Importance of Plant Galls to the Diet and Nutrition of a Frugivorous Primate, Varecia variegata
ABSTRACT
enFrugivorous primates may consume unusual food items, such as plant galls, to meet their nutritional requirements; yet, the contributions of these dietary components to their diet, nutrition requirements, and energy intakes are still unclear. We documented the importance of plant galls in these aspects for black-and-white ruffed lemurs (Varecia variegata) in a Malagasy rainforest. Using daily observation data of lemur foraging and nutritional analyses of their consumed items, we measured (1) the proportion of galls in their diet, (2) the rate at which they acquire nutrients and energy from galls compared to other food items, and (3) the changes in their diet patterns and acquisition of nutrients and energy with the consumption of galls. We also investigated whether they are more likely to consume fruits on trees with galls and characterized whether galls have similar characteristics as fruits. We found that plant galls constituted 12.96% of the lemur feeding occurrences; and on some days, lemur diets comprised galls only. Also, the lemurs acquired from galls higher protein than any other food items and higher sugar, fiber, and energy than leaves, but lower lipid than fruits and similar nutrients and energy as from flowers. The addition of galls in their diet significantly reduced their consumption of ripe fruits on a daily basis and increased the acquisition of protein, sugar, fiber, and energy. Also, lemurs were more likely to consume fruits on trees with galls than expected, likely due to similarities in color and nutrient components between galls and fruits in this system. These findings enhance our understanding of the nutritional needs and food selection behavior of primate frugivores. Such knowledge has implications for developing strategies to maintain primate populations in their natural environments and potentially to ensure the welfare of captive animals in ex-situ conservation settings.
Abstract in Malagasy
mgIreo gidro mpihinam-boa dia mety mihinana ravina, felana, ary taho, mba hamenoany ny otrikaina sy ny angovo ilainy isan'andro ankoatry ireo efa azony avy amin'ny voankazo. Ny sasany amin'ireo biby ireo dia mety mihinana lagaly amin'ny ravina zava-maniry, izay singa tsy dia mahazatra miendrika karazana vonto ateraky ny fitomboan'ny isan'ny selan-java-maniry rehefa misy bibikely manatody eo aminy. Mbola tsy dia tena fantatra anefa ny mety ho vokatry ny fihinanan'ireo biby mpihinam-boa izany lagaly amin'ny zava-maniry izany. Noho izany, ity fikarohana nataonay ity dia nentina handinihina ny mety ho tombotsoa azon'ny varijatsy, izay fantatra amin'ny anarana siantifika hoe Varecia variegata, amin'ny fihinana izany. Tao amin'ny ala mandon'Ihofa, faritra atsinanan'i Madagasikara no nanaovanay ity fikarohana ity, hamantarana ny hoe 1) firy ny tahan'ny lagalin-java-maniry ao anaty sakafony, sy ny otrikaina ary angovo entiny ho azy ireo? 2) mirona hihinana voa amin'ny hazo misy lagaly ve ireo varika ireo? ary farany, 3) manana toetoetra ivelany sy anatiny itovizana ve ny lagaly sy ny voankazo? Hita tamin'ny fotoana nanaovanay ny fikarohana fa ny 12.96% ny sakafon'ny varijatsy dia lagaly, ary an'ny andro sasany dia tsy misy afa-tsy lagaly ny sakafony. Ho fanampin'izay, mitondra proteina betsaka kokoa noho ny voankazo ny fihinanany lagaly ary manome angovo ambony kokoa. Nahita koa izahay fa mirona hihinana voankazo bebe kokoa amin'ny hazo misy lagaly ny varijatsy, izany dia mety noho ny lagaly manana loko sy tahan'otrikaina sasany mitovy amin'ny an'ny voankazo. Ireto voka-pikarohana ireto dia mampitombo ny fahalalana misy momba ireo gidro mpihinam-boa sy ny otrikaina sy angovo ilainy ary ny fisafidianany sakafo. Mety hanampy ihany koa amin'ny famolavolana paikady hitandrovana ireo gidro ireo eo amin'ny toerana voajanahary misy azy izany voka-pikarohana izany.
1 Introduction
Characterizing the nutritional needs and requirements of primate populations in their natural environments is fundamental for understanding their biology and health, given how nutrition can influence their physiological and metabolic functions (Felton et al. 2009; Oftedal et al. 1997). Insights about primate nutritional ecology can further contribute to advancing current knowledge about primate ecology, specifically regarding niche partitioning, competitive abilities, and social structure (Ganzhorn 1988; Hohmann et al. 2006; Leighton 1993). Also, like any other wildlife facing environmental changes, it is crucial to know if primates can continue to obtain adequate nutrition to sustain an already frail existence (Felton et al. 2009; Rothman et al. 2012). Thus, such knowledge can also be used for optimizing informed actions aimed at the conservation and restoration of their habitats (Chapman et al. 2004; Felton et al. 2010) and for addressing the nutritional requirements of captive individuals for their wellbeing in ethically ex-situ conservation settings.
Advances in primate nutritional ecology have revealed that nonhuman primates often devote most of their feeding time to one or two main food types, but also target other discrete food types to supplement their main diet (Britt 2000; Chapman 1995; Irwin et al. 2025; Overdorff 1988). They occasionally consume such other food types to obtain additional or specific nutrients that might be missing from their main food source. For example, some arboreal, frugivorous lemurs descend to the ground to consume soil, which can aid in gut detoxification by neutralizing toxins from certain plants they eat, provides essential minerals like manganese and iron enhancing enzymatic activities, and aids digestion by introducing beneficial microbes into their gut (Borruso et al. 2021; Britt 2000). Other supplementary food types in primate diets can include unusual structures on plants such as galls, which are abnormal growths caused by small organisms such as insects, nematodes, mites, fungi, bacteria, or viruses (Blanche 2012; Bryer et al. 2015; Deluycker 2012; Smith et al. 2022).
