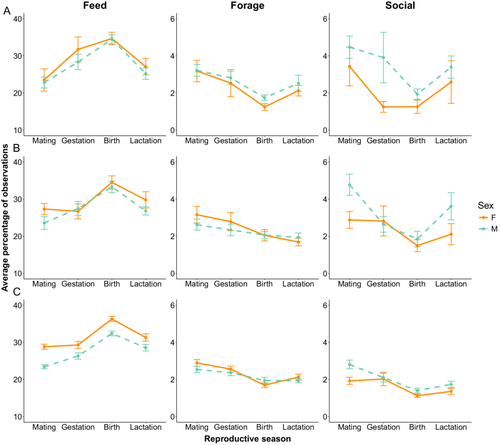
3.4 Seasonal Variation in Activity Budget (H3)
Seasonal variation in activity followed similar trends between males and females among all age classes (Figure 4; Table S6). All sifaka were more likely to be observed feeding in the birth season compared to all other seasons (p's < 0.001; Table S7). The likelihood of foraging varied across seasons among all age-sex classes with sifaka significantly more likely to be observed foraging in the mating season than in the birth (p's < 0.01) and lactation seasons (p's < 0.05). All age-sex classes were less likely to engage in social activity during the birth season. For (P3.1) juveniles and subadults, this difference was significant compared to all other seasons (p's < 0.05). For (P3.2) adults, this difference was significant when compared to the mating and gestation seasons (p's < 0.001) but not compared to the lactation season (p = 0.864). Subadults and adults were also significantly more likely to be social in the mating season compared to any other season (p's < 0.01). Seasonal differences in resting and traveling were less consistent across age-sex classes. For subadults and adults, both male and female sifaka were less likely to rest in the birth season than any other season (p's < 0.01). (P3.2) Adult males and females were most likely to travel in the mating season than any other season (p's < 0.01). (P3.1) Juveniles did not show consistent seasonal patterns in resting or traveling, nor did subadults for traveling.
4 Discussion
We investigated the effect of age class, sex, and seasonality on activity budgets in Verreaux's sifaka. We found support for H1, because both juveniles (P1.1) and subadults (P1.2) invested more heavily into social activity compared to adults, likely to gain experience in social interactions and begin to form bonds as they become independent from caregivers (Pusey 1990). Contrary to H2, we found that (P2.1) sex differences in activity budgets did emerge among immatures. Inconsistent with H3, (P3.1, P3.2) all age classes displayed similar seasonal variation in activity. Males and females displayed the same seasonal trends in activity among all age classes.
4.1 Overall Activity Budget
The overall activity budget of our study population was consistent with both other Verreaux's sifaka populations (Kirindy Forest: Dinter et al. 2021; Norscia et al. 2006; Ifotaka: Kanuka 2014; Berenty Reserve: Markham and Gould 2018; Mahavalo: Wilson and Ferguson 2014) and other wild sifaka species (P. diadema: Farmer 2024; Bonadonna et al. 2024; Powzyk 1997; Rahalinarivo et al. 2023; P. edwardsi: Arrigo-Nelson 2006; P. coronatus: Pichon and Simmen 2015). The tendency for resting and feeding to comprise the majority of activity budget is also consistent with species across the primate order (Strepsirrhines: Vasey 2005; Platyrrhines: Souza-Alves et al. 2024; Cercopithecoids: Canteloup et al. 2019; Hominoids: White 1992).
4.2 Effects of Age Class on Activity Budget
Sifaka activity budgets varied by age class with the strongest effects of age on social activity. Both (P1.1) juveniles and (P1.2) subadults were significantly more likely to be social compared to adults, suggesting the importance of social activity in these early developmental stages. While direct comparative research on social activity between adults and immatures is limited, younger individuals seem to prioritize social behavior more than adults across primate species (Cebus spp.: Back et al. 2019; O'Brien 1993; Macaca mulatta: Liao et al. 2018; Pongo: Galdikas 1985, 1995). Additionally, our post-hoc analyses indicated that, controlling for overall group size, the presence of immatures in a group increases the likelihood that individuals devote time to social activity. Therefore, immature individuals may drive up the amount of social activity within a group by not only engaging in more social activity themselves, but also by impacting social activity of surrounding group members.
For juveniles, social activity may be essential because of the lasting effects of early sociality on future social and reproductive outcomes, and juveniles may increase feeding to offset the energetic costs of higher social activity. In primates, social adversity and isolation in early life can have detrimental negative effects on social cognition, survival, and reproduction in adulthood (Alberts 2019; Anderson and Mason 1978; Tung et al. 2023). Social activity may be especially important for sifaka because strepsirrhine juvenile periods are shorter relative to other anthropoid species (Pereira and Fairbanks 2002). Contrary to P1.3, juveniles in our study population did not differ from adults in feeding or resting activity. Despite needing energy for growth in the juvenile period (Kramer and Ellison 2010), smaller body size and lack of sexual maturity may result in lower absolute energetic requirements for juveniles compared to adults (Emery Thompson 2017; Janson and van Schaik 1993; Pontzer 2015). The importance of engaging in social activity during the juvenile period may then outweigh the energetic costs.
