Physiologic Consequences of Housing Adult Male Cynomolgus Monkeys (Macaca fascicularis) in Heterosexual Pairs: A Pilot Study Using Implanted Biotelemetry
ABSTRACT
Facilities may forgo attempting to socially house adult males due to fear of animal injury, study disruptions, and confounding data results. To leverage the potential advantages of male–female pairs, and to understand the impact on measures typically used in safety pharmacology studies, we measured activity as well as physiologic parameters in vasectomized male-female pairs: heart rate, blood pressure, and body temperature. Seven singly housed males that were previously implanted with telemetry were vasectomized and paired with females. Data were collected before and after pairing at specific timepoints in the first study. A second study employed four nonvasectomized, singly housed males to determine if the physiologic effects observed in the first study could be due simply to the increased cage size due to pairing. Results indicated that pair-housing with a female resulted immediately in a significant increase in blood pressure, body temperature, and heart rate. Over the course of a week of being paired, the males showed a significant decline in blood pressure; body temperature remained elevated, though at a lower level than during the immediate response. The second study suggested that increased cage size alone could not account for the immediate results in Experiment 1, inasmuch as no effects were found on our measures. Although the sample sizes for the studies were small, we discuss how our results are similar to, and differ from, previous studies, as well as the clinical significance and welfare implications. This information may be useful in designing long-term studies using sexually mature males while providing stable social support to animals.
Summary
-
Pairing of vasectomized, young adult male long-tailed macaques with females resulted in an immediate increase in blood pressure, body temperature, and heart rate, as assessed by implanted telemetry.
-
By the end of a week of living together, males' blood pressure dropped below that of when the males were housed singly, and body temperature remained somewhat elevated.
-
Our second experiment suggested that the physiological changes that we saw were related to the pairing, and not simply to the larger size of the pairing cages.
1 Introduction
Research has demonstrated the benefits of social housing to macaques (see review by DiVincenti L and Wyatt 2011). Studies have shown, for example, that pair housing, compared to single housing, results in a reduction of abnormal behaviors, ability to cope with stress, and ability to display species typical behavior. In a study of pair-housed adolescent male rhesus macaques, paired animals exhibited fewer undesirable behaviors such as self-clasping, fecal smearing, hair plucking and hunched posture (Jackson et al. 2023). In the same study, hunched posture associated with depression was observed in more single than paired subjects. Another study found that abnormal behavior decreased in paired housing in both male and female cynomolgus macaques (Koyama et al. 2019). Cassidy et al. (2020) reported that female rhesus exhibited higher levels of inactivity and self-directed behavior when separated due to intermittent pair-housing in comparison to continuous pair housing. Stressful events that commonly occur in a research setting can also be mitigated by pair housing which has a social buffering effect (Gilbert and Baker 2011; Baker et al. 2012). As reviewed by Schapiro and Bloomsmith (1994), grooming and play were the most common socially directed activities in paired, juvenile rhesus macaques.
So clear are the welfare benefits of social (pair) housing, that regulatory agencies, such as the United States Department of Agriculture (USDA), have made social housing the default condition for nonhuman primates (Animal Welfare Act 2008). Exceptions to social housing are allowed only in cases where animals are overly aggressive, are incompatible, are considered by the attending veterinarian as having health issues, or for scientific justification. The Guide for the Care and Use of laboratory Animals (Institute of Laboratory Animal Resources US. Committee on Care, & Use of Laboratory Animals 2011) states that single housing should be the exception and justified based on experimental requirements or veterinary or health related concerns.
Some facilities observe aggression and difficulty in maintaining male macaques in pairs in comparison to female - female or male - female pairs. Crockett et al. (1994) evaluated the effects of same-sex pair housing on psychological well-being of adult male and female cynomolgus macaques and found that while 100% of female pairs were compatible, only eight of fifteen male pairs were still together after 2 weeks. Truelove et al. (2017) reported that pairing males with females had the highest success rate. Paired males may see a decrease in abnormal behavior after initial introductions, but in one study (Doyle et al. 2008), the reduction of abnormal behaviors was not statistically significant after three to 5 months. In a retrospective study (Logan and Sayers 2024), adult male pairs were reported to have a 72% success rate of pairing, defined as 2 weeks in full tactile contact without aggression or injury. Kezar et al. (2022) observed that, using a gradual phased introduction, adult male rhesus were paired with only a 45% success rate; when male pairs were introduced using diazepam before full contact, however, the success rate of introductions was 94%. Longer duration of single housing, as well as increased age were predictive of failure when males were introduced gradually. McGrew (2017) has described commonly employed pairing strategies to facilitate positive, and minimize or eliminate negative, outcomes.