Consumption of galls has been documented across various primate taxonomic groups, including apes (e.g., Gorilla gorilla beringei; Vedder 1984), lemurs (e.g., Propithecus diadema; Powzyk and Mowry 2006), and monkeys (e.g., Cercopithecus ascanius; Bryer et al. 2015) (see Table S1 for additional examples). Yet, studies investigating the contribution of this type of food to the nutrition of primates are scarce. The limited studies on this topic demonstrated that galls can contain high fiber and minerals (such as calcium, magnesium, and potassium) and moderate levels of lipids, Nonstructural carbohydrates, and energy (Bryer et al. 2015; Du Bour 2018; Weiblen et al. 2010). Additionally, plant tissues with galls may have high nutrient levels such as amino acids, nitrogen, phosphorus, and potassium because certain insects that induce the formation of galls can control the quality of gall tissue for their own benefit (Chen et al. 2020; Diamond et al. 2008; Hartley 1998; Suzuki et al. 2009). Thus, examining the contribution of galls as well as other unusual food items to primate diet can provide a better understanding of dietary change, adaptation to seasonal food availability, and the role of fallback foods. Certain animals may consume these galls to provide them with additional energy and/or critical nutrients that are otherwise difficult to find, or as an alternative food when their preferred resources are scarce. This might be the case for certain frugivores that rely on unpredictable fruiting resources in their ecosystems (Gautier-Hion and Michaloud 1989; Lambert and Rothman 2015; Marshall and Wrangham 2007; Overdorff 1996; Wright et al. 2005). Documenting the consumption of galls and quantifying their nutritional composition may, thus, provide critical insights into whether animals select for galls to meet their nutritional needs.
To meet their daily nutritional needs, primates may exhibit a variety of foraging behaviors and preferences that allow them to find and select specific food. Primate frugivores, especially those that rely on visual cues to find and select fruits, may be attracted to galls on plants because of specific physical characteristics that make them resemble fruits. Gall morphology, such as conspicuous shape and color against the foliage background, may be visually attractive or confused with fruits (Isaias et al. 2013; Margulis and Fester 1991). Similar to fruits, the fleshy shape of galls may also advertise their nutrient content, which may influence the frugivore's food selection and tree visitation rates to meet their daily nutrient and energy requirements. Although it is well-accepted that nutrient content of plant items drives feeding behavior and selection, it is often unclear which specific nutrients are prioritized by the animal (but see: Beeby et al. 2023; Felton et al. 2009; Takahashi et al. 2021). For example, some frugivorous birds are more likely to forage and eat fruits with high lipid content and energy, but with moderate sugar to support flight demand (Lei et al. 2021); however, the dietary items of frugivorous primates in some regions are protein-limited and, thus, they may seek other food items that can provide such requirements (Donati et al. 2017; Ganzhorn et al. 2009; Irwin et al. 2025). Therefore, if galls can provide the same or even more nutrients than fruits, frugivores may be more likely to visit trees with galls and/or consume galls. Determining what characteristics of galls may be attractive to frugivores can help understand frugivore selection of fruits, as well as selection of their supplementary foods, and the broader factors that influence their foraging strategy.
In this study, we aim to investigate how the consumption of plant galls benefits a highly frugivorous lemur species, black-and-white ruffed lemurs (Varecia variegata), and influences their foraging patterns. This species inhabits the eastern rainforests of Madagascar and is classified as Critically Endangered in the International Union for Conservation of Nature (IUCN) red list (Baden et al. 2019). They spend up to 92% of their feeding time consuming fruits (Britt 2000 and this study - details in Figure S1). This species was also observed to consume galls occasionally (Beeby et al. 2023, Razafindratsima unpbl), making it a good system to address our main goal. Understanding the contribution of galls to their diet and nutrition can provide critical insights regarding potential changes and flexibility in their diets that may be critical for lemur conservation actions in the face of increasing environmental changes. We first assessed the contribution of galls to the diet, nutrition, and energy intake of these lemurs by measuring (1) the proportion of galls in their diet relative to other food items (fruits, leaves, and flowers), (2) the rate at which they acquire nutrients and energy from gall feeding compared to feeding on other food items, and (3) the change in diet patterns and acquisition of nutrient and energy on days when they consumed galls versus when they didn't. Then, we determined whether these lemurs visit and consume fruits on trees with galls more often than expected by chance. Finally, we characterized whether the galls in this system have similarities in traits with fruits (color, size, nutritional content).
2 Methods
2.1 Study Site and System
We conducted this study between January and October 2021 in the rainforest of Ihofa in the eastern part of Madagascar (18°45′ S and 48°24′−48°25′ E), located at 200 m to 1,200 m altitude. This forest is part of the Corridor Ankeniheny-Zahamena, situated near the Mantadia National Park, in the region of Alaotra Mangoro, and is currently managed by local communities. We carried out the project in three sites in this forest (Ambatabe, Bekalakody and Bevolohoto), where we were able to find black-and-white ruffed lemur groups (see map in Figure S2). We identified one group of black-and-white ruffed lemurs in each site; the group in Ambatabe was composed of five individuals, the one in Bekalakody had four and there were two individuals in the group in Bevolohoto. At each site, we randomly selected one individual adult in the group as a focal to collect data throughout the study; our focal individuals were composed of two males and one female. To differentiate the individuals, we relied on a noninvasive technique, that is, without darting animals to put a collar on. With this approch, we identified the focal individual based on particular characteristics, for example, extent of the black color on their fur relative to the white part. To ensure consistency with the identity of the focal animal that we observed, our 5-person team spent a week following each group at the beginning of the project to familiarize ourselves with the different individuals within the group and allow the lemurs accustomed to our safe presence. Then, at the start of each observation day, when we located an individual lemur, we visually checked, with binoculars, for the specific characteristics unique to each lemur to identify them accurately before collecting data. We often find them in the same sleeping site where we left them the previous day, making it even easier to know that we are following the right group.