Subadults must prioritize social activity as they prepare to transition into adult roles within social groups. In Verreaux's sifaka, males typically disperse from their natal groups during this period (Richard et al. 1993; Leimberger and Lewis 2017) and must not only form new relationships, but also learn about the social structure and “rules” of the group (Pusey 1990). Social activity still holds importance for subadult female primates that do not disperse from their natal groups because they must integrate into adult hierarchies (Nautiyal et al. 2024). Subadult sifaka compensated for high levels of social activity by decreasing time spent feeding, possibly as a result of dominance status in sifaka because subadults are subordinate to adults (Voyt et al. 2019) and may have lower feeding priority. Subadults may also reduce feeding to increase time available for social activity because the costs of not investing in social integration may be especially high during this stage. Social instability in subadulthood can negatively impact social competence and reproductive outcomes in adulthood (Pan troglodytes; Reddy et al. 2021, 2022). Subadult sifaka may therefore prioritize this aspect of behavioral development to maximize future reproductive success at the expense of energy conservation in this stage. Importantly, despite the attainment of sexual maturity, subadults prioritized social activity more than adults indicating a functional distinction between these two stages.
Consistent with our predictions, (P1.3) adult sifaka were least likely to devote time to social activity out of all age classes and fed more than subadults. Devoting time to social activity may be too energetically costly for adult primates with females investing in gestation (Touitou et al. 2021), lactation (Rangel Negrín et al. 2021), and infant care (Ross 2001) and males investing in traits related to male-male competition and mating effort (Bueno and Lewis 2024; Higham and Maestripieri 2014; Lawler et al. 2005). While animals might be expected to decrease resting to increase the time available for other activities such as feeding (Dunbar 1992), adult sifaka appeared to decrease social activity first. For adults in this species, maintaining adequate rest may be especially necessary for maximizing energy in a seasonal environment where resource availability is highly variable (Axel et al. 2024; Veilleux et al. 2024). While many primate species display a trend of increased resting with age (Cercopithecoids: Hendershott et al. 2016; Zhou et al. 2022; Platyrrhines: Mello et al. 2024; Prates and Bicca-Marques 2008), this strategy may be insufficient for adult sifaka given that they are highly folivorous (Lewis and Kappeler 2005) and live in a deciduous forest. More research is needed to determine whether this inconsistency between sifaka and other primates is due to greater energetic constraints or a reduced importance of social activity.
4.3 Effects of Sex on Activity Budgets Within Age Classes
We detected fewer sex differences in activity budgets for juveniles and subadults compared to adults. This result partially supports our prediction that activity varies by sex predominantly in (P2.2) adults due to divergence of roles with the onset of regular reproduction (Kappeler 2017). Male sifaka were more likely to engage in social activity than females for all age classes, although possibly for different reasons. Play is the primary social behavior among juvenile primates (Fagen 1993), and males tend to engage in play more than females as preparation for adult fighting behavior (Theropithecus gelada; Barale et al. 2015; Macaca fuscata; Eaton et al. 1986; Shimada and Sueur 2018). For subadult sifaka, males may devote more time to social activity than females because they are the obligate dispersing sex in this species (Leimberger and Lewis 2017; Richard et al. 1993) and therefore must invest more heavily into social relationships as they negotiate their way into multiple groups throughout their lives.
In female-philopatric primates, adult males may be more likely to engage in social activity than females because they must invest in social relationships to maximize their mating opportunities (Jack and Fedigan 2004; Teichroeb et al. 2011). To gain reproductive access to females, adult males may disperse secondarily (Papio cynocephalus; Alberts and Altmann 1995), visit other groups (Aotus azarae; Corley and Fernandez-Duque 2023; Propithecus verreauxi; Norscia et al. 2009), or invest in social bonds with alpha males (Macaca arctoides; Toyoda et al. 2022; Cebus imitator; Wikberg et al. 2022). In our study population, alpha male sifaka hold tenure for approximately 3.6 years, and subordinate males transfer groups even more frequently (Leimberger and Lewis 2017). Adult males may also continue to invest in male-male relationships to improve group stability in the presence of outgroup males (Antonacci et al. 2010). Therefore, as adults, males may need to engage in social behavior more frequently than females to maximize reproductive success or group stability.
Contrary to P2.2, sex differences in non-social activity emerged before adulthood. Juvenile females were more likely to rest than males perhaps as an energy conservation strategy in preparation for rapid growth in the subadult period. Female sifaka have faster hindlimb growth rates than males starting at 2 years old and faster growth in both hindlimbs and forelimbs at 3 years (Bueno et al. in review). During this peak in growth rates, subadult females shifted from spending more time resting to feeding. This activity shift may also be due to the fact that subadult females can conceive offspring (Richard et al. 2000, 2002), and may invest in energy intake similarly to adult females even though the likelihood of infant survival is low (Richard et al. 2002). By adulthood, females fully shift energy-maximizing strategies to not only feed more than adult males, but also rest less. Increasing feeding may be essential for females to create an energy surplus for reproduction (Dolotovskaya and Heymann 2020; Rangel Negrín et al. 2021), and they must sacrifice rest accordingly. This activity trend has been previously reported for Verreaux's sifaka (Koch et al. 2017) and other primates (Strepsirrhines: Vasey 2005; Platyrrhines: Prates and Bicca-Marques 2008; Cercopithecoids: Zhou et al. 2022).