The cynomolgus macaque is a Nonhuman primate (NHP) representing one of the most commonly used large animal models in preclinical, safety toxicology studies, as it shares anatomic and physiologic similarities to humans (Carlsson et al. 2004; Li et al. 2023; Niehoff et al. 2010). In our safety pharmacology studies, typical study design includes the use of paired males that are implanted with telemeters when they are subadults or approaching sexual maturity. As attrition and study reassignment occurs, males may reach sexual maturity and display more aggression to maintain their hierarchy, making it more difficult to maintain them in same-sex pairs for these studies. Maintaining male and female pairs can be more manageable. Pairing males with females may help mitigate aggressive behavior and can lead to a more stable and less stressful environment for the subjects, ultimately improving the quality of the data collected. We sought to leverage the often-easier task of male-female pairing to create longer term, stable pairs as an option to address the social housing needs of sexually mature males that might be overlooked in social housing decisions.
To understand the behavioral and physiological consequences of this pairing paradigm, we measured heart rate, blood pressure, body temperature, and physical activity, which are the principal measures used in safety pharmacology studies. Physiological measures such as these have rarely been employed as outcomes of social pairing studies, presumably because acquiring these data in a fashion that is uncontaminated by the measurement process itself, requires implantable biotelemetry or use of a tether system. Two relevant studies did utilize such technologies: Coelho et al. (1991) used a tether and catheter system to measure HR and blood pressure (BP) in adult male baboons, and Doyle et al. (2008) used implantable telemetry to assess heart rate (HR) in young adult male rhesus monkeys. In both cases, relationships were found between social housing and these physiological measures (results described in detail in General Discussion).
In the present report, we performed an experiment using seven, Mauritian sourced, cynomolgus macaque males that were part of a safety pharmacology colony and had been previously implanted with telemetry devices. These animals were selected based on good health, and a history of single housing in a male only study room. Females were selected from the colony and had no history of injurious aggression and had only been paired with other females in different mixed-sex rooms. To prevent breeding, males were vasectomized before pairing and data were collected before and after pairing at specific timepoints. We were interested in two questions. First, what is the immediate response to pairing? We compared days when the males were unpaired with the first day of pairing, and we expected we might see elevations in blood pressure (BP), body temperature (BT), and physical activity (PA), but not heart rate (HR), as others (Doyle et al. 2008) reported no elevations in HR upon initial introduction. The second question was, what are the longer-term benefits of pairing? We examined data collected after approximately a week of continuous social housing, and expected that pairing would result in a decline in HR and BP. Assuming animals were compatible, we expected that pairing might result in elevated BT, as animals huddled together. We were less certain in our predictions of physical activity; if animals were active while housed alone, then activity might decline as animals spent more time grooming, huddled, etc. On the other hand, if animals were showing a more huddled posture while housed singly, activity might increase upon pairing.
Prior studies of the benefits of pairing are usually confounded, in that the paired condition also includes an increase in available space (e.g., a barrier is removed between two adjacent cages, resulting a doubling of floor space). For this reason, we conducted a second study, using four non-vasectomized males, and compared data when animals were housed in a single-size cage versus when they were housed in a double-size cage (where height remained the same, but floor space was doubled). If the benefits of pairing were due only to the increased cage size, we would expect to see differences in our measures in the double-cage condition.
2 General Methods
In this section we describe subjects, materials, methods, and procedures that were common to both studies.
2.1 Subjects
All cynomolgus macaques (Macaca fascicularis) were of Mauritian origin with a specific-pathogen-free status to exclude cercopithecine herpesvirus 1, simian beta-retrovirus 1-5, simian T-lymphotropic virus, simian immunodeficiency virus, and Mycobacterium tuberculosis. All animals were housed and maintained in accordance with the “Guide for the Care and Use of Laboratory Animals” (Institute of Laboratory Animal Resources US Committee on Care, & Use of Laboratory Animals 2011). The facility is accredited by the Association for the Assessment and Accreditation of Laboratory Animal Care International. All work performed on animals was in accordance with regulations and established guidelines and were reviewed and approved by an Institutional Animal Care and Use Committee. In addition, all procedures were in compliance with the American Society of Primatologists Principles for the Ethical Treatment of Nonhuman Primates.