2.2 Observations of Lemur Diet
We followed the focal animals for a total of 82 days (766.37 h) in Ambatabe, 71 days (597.87 h) in Bekalakody, and 85 days (547.03 h) in Bevolohoto. The lemur follows consisted of five successive days per week at each site, alternating between sites every week. Each direct observation took place from morning (at around 07:00 a.m.) until the group became inactive in the evening (at around 06:00 p.m.) or until we could no longer locate the group (total observation time: 1911.27 h). We used binoculars to watch the focal individual and collected data on each feeding event whenever it occurred (ad libitum sampling, Altmann 1974). We recorded the name of the plant species, geographic coordinates, start and end of feeding times, plant items consumed (unripe fruits, ripe fruits, flowers, leaves, and galls), the number of times that the lemur put a food item into its mouth and actually chewed and swallowed it (“bites”), and an estimate of the number of items per bite referred here as “intake sample”. For leaves, this intake sample refers to the proportion of leaf consumed based on what we observed as leftover on the leaf. We multiplied the bites by the intake samples to obtain a value of a “feeding bout”. Note that for gall consumption, these lemurs consumed galls on leaves only (see Figure S3 for photo illustrations), although some twigs also had galls; thus, the galls we mention in this study are those that were on leaves. To discriminate between feeding on galls or leaves, we closely observed what the focal individual picks up and puts in its mouth. If it consumes leaves that contain galls, we consider this as feeding on galls even though the lemur eats both the leaf and the gall together. We are confident that we were able to identify the specific food items that the lemurs consumed given that we had multiple people watching with binoculars and confirming what each person saw, and we often stood in an elevated structure facing the tree on which the lemur was feeding, whenever possible, to get a clear view. However, we recognize that human errors may be unavoidable. For each feeding tree, we measured the diameter at breast height (DBH in cm, taken at 1.30 m from ground level) and visually estimated its height (in m). We collected samples of unknown plant species for later identification at the National Herbarium of Madagascar in Antananarivo.
2.3 Macronutrient Content in Lemur Diet
In the field, we sampled about 100–200 g of wet weight of each food item per plant species consumed. Given that the galls that the lemurs consumed were always on leaves and the lemurs ate the whole leaf with the gall, we analyzed the galls along with the leaves still attached to them. In case of unavailable or insufficient items for a given species, we sampled the same item from other trees of the same species anywhere at the site. We weighed wet samples before drying them in a traditional charcoal oven. We kept the oven open during drying to allow air circulation and to keep temperature below 50°C, which we monitored using a thermometer. We considered samples as dry after having a constant daily weight for approximately 5 days depending on the thickness of the sample. We put the dried samples in paper bags and stored them in a dark box containing silica gel beads before taking them to the laboratory of FOFIFA (Centre National de Recherche Appliquée au Développement Rural, Madagascar) in Antananarivo for nutritional analyses.
In the laboratory, we evaluated macronutrient contents in the different food items using near-infrared reflectance spectroscopy (NIRS) method. This technique uses the reflectance of the food item at a certain wavelength to estimate its nutritional values (de Oliveira et al. 2014; Rothman et al. 2009, 2012) (see Supplementary Information for detailed methodology). FOFIFA used their existing reference database of nutrient values for Malagasy plant samples to perform the NIRS. We obtained estimated values of all nutrient and component concentrations in percent of dry matter such as lipid, crude protein, sugar, NDF (Neutral Detergent Fiber), ADF (Acid Detergent Fiber), cellulose, hemicellulose, lignin, and ash. We recognize that not all crude protein in the food may be available to the lemurs; but we currently do not have a measure of available protein, and the difference between crude and available protein is likely to be less for frugivores than for folivores, due to the high tannin content in some leaves (Wallis et al. 2012).
We were unable to obtain samples for some food items for certain species to analyze their nutritional contents (~35% of the types of food item and species consumed missing nutritional data). We imputed these missing values using a Multivariate Imputation by Chained Equations (MICE) with the package mice (version 2.9) (Buuren and Groothuis-Oudshoorn 2011) in R v4.2.2 (R Core Team 2022). MICE can preserve nonlinear relationships among the traits, allowing the imputed value to fall within the range of the available data (Johnson et al. 2021) and is considered a robust method for handling missing data (Buuren 2018; Buuren and Groothuis-Oudshoorn 2011). The MICE function generates multiple imputations (50 iterations) for the missing values of each trait based on the available values for that specific trait. We selected the Predictive Mean Matching (pmm) method as the imputation model, which is appropriate for numeric data (Buuren 2018). For each missing entry, this method first selects a set of complete cases with predicted values similar to the predicted value of the missing entry (candidate donors), then randomly selects one of these donors and uses the observed value from the donor to replace the missing value (Buuren and Groothuis-Oudshoorn 2011).
2.4 Acquisition Rate of Nutrient From Galls Versus Other Items
We assessed the rate at which lemurs acquired daily nutrient intakes from galls relative to other food items by examining how the acquisition rates of different macronutrients (lipid, protein and sugar), and fiber (NDF) from each item consumed varied among these items.
is the daily intake of nutrient y (expressed in grams); B is the number of feeding bouts in the day; is the duration of feeding bout i (expressed in minutes); is the average intake rate (units/min) for food (plant item part and species combination); is the mass of one dried intake unit for food x expressed in grams; refers to concentration of nutrient y in food item (percent of dry matter). Then, for each item consumed (ripe fruit, unripe fruit, gall, leaf, flower, and other), we measured the acquisition rates by dividing the values of these intakes by the time spent consuming the item in a given day.