Sex differences in traveling also emerged early in life. Juvenile males were more likely to travel than females. In some female-philopatric primate species, mothers may reject male offspring more than female offspring (Macaca spp.; Eaton et al. 1986; Kulik et al. 2016), which may necessitate juvenile males to explore their physical and social environments more than females. By adulthood, however, female sifaka were more likely to be observed traveling than males. Overall, sex differences in energy balance strategies are not constrained to adulthood nor are they consistent across life stages, and further studies should investigate the direct effects of sexual maturity on the ontogeny of activity budgets for both sexes.
4.4 Seasonal Variation in Activity Budget
All age classes displayed similar seasonal variation in activity, contrary to our prediction that (P3.1) juveniles and subadults do not display this variation due to the absence of the energetic demands of seasonal reproduction. Additionally, (P3.2) adults males and females did not deviate in seasonal variation in activity. Instead, male and female sifaka across all age classes were least likely to engage in social activity and foraging and most likely to feed during the birth season when leaves are senescing (Axel et al. 2024; Lewis and Axel 2019). The sifaka active period is shorter in the birth season than during the rainy mating season, when sifaka are active before sunrise and after sunset (Erkert and Kappeler 2004; Norscia et al. 2006). Because we were unable to collect data in the dark, the time spent feeding over the full 24-h period during the rainy (i.e., mating) season may be greater than our estimations (Norscia et al. 2006). Sifaka may cope with the low food availability in the birth season by increasing food intake during daylight hours at the expense of other energy-consuming activities, such as searching for preferred food resources or social activity. Sifaka at Kirindy Forest also spend more time feeding in the dry season (Norscia et al. 2006) when nutrient intake is low (Koch et al. 2017). Recent data suggest that female reproductive status does not impact activity budget in howler monkeys (Mello et al. 2024), but more work is needed to quantify how the demands of reproduction alter a female's allocation of energy to particular activities. Thus, our data are more consistent with the idea that resource availability, rather than reproductive seasonality, drives patterns of activity across seasons, which would hold true despite any age or sex differences. Comparing these trends in rainforest-living sifaka species that face less drastic seasonal environments would further clarify this point.
4.5 Conclusions and Future Directions
In total, our results suggest that social needs shift throughout development with social activity holding a particularly high importance in the early stages of development and as individuals approach adulthood (Pereira and Leigh 2003). Patterns of energy allocation may partially be dictated by the energetic demands of reproduction because adults display greater tendencies to invest in activities that maximize energy conservation. However, other evidence of this connection is mixed, particularly when considering seasonal trends in activity across age-sex classes. Our conclusions were partially limited by the absence of data for immatures in the early years of our data set and the inability to fully capture the active period during the rainy season. Nevertheless, the data set provides comprehensive evidence of differences in activity budgets, and thus energetic priorities, between specific age classes in a wild primate. Additionally, our findings support the hypothesis that subadulthood is a unique stage characterized by a combination of traits found in both adulthood, such as sexual maturity, and the juvenile period, such as social development (Pusey 1990). Future studies should investigate more specific age differences, such as in play and grooming behaviors, foraging efficiency, and food choice, to further elucidate the needs characterizing each age class.
Author Contributions
Catherine A. Byun: conceptualization, writing – original draft, writing – review and editing, data curation, formal analysis. Meredith C. Lutz: writing – review and editing, formal analysis, data curation. Rebecca J. Lewis: conceptualization, funding acquisition, methodology, writing – review and editing, data curation, supervision, project administration.
Acknowledgments
This manuscript was greatly improved by the suggestions from Jessica Rothman, Ivan Norsica, and an anonymous reviewer. We would like to thank the Malagasy government, MICET, CAFF/CORE, Madagascar National Parks, University of Antananarivo, staff of the Ankoatsifaka Research Station, Sifaka Research Project Assistants and graduate students who collected the majority of data over the years. Data collection for this project was financed by the University of Texas at Austin, The Leakey Foundation, Primate Conservation Inc., National Science Foundation BES# 1719654, and multiple private donors.
Ethics Statement
All data collection complied with the protocols approved by the IACUC at the University of Texas at Austin and adhered to the American Society of Primatologists Principles for the Ethical Treatment of Nonhuman Primates. All research followed the American Society of Primatologists Code of Best Practices for Field Primatology. All research adhered to the legal requirements of Madagascar where research was conducted.
Conflicts of Interest
The authors declare no conflicts of interest.
Open Research
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.