Males were sexually mature and housed in an indoor facility. Males were housed singly in Experiments 1 and 2 due to documented incompatibility or aggression following isosexual pair housing attempts or a breakdown of compatibility following a period of successful paired housing. Males were part of a Safety Pharmacology colony and had been previously implanted with a telemetry transmitter (details below). The transmitter is social-housing compatible within a wide range of enclosures.
2.2 Housing and Husbandry
One small, dedicated study room was used for both studies. There were two to three banks of cages facing each other and no more than twenty animals housed in the room; all animals had a view of each other. Animals were housed in stainless steel cages measuring 30″ × 30″ × 32.5″ (L × H × W) when housed singly. During the pairing conditions of Experiment 1 and the large cage condition of Experiment 2, the divider between adjacent cages was removed, resulting in a doubling of the width. A standard diet of pelleted food (Certified Purina Primate Diet 5K91) was provided once per day. Husbandry staff hand fed fresh produce twice daily at approximately 7:00 AM and 1:00 PM. Water was provided ad libitum. Environmental conditions were maintained between 18° and 25° C, 30%–70% relative humidity, a minimum of 12 air changes/hour, and a 12-h light/dark cycle (6 AM/6 PM lights on/off).
2.3 Telemeter Implant Placement
Each male underwent a surgical implant procedure for the placement of a DSI model L21 telemetry device (Data Sciences International, Saint Paul, MN), capable of transmitting arterial blood pressure (BP), left ventricular pressure (LVP), body temperature, electrocardiogram (ECG), and activity data. One pressure catheter was placed in the femoral artery and advanced to the descending aorta and a second pressure catheter was placed into the left ventricle through the apex. The body of the transmitter was implanted into an intramuscular pocket on the left flank of the animal. Telemetry implant procedures were performed, with minor modifications, according to Data Science International recommendations. Multimodal and pre-emptive analgesia was used for the surgical procedure and during post operative recovery. Animals were assessed daily by veterinary technicians for 14 days. Activity, behavior, food consumption and wound site appearance were used to ensure adequate analgesia.
2.4 Physiological Data Collection
Telemetry data were transmitted to transceivers located in close proximity to the associated animals. The acquired signals were passed through a communication link controller to the computer-based data acquisition system. Animals that participated in these studies may have had transmitters that did not have all channels functional. This often was represented by a poor or nonfunctional blood pressure signal (which did not impact any other aspects of data collection). When the BP signal was poor or nonfunctional, HR was derived from the ECG signal. This process was used for all the telemetry collections. Data were collected for nearly 24 h each day, but for analysis we extracted two 4-h time bins (2–6 PM, 12-4 AM) when care staff were not in the room, and when routine husbandry was not being performed, to reduce variability in the physiologic data.
2.5 Data Analysis
Ponemah Review was used for post-acquisition analysis of telemetered signals. Measurements derived from these signals include mean blood pressure (calculated using the area under the curve of each cycle), HR, body temperature, and activity (measured in arbitrary units, which is a proprietary unit of measure for the activity sensor). HR was calculated either by the number of BP events, or when the BP signal was problematic, from the ECG data using the R-R interval. Data were initially reduced to 1 min means and further processed to provide 15-min contiguous mean values. The 15-min mean data were then averaged by binning into two periods, ~ 2–6 pm and ~12-4 am ( ± 15 min) (referred to as afternoon and night-time, respectively). The binned data were used for statistical evaluation.
2.6 Statistical Analysis
For all analyses, there were four dependent variables: mean blood pressure (BP), body temperature (BT), heart rate (HR), and physical activity (PA). We examined linear mixed effect models for both experiments with the hypothesis that the telemetered signals depended on the housing condition and time of day (night-time: 12–4 a.m. or afternoon: 2–6 p.m.) while controlling for the random effects of animals.
Experiment 1: Immediate and longer-term effects of pairing.