We compared these nutrient acquisition rates from galls versus other food items by performing Linear Mixed Effects Models (LME) using the R-package lmerTest (Kuznetsova et al. 2017), in which we set the item “galls” as a reference level for all comparisons. We considered the acquisition rate as the response variable and the food type as fixed effects. Also, we treated as random effects the following variables: average tree height, average DBH, and the number of tree species that the lemur consumed per day. These variables were included as random effects because foraging behavior and dietary choices may depend on plant size, particularly for large-bodied animals (Semel et al. 2022). Larger trees may produce more fruits, making them more attractive, and provide greater structural support for larger frugivores, biasing their feeding selection. Additionally, the diversity of plant species in the diet can influence feeding patterns (J. P. Herrera 2016; Yamashita 2002). A more diverse diet might result in smaller quantities of each plant species and food types being consumed, while a less diverse diet could lead to larger quantities of one or a few plant species and food types being eaten (Chaves et al. 2023). We excluded from the analyses any outliers that we observed from an initial visualization of the data. To do so, we applied the interquartile range (IQR) criterion to identify and remove these outliers (Vinutha et al. 2018).
2.5 Acquisition Rate of Energy From Galls Versus Other Items
CP represents Crude Protein. TNC is the Total Nonstructural Carbohydrates for each food, calculated as TNC = 100 – (Lipid + CP + Ash + NDF). All values are expressed in % of dry matter. Dig. cellulose & Dig. hemicellulose refers to the digestibility for cellulose and hemicellulose respectively, which we obtained from the literature on captive Varecia variegata fed diet 30ADF, that is, diet with fiber concentration 30% ADF (Edwards and Ullrey 1999): 0.119 and 0.403 respectively. Diet 30ADF maintains captive Varecia body weight at 4.10 kg (Edwards and Ullrey 1999), which is approximate to the average body weight at 3.7 kg of a wild Varecia (Baden et al. 2008). Similar to the analysis with the acquisition rates of nutrients above, we also performed an LME to compare the energy acquisition rates from galls versus other food items. We also excluded outliers from the data using the IQR approach.
2.6 Changes in Diet Patterns, Nutrient, and Energy With the Consumption of Galls
We quantified whether the addition of galls to the lemur diet influences the shift in feeding preference or the quantity of food consumed each day. To do so, we first compared the percentage of feeding occurrences on each item between the days that they consumed galls (N = 124 days) and the days that they did not consume galls (N = 114 days). Then, we compared the total weight of food consumed on days with versus without galls in their diet, calculated as intake rate multiplied by the mass of one dried intake unit for specific food item and plant species combination. We run Generalized Linear Models with a gamma distribution to test for the statistical significance of the observed differences. For the total weight of food consumed data, we removed outliers using IQR approach before running the model.
Additionally, we assessed how much the addition of galls in their diet contributes to the acquisition of nutrients and energy of these lemurs on a daily basis. We compared the total acquisition rates gained from all food items for each nutrient type (lipid, protein, sugar, fiber) and energy between those two categories of days (days with galls vs. days without galls). We used data without outliers and also performed GLM with a gamma distribution to test for the statistical significance of the differences.
2.7 Lemur Feeding Tree Selection
We determined whether these frugivorous lemurs are more likely to visit and consume fruits from trees with galls than expected by chance by examining the association between fruit consumption on gall-infected and noninfected trees (“observed”) relative to the availability of these categories of tree infection in their habitats (“expected”), using a Chi-square test.
We assessed the presence of galls on each tree in which the focal individual fed during the aforementioned lemur follows. After the lemur finished eating and left the feeding tree (immediately after or on the following day if needed), we used a telescoping pole or climbed the tree to collect leaf and twig samples. We randomly collected 100 to 200 leaves per individual tree, depending on availability, making sure that we did not collect more than 5% of the leaves and/or twigs on a given tree. We checked for the presence of galls on these leaves and twigs. We considered any tree without galls as “noninfected” and any tree with galls as ‘infected’. We obtained the availability of each category of tree infection in each site by assessing the presence of galls on each tree within botanical plots. To do so, we set up 10 plots of 2 m × 50 m alternated along a linear transect of 500 m in each site (Gentry 1982; Phillips et al. 2003). Within each plot, we identified and tagged each tree with a DBH ≥ 5 cm. We used the same approach as with the lemur feeding trees for gall assessment.
2.8 Comparison of Gall and Fruit Characteristics
To characterize whether galls share similarities in characteristics (color, size, and macronutrient) with fruits, we randomly collected up to five galls and five fruits from trees on which lemurs consumed fruits and from all trees in the botanical plots (including those on which the lemurs did not feed), as available. In total, we sampled 1443 galls from 41 tree species and 399 fruits from 22 tree species (Table S2). We visually determined and categorized the color of each of these galls and fruits using broad color categories based on human perception of color: Red, Blue, Yellow, Green, Orange, and Purple. We did not differentiate between variations in color saturation (e.g., between dark or light red). We also measured the size of each fruit and gall along three dimensions using a caliper: length (along the longest axis), width (second longest axis perpendicular to the length), and thickness (the thinner side of the gall or fruit); for gall measurement, we did not include the leaf or twig to which the gall was attached. For round-shaped galls or fruits, the width and thickness values were the same. We represented size by calculating volume as: length × width × thickness (Pinheiro et al. 2023).