The goal of Experiment 1 was to determine the immediate (day of social pairing) and longer-term (nearly a week after pairing) effects of pair-housing on physiological measures by comparing our outcome measures when the males were alone, or when paired with the females.
3 Methods
3.1 Subjects
A total of seven healthy males were examined and had a complete blood count and serum chemistry performed to assess health and suitability for study. The animals were vasectomized (see below) and recovered from surgery in approximately 2–3 weeks. A portion of the vas deferens that was removed was also submitted for histopathology verification of successful sterilization. Animals were housed in a male only room during the postoperative recovery and were not paired with females until after 63 days postvasectomy. Mean age of the males at time of pairing was 5.3 years (range: 4.8–6.0 years) and weights ranged between approximately 5.0–8.0 kg. Approximately 1 week before pairing, a urine sample was collected, and absence of spermatozoa was confirmed by direct examination of the sample under a microscope.
Females were examined during routine biannual physical examination and had complete blood count and serum chemistry data reviewed to assess health status and suitability. These animals were part of a drug safety standing colony and were not implanted. They were sexually non-naïve and pair-housed with other females in another room in the same corridor. There had been no documented aggression in any female used in the study. Mean age of the females at time of pairing was 5.9 years (range: 3.4–7.2 years).
Pairing was performed by placing the female in the adjacent cage with the male with a grid partition of 1” square mesh between the male and female. A veterinarian and veterinary technician observed for signs of aggression between the animals before removing the partition. If no signs of aggression were observed, the partition was removed within the hour. Once the partition was removed, animals were observed for signs of affiliative behavior and aggression. Pairs that showed grooming, resting, huddling, and no signs of fear towards each other were allowed to remain in pairs. Animals that showed excessive fear, or aggression towards each other, would have been separated; this was not observed, however, among our seven subjects.
3.2 Experimental Design
Because the animals were part of a related study, Experiment 1 was conducted in two waves, with slightly different timing of the physiological recording. For both waves, males were recorded on Day 1 while in their familiar cages in an all-male room. Females were subsequently moved into the room, and recording occurred on Days 4, 8, 36, and 37 (Wave 1) or Days 4, 8, 38, and 39 (Wave 2). On Day 45 (both Waves), the females were relocated to adjacent cages and paired with the males in the double-wide cages (see previous section) in the same room, and recording occurred on those days as well as two follow-up days: Days 50 and 51 (Wave 1) or Days 51 and 52 (Wave 2). Because the days that were discrepant between the two waves were +/- 2 days, we aligned all data using the Wave 1 designations.
3.3 Vasectomy Surgical Procedure
Vasectomy was performed 365-798 days following the telemetry implant surgery. Vasectomy procedures were performed as previously described (Ekanayake-Alper et al. 2018). Briefly, bilateral 2- to 3-cm prescrotal incisions were made over the palpable spermatic cord and a 1–2 cm segment of vas deferens was excised and the transected ends of the vas deferens were ligated and cauterized. Multimodal and pre-emptive analgesia was used for the surgical procedure and during post operative recovery. A combination of meloxicam (0.2 mg/kg IM) and highly concentrated buprenorphine (0.24 mg/kg SC) was used post operatively for analgesia. Animals were assessed daily by veterinary technicians for 10 days. Activity, behavior, food consumption and wound site appearance were used to ensure adequate analgesia.
3.3.1 Pair Management
Once the partition was removed and males and females were placed together, the pairs were monitored by a veterinarian and veterinary technician for at least 1 h. Some behaviors noted were mutual grooming, genital inspection, mounting and copulation, food sharing and mutual threat displays. On subsequent days, informal, daily observations were performed by husbandry staff to ensure pairs continued to display signs of compatibility and no signs of injury.
3.4 Data Analysis
We were interested in two questions. To determine the immediate response to pairing, we contrasted the unpaired condition (Days 1, 4, 8, 36, 37) with the first day of pairing (D45). To examine the longer-term effects of being paired, we contrasted the unpaired condition (as above, Days 1, 4, 8, 36, 37) with the two later days in the paired condition (Days 50 and 51). For each analysis, we used linear mixed effects models to examine three specific contrasts for each of our outcome measures: unpaired versus paired during the afternoon period (2–6 PM), unpaired versus paired during the night-time period (12–4 AM), and an interaction term to determine whether pairing might have significantly different effects based on time of day. (For all analyses, time was a significant main effect: BP, BT, HR, and PA were all significantly lower during the night-time period compared to the afternoon period (all p < 0.001). Because these results were not particularly of interest to us, they will not be discussed further; the separate data for the afternoon and night-time periods are shown in the figures, however).