We compared the color categories of galls versus fruits by describing the frequency distribution of the color categories. For size, we compared the average volume between galls and fruits using Welch Two Samples t-test (Ahad and Yahaya 2014). We identified and excluded from this analysis any outlier using the interquartile range (IQR) method (Vinutha et al. 2018). We also performed a Welch Two Samples t-test to compare the differences in sugar content in galls versus fruits using the available data (i.e., values obtained from the MICE imputation not included) from the nutritional analyses described above (N = 6 galls and 24 fruit samples). For the differences in lipid, protein and fiber, which did not follow the normal distribution, we performed Mann-Whitney U test (Nachar 2008).
3 Results
3.1 Contribution of Galls Versus Other Food Items to Lemur Diet
During our study period overall, black-and-white ruffed lemurs fed on 42 known plant species (including trees, lianas and epiphyte), two unidentified tree species, and one unidentified liana species (Table S3). They consumed different parts of the plants; the majority of their feeding occurred on ripe fruits (81.2%), followed by galls (12.96%), leaves (4.32%), unripe fruits (1.06%), flowers (0.38%), and other parts (0.07%) (Figure S1; N = 1319 feeding occurrences). These percentages slightly varied across months during our study period (χ2 = 238.39, p < 0.001, Figure S4) with the consumption of fruits relatively high across all months, while gall consumption varied, ranging from 3.54% to 27.42% per month). Among the tree species consumed, three were for the consumption of galls only, three for both galls and fruits, one for galls, fruits and leaves, and the rest for the consumption of fruits and/or other items (Table S3).
At the daily level, the lemurs devoted on average 14.47% of their feeding time to galls (up to 100%), while 79.97% of time spent feeding on ripe fruits (up to 100%), 1.30% on unripe fruits (up to 66.7%), 3.93% on leaves (up to 33.3%), and 0.29% on flowers (up to 30%). Thus, on some days, these lemurs ate only galls or only fruits for the entire day.
3.2 Macronutrient, Fiber, and Energy Acquisition Rates From Galls Versus Other Items
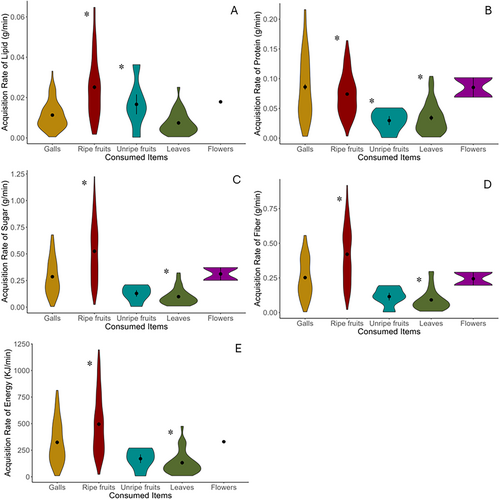
The acquisition rates of macronutrient, fiber, and energy varied among the food items consumed by black-and-white ruffed lemurs (Table 1; Figure 1). Specifically, the acquisition rate of lipid from galls was significantly lower than that from ripe fruits and unripe fruits. However, galls delivered higher acquisition rates of protein than ripe fruits, unripe fruits, or leaves. Additionally, the acquisition rates of sugar, fiber and energy from galls were much lower than those from ripe fruits, but considerably higher than those from leaves. Galls and flowers did not show a significant difference in nutrient and energy acquisition rates.
| Food item | Lipid | Protein | Sugar | Fiber | Energy |
|---|---|---|---|---|---|
| Ripe fruits | β = 0.01 | β = −0.02 | β = 0.24 | β = 158.60 | β = 0.17 |
| t111.27 = 9.38 | t176.70 = −3.29 | t145.77 = 8.00 | t158.60 = 5.26 | t142.28 = 7.51 | |
| p < 0.001 | p = 0.001 | p < 0.001 | p < 0.001 | p < 0.001 | |
| Unripe fruits | β = 0.02 | β = −0.06 | β = −0.07 | β = −72.51 | β = −0.06 |
| t175.48 = 2.01 | t246.22 = −2.11 | t234.27 = −0.42 | t235.67 = −0.44 | t227.83 = −0.50 | |
| p = 0.04 | p = 0.03 | p = 0.67 | p = 0.66 | p = 0.61 | |
| Leaves | β = −0.00 | β = −0.05 | β = −0.18 | β = −193.60 | β = −0.16 |
| t137.68 = −1.32 | t224.37 = −5.09 | t200.90 = −2.84 | t208.31 = −3.055 | t200.77 = −3.34 | |
| p = 0.18 | p < 0.001 | p = 0.005 | p = 0.001 | p = 0.001 | |
| Flowers | β = −0.00 | β = −0.01 | β = 0.02 | β = −64.06 | β = −0.02 |
| t168.85 = −0.07 | t246.74 = −0.97 | t235.46 = 0.11 | t235.28 = −0.28 | t230.54 = −0.15 | |
| p = 0.94 | p = 0.66 | p = 0.91 | p = 0.78 | p = 0.88 |
3.3 Changes in Diet, Nutrient and Energy With the Addition of Galls in the Diet
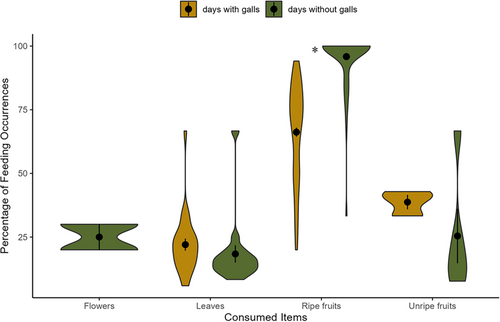
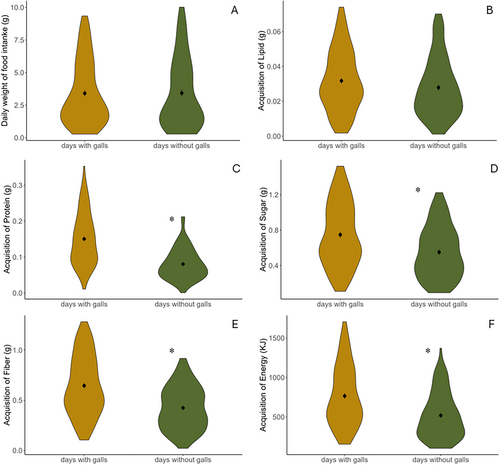
We found that the percentages of food items consumed on days with versus without galls were significantly different (t = 6.98, p < 0.001). This difference was mainly driven by ripe fruits, such that the addition of galls in the daily diet of lemurs led to significantly reduced consumption of this type of food (Figure 2, p < 0.001). However, the percentages of leaves and unripe fruits consumed did not significantly differ between the days with and without galls, although they consumed more of these food items on days with galls (Figure 2; leaves: t = −0.03, p = 0.98; unripe fruits: t = 1.29, p = 0.20). Despite the shift in more consumption of fruits, the addition of galls in the diet did not significantly affect the total weight of food consumed (Figure 3A; t = 0.06, p = 0.95) and the acquisition rate of lipid (Figure 3B; t = −1.72, p = 0.09). However, it significantly increased the acquisition rates for protein (Figure 3C; t = −9.47, p < 0.001), sugar (Figure 3D; t = −4.65, p < 0.001), fiber (Figure 3E; t = −6.65, p < 0.001) and energy (Figure 3F; t = −5.56, p < 0.001). We also found that lemur consumption of flowers occurred only on days without galls in their diet.
3.4 Lemur Feeding Tree Selection
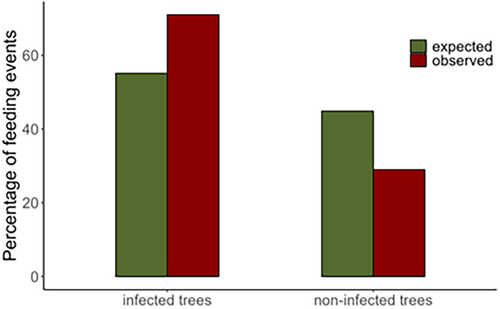
We found that lemur consumption of fruits on infected trees was significantly higher than expected based on what is available in their habitats (χ2 = 4.76, p = 0.03; Figure 4).
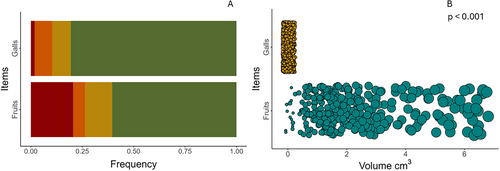
3.5 Comparative Analyses of the Characteristics of Galls Versus Fruits
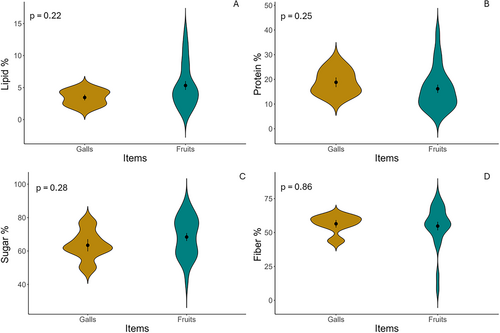
Overall, the galls and fruits in this system displayed similar color categories, although the distributions of the color categories were different (Figure 5A). However, when comparing the color category of the galls and fruits on the same trees, we found that only two individual trees of Grewia cuneifolia (Malvaceae) had galls and fruits in the same category, which was green (based on an assessment of 370 individual trees in the plots and on which the lemurs fed; Table S2). Also, on average the galls were significantly smaller in volume than the fruits (t = 25.39, df = 344.02, p < 0.001; Figure 5B). Regarding the nutrients, galls were statistically similar to fruits in terms of lipid (w = 96, p = 0.22), protein (w = 49, p = 0.25), sugar (t = 1.13, df = 9.67, p = 0.28), and fiber contents (w = 0.68, p = 0.86) (Figure 6A–D).
4 Discussion
Unusual food items like plant galls can provide important nutritional value to primate frugivores as a complement, or even alternative, to fruits. The black-and-white ruffed lemurs at Ihofa, which are highly frugivorous with 81.2% of fruits in their diet during our study period (Figure S1), showed substantial consumption of plant galls, and these galls had significant consequences for observed acquisition rates of nutrients and energy. The rates of acquisition for some critical nutrients, particularly protein, were higher from galls than from other food items including ripe fruits that constituted the bulk of their diet. Given their broadly fruit-based diet, it is likely that protein is a limiting nutrient for growth and reproduction (Donati et al. 2017). We also found that galls were used to supplement or replace ripe fruits as the consumption of fruits was lower on days that had galls in their diet. This shift suggests that galls may be used to obtain additional nutrients that may be lacking with the absence of fruits in the diet. Despite the total weight of food intake being the same on days that the diet contained galls or not, lemurs acquired higher levels of nutrients (specifically protein, sugar and fiber) and energy on days when their diet included galls. These results suggest that V. variegata may balance their diet and nutrition by consuming galls during periods of preferred food scarcity. This finding is consistent with the research of Beeby et al. (2023), which highlights the ability of this species to adjust their nutrient intake in response to seasonal variations in food availability. In addition, the lemurs fed fruits on trees with galls more than expected by chance. Although the reasons for this attraction to infected trees were not systematically investigated, warranting further studies, it is possibly driven by the similarities of fruits and galls in terms of color in the system, with galls acting as an attractant and imitating larger crop size. Indeed, frugivores, including lemurs, use visual cues to detect and visit particular fruiting plant species (Corlett 2011; Flörchinger et al. 2010; Melo et al. 2011; Nevo et al. 2018; Valenta et al. 2013, 2018; Willson and Whelan 1990).