4 Results
4.1 Immediate Responses to Pairing
We found significant effects of the pairing condition for BP, BT, and HR, but no effect for PA on the day of pairing (D45). See Figure 1.
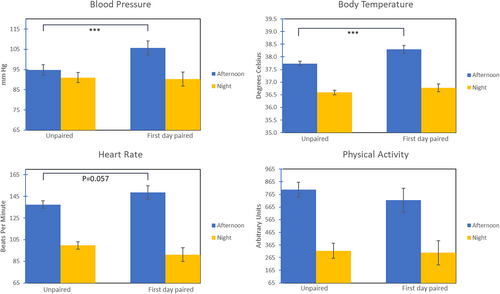
BP: We found a significant interaction of pair condition by time (t(56) = −3.04, p = 0.004). BP was significantly elevated by nearly 11 mmHg on the first day of pairing during the afternoon compared to when the males were housed singly (p < 0.001) but was relatively unchanged during the night-time (see Figure 1A).
BT: BT increased significantly upon initial pairing during the afternoon, as indicated by a significant effect of pair condition (t(84.4) = 3.95, p < 0.001). When paired, the males' BT was elevated by 0.56°C (Figure 1B).
HR: We found a significant interaction of pair condition by time (t(84.01) = −2.41, p = 0.018). As shown in Figure 1C, HR was 11 bpm higher in the afternoon on the first day of pairing compared to when the males were unpaired, though statistically, this was only a trend (p = 0.057), whereas during the night-time HR non-significantly declined by about 8.5 bpm (p = 0.146).
4.2 Longer-Term Responses to Pairing
Nearly a week following the initial pairing, we found significant effects for BP and BT only; contrasts for HR and PA were not significant. See Figure 2.
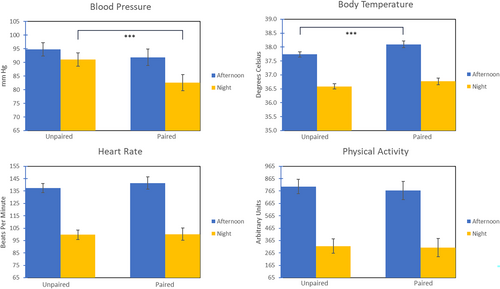
BP: BP decreased by 8.3 mmHg during the night-time period when animals were paired compared to when they were unpaired (t(56.26) = −3.69, p < 0.001).
BT: When animals were paired, BT was significantly elevated in the afternoon (t(84.68) = 3.34, p < 0.001) by 0.36°C, compared to when animals were unpaired.
5 Discussion
Pairing of vasectomized males with females resulted in an immediate increase in BP, BT, and HR, and these effects were particularly strong in the afternoon period, which occurred only a few hours after the pairing. Nearly a week after the initial pairing, BP showed a significant decline during the night-time period, while BT remained elevated, albeit at a lower level than was seen following the initial pairing.
We note that in the present study, there is a confound between social condition and housing condition–pairing was accomplished by doubling the size of the available space. Is it possible that the effects on BP, BT, and HR were due solely to an increase in cage size, and not to the presence of the social partner? Line et al. (1989) reported no differences in HR or PA in their study of adult female rhesus monkeys housed in cages of different size. The largest cage used by Line et al. (1989), however, was smaller than the large cages used in our facility to house social pairs. To determine whether the results obtained in the previous study could be due to changes in cage size, singly-housed, implanted animals were recorded while in their usual individual cages, and again after the barrier had been removed and the amount of available space was doubled.
Experiment 2: Effects of cage size on physiologic data in non-vasectomized males.
In Experiment 2, we recorded animals' physiological responses to a doubling of their cage size.
6 Methods
6.1 Subjects
Four young adult nonvasectomized males (mean age: 5.07 years, range: 4.25–6.59) served as subjects. All subjects were implanted with the telemetry device as described in the General Methods and had a history of incompatibility with previous partners or no suitable partner available.