Consumption of plant galls by frugivorous and folivorous lemurs has been reported in other systems, but is not very common (Du Bour 2018; Powzyk and Mowry 2006; Beeby et al. 2023, Razafindratsima unpbl.). For instance, Beeby et al. (2023), working with the same species of V. variegata in the Ranomafana National Park in eastern Madagascar, observed only one gall consumption event over a 12-month period. In the same location, gall consumption constituted < 0.15% of feeding occurrences for two other frugivorous lemurs, Eulemur rubriventer and E. rufifrons, monitored in their natural environments for almost 2 years (Razafindratsima unpbl.). For E. rubriventer in Tsinjoarivo, consumption of galls occurred only during the lean season and constituted only 0.9% of their lean season diet (Du Bour 2018). However, our data show that V. Variegata in the rainforest of Ihofa devoted 12.96% of their feeding time to galls during our 9-month study, though the consumption of galls fluctuated across months. The observed differences in the consumption of galls among the different lemur species may be due to differences in habitat structure across these studies. In fact, habitat structure can influence the distribution of plants that produce galls, as well as the gall-inducing insects (Egan and Ott 2007; Fernandes et al. 2005; Ribeiro-Mendes et al. 2002).
As the galls in this system had similar concentrations of nutrients as fruits, consuming galls may help these lemurs better sustain their daily activities and support their daily nutritional requirements. This is critical because they may be limited in nutrients as they rely on unpredictable fruiting resources (Overdorff 1996; Wright et al. 2005). In addition, fruits in tropical forests, especially in Madagascar, are usually low in proteins that can provide critical energy (Donati et al. 2017; Ganzhorn et al. 2009; Rode et al. 2006). Nevertheless, galls do not necessarily need to have higher nutrients than fruits to be useful for lemur nutrition; the addition of galls in the diet may help supplement important nutrients, especially during periods when they might be more available for consumption.
The similar acquisition rates of nutrients and energy of galls and flowers suggest both items may have comparable nutritional value, potentially providing similar benefits to V. variegata. However, given the small sample size we have for flowers in this study, we are unable to make such a comparison. Flowers have been identified as among the fallback foods for many lemur species, including V. variegata (Beeby et al. 2023; Irwin et al. 2014, 2025). The consumption of flowers in this study occurred only on days when galls were absent from their diet. Additionally, the consumption of both items occurred at different times of the year, with an increase in flower consumption corresponding to a decrease in gall consumption (Figure S4). This observed pattern suggests a flexible dietary strategy, allowing V. variegata to adapt their diet based on the availability of different food sources. It also highlights the importance of both galls and flowers as alternative food resources, helping lemurs maintain their energy balance and overall health when fruits are scarce.
The increased energy in the diet of the lemurs in this study resulting from the consumption of galls may be due to the high concentration of certain macronutrients that may significantly contribute to daily caloric intakes (Felton et al. 2009). Our findings indicate that galls contribute more to protein and energy, but not lipids, likely due to their composition being similar to the leaves on which they are attached. Since leaves generally have lower lipid content and higher protein levels than reproductive plant parts (Irwin et al. 2014), and lemurs in our study consumed galls still attached to the leaves, the nutritional contents of these consumed galls might be influenced by the nutritional content of the leaves. Moreover, the insects' presence within the galls could modify the protein profile. For instance, they might release enzymes or other proteins into the gall tissue, or their feeding behavior could prompt the plant to synthesize additional proteins, further elevating the amount of protein available to the lemurs (Costa et al. 2021). In contrast, lipid production in the plant tissue is less affected, likely because lipids primarily serve structural functions or act as long-term energy reserves, which may not be as advantageous for the insects (Costa et al. 2021). Additionally, as the vast majority of galls on epigeal plant organs are induced by insects (de Araújo et al. 2021), they may bring additional protein to the lemur diet if the insects did not vacate the galls before lemur consumption (Barnett et al. 2017; Bryer et al. 2015); although the presence of insects in the galls was not thoroughly checked in this study. Indirect insectivory may actually be common among primate frugivores as they may also be ingesting a significant amount of immature insects inside the fruits as well (Redford et al. 1984; Rowe et al. 2021). While the consumption of galls by black-and-white ruffed lemurs was much lower compared to fruits (only 12.96% vs. 81.2%), the acquisition rate of protein from galls was much higher than that from fruits, although the protein content in galls was not significantly higher than fruits (but the mean was 2.64% higher). Thus, despite low protein content, the consumption of galls can help lemurs with their daily protein requirements, although our small sample size for comparing nutrients in galls versus fruits may be part of the reason of the non-significance. This increase in protein acquisition may be due to several factors, including the nutritional characteristics of leaves still attached to the galls, the physiological processes in gall cells during some stage of their formation, the presence of insects within the galls (Desnitskiy et al. 2023; Ferreira et al. 2022; Miller and Raman 2019; Rohfritsch 2010), or because of other factors warranting further study. Specific investigation into the contributions of insects to the nutrition of ruffed lemurs may also provide a better understanding of potential indirect insectivory in these highly frugivorous species.
The tendency of black-and-white ruffed lemurs to feed on trees infected with galls may be due to the relatively higher abundance of infected trees than noninfected in the habitat (Figure 4) or potentially the similarities in color of galls and fruits in this system, that is, the galls become as conspicuous as fruits from the foliage. Thus, given that fruit color play a key role in attracting animal frugivores as they play critical role as seed dispersers (Lomáscolo and Schaefer 2010; Schaefer 2011), the color resemblance between galls and fruits may appear to indicate a large crop size on the tree, increasing lemur attraction to the tree. Such potential influence of other non-fruit food items in food selection among primate frugivores warrants further study. If this food selection pattern is maintained during low fruit availability, galls may present a high-quality fallback food during such a period. When fruit abundance is low, the foraging behavior and decision of frugivores may entirely rely on the spatiotemporal distribution of fallback food (Hanya and Chapman 2013; Irwin et al. 2014), which are often available when the preferred resources are scarce (e.g., figs for the case of red-tailed monkeys, blue monkeys and mangabeys in Kibale, Uganda) (Chapman et al. 2005), or protein-rich food items such as leaves (Beeby et al. 2023; G. L. Herrera et al. 2002; Irwin et al. 2014). A better understanding of the foraging ecology of frugivores over time and across seasons with varying availability of primary and fallback food resources, and whether they increase or decrease energy expenditure in the face of scarcity, will be important to understanding variations in their nutritional composition, requirements, and balancing.
In conclusion, our findings suggest that the nutritional needs and foraging behavior of highly frugivorous lemurs may depend on other plant food items in addition to fruits. Also, our study provides new insights into the understanding of the importance of insect-induced galls in the diet and nutritional needs of frugivorous primates, with some conservation implications. By presenting a broader picture of the nutritional ecology of black-and-white ruffed lemurs, we are offering a basis for better understanding their foraging ecology and behavior and opening an avenue for investigating in-depth the importance of insects in their diet. Moreover, in addition to our basic knowledge of nutritional profiles of the frugivorous black-and-white ruffed lemurs, understanding their consumption of galls could also help in selecting plant species for the restoration of their habitats. Some plant species were consumed for their galls only, indicating a unique dietary preference that could be critical for their nutrition and overall lemur health. This insight is particularly valuable for conservation efforts, as black-and-white ruffed lemurs are Critically Endangered (Baden et al. 2019). Such knowledge can be used to guide the selection of plant species that are important food sources for V. variegata in habitat restoration efforts, with particular attention to those that often contain insect-induced galls. Therefore, ensuring the presence of gall-producing plants in restoration projects could enhance the success of habitat restoration and support the sustainability of these lemur populations. By prioritizing the inclusion of these specific plant species, conservationists can create more resilient and self-sustaining ecosystems that cater to the lemurs' natural dietary needs. Furthermore, this approach can also contribute to the preservation of plant diversity and ecological balance within the restored habitats, ultimately benefiting a wide range of species and promoting biodiversity conservation.
Author Contributions
Rindra H. Nantenaina: conceptualization (lead), data curation (lead), formal analysis (lead), funding acquisition (lead), investigation (lead), methodology (lead), project administration (lead), resources (lead), supervision (lead), validation (lead), visualization (lead), writing – original draft (lead), writing – review and editing (lead). Mitchell T. Irwin: methodology (equal), validation (supporting), writing – review and editing (equal). N. Nancia Raoelinjanakolona: investigation (supporting), methodology (equal), project administration (supporting), validation (supporting), writing – review and editing (equal). Verohanitra M. Rafidison: conceptualization (supporting), methodology (supporting), supervision (supporting), writing – review and editing (supporting). Vonjison Rakotoarimanana: conceptualization (supporting), methodology (supporting), supervision (supporting), writing – review and editing (supporting). Walter S. de Araújo: methodology (equal), validation (supporting), writing – review and editing (equal). Onja H. Razafindratsima: conceptualization (equal), formal analysis (equal), funding acquisition (equal), investigation (supporting), methodology (equal), resources (equal), supervision (lead), validation (equal), writing – original draft (equal), writing – review and editing (equal).
Acknowledgments
This study was funded by the Rufford Foundation for Nature Conservation (Nos. 25325-1 and 31073-2) and Idea Wild to R.H.N., as well as support from the University of California Berkeley and Dr. Peter Long at Oxford Brookes University. We thank the community-based management association VOI FITAMA and the Ministère de l'Environnement et du Développement Durable in Madagascar for research permits (Permits No 002/21 and No 325/21/MEDD/SG/DGGE/DAPRNE/SCBE.Re). We are grateful to the association Ary Saina for logistics. We would also like to thank Evangeliste Randriamanantena, Nirina Randrianantenaina, Mamitiana Randriamanantena, Gabriel Rafaliarimanana, Elicien Lovasoaniaina, Kotonandrasana Ndrenasoa, and Benoro Ratobivelo for their expertise in the field. We owe many thanks to the team of FOFIFA Ampandrianomby led by late Dr. Tsirinirina Razafinarivo for helping with the nutritional analysis. We thank Dr. Tsiory Andrianavalona for lending us some field materials. We would like to thank Mad Randrianasolo and Anjaratiana Tafitamahandry at Association Mitsinjo, Benja Rakotonirina and Elyna Mahitasoa at the Institut Malgache de Recherches Appliquées (IMRA), and Razafindrahaja Vololotahiana at the National Herbarium of Madagascar at the Parc Botanique et Zoologique de Tsimbazaza (PBZT) for helping plant species identification. We also thank Angelo Andrianinaina and the Coding for Conservation program (grant award #102825) - C4C team led by Dr. Cara Brook for their valuable feedback in the statistical analysis. Special thanks to the members of the Razafindratsima lab at the University of California Berkeley for their insightful comments that improved an earlier version of this manuscript. Research complied with the protocols approved by the Ministère de l'Environnement et du Développement Durable in Madagascar for research permits (Permits No 002/21 and No 325/21/MEDD/SG/DGGE/DAPRNE/SCBE.Re) and adhered to the American Society of Primatologists (ASP) Principles for the Ethical Treatment of Nonhuman Primates.
Open Research
Data Availability Statement
The data that support the findings of this study will be made available in a public repository upon acceptance of the manuscript.