6.2 Experimental Design
A crossover design was utilized, in which two animals experienced the smaller cage on Day 1 and the larger cage on Day 2, while the second set of animals experienced the conditions in reverse order. As with the previous Experiment, physiological and activity data were recorded continuously, with the data binned for two time periods: 12–4 AM and 2–6 PM.
7 Results
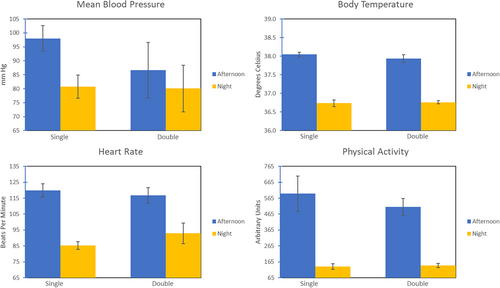
Using linear mixed models, we found no significant effects for cage size for any measures: BP (p = 0.228), HR (p = 0.558), BT (p = 0.482), PA (p = 0.55). As in our previous analyses, time of day effects were evident for all measures: BP (p = 0.031), HR (p < 0.001), BT (p < 0.001), and PA (p < 0.001) were all significantly lower during night-time compared to the afternoon (See Figure 3).
8 General Discussion
Social housing is now considered the default for primates housed in laboratories (Institute of Laboratory Animal Resources US. Committee on Care, & Use of Laboratory Animals 2011). Many studies have shown the behavioral benefits of pair housing (reviewed in DiVincenti L and Wyatt 2011; Truelove et al. 2017), though fewer have identified the physiological benefits (Capitanio et al. 2023). We were interested in demonstrating the effects of pair housing on outcome measures that that can index stress, and that are important in safety pharmacology studies with nonhuman primates, namely heart rate (HR), body temperature (BT), blood pressure (BP), as well as physical activity (PA), using implantable biotelemetry technology. Our interest was in pair-housing of young adult male long-tailed macaques, as pair-housing with this age/sex class tends to occur with less frequency in our facility, due to expectations (or actual occurrences) of wounding aggression.
Pairing of males with females resulted in an immediate elevation of BP, BT, and HR, and this was especially evident during the active, afternoon period. This period occurred only a few hours after the initial pairing. As there were no corresponding changes in males' PA, we suspect these effects may be mediated by physical contact/interaction between the males and females–grooming, mounting, and sitting in proximity or contact–and/or simply the social change in the males’ environment (i.e., not specific to the sex of the partner). Because we found no effects on our measures in Experiment 2, of simply increasing the cage size, we do not believe that the immediate results from Experiment 1 were due solely to the doubling of cage size that was concomitant with the pairing procedure. Following nearly a week of cohabitation, however, we found that BP decreased during the night-time period compared to when the animals were unpaired, while BT remained elevated, albeit at a reduced level compared to the initial response to pairing. Below we discuss three topics relating to our results: consistency of our results with previous studies, the potential clinical significance of our results, and the implications for welfare.
8.1 Consistency With Previous Studies
Two studies, mentioned above, are relevant to our findings. Doyle et al. (2008) studied male rhesus that were paired with each other, and found that “settled pairs” (i.e., pairs that had been together for at least 20 weeks) had significantly lower HR compared to when the animals were in protected contact. No significant effects for HR were reported for the first day of pairing, whereas we found a significant elevation on the first day of pairing in our study. There may be several reasons for this discrepancy. One important difference is a species difference. A series of studies by Clarke and colleagues showed that long-tailed macaques showed greater behavioral and cardiovascular responsiveness compared to rhesus (Clarke et al. 1988, 1994). A second difference was that Doyle et al. (2008) paired males together, whereas we paired males with females. It's possible that the opportunity for sexual interactions in our study led to greater arousal in our males, manifested in elevated HR. In fact, behavioral observers did note, in our study, sexual activity in many of the animals upon pairing. Perhaps the most important difference is that, before experiencing full contact, the animals in Doyle et al.'s (2008) study experienced 24 h of protected contact in which the future partners were housed together but with a grate between them in order for the animals to become familiar before pairing. In our study, females were moved into the room housing the males on D4, and they were visible to the males up until D45, the day of pairing. They were then placed into the adjacent cage to the males for an hour of protected contact before the partition was removed. The significant HR elevation on the day of pairing in our study disappeared, however, as shown in our nonsignificant result for HR in the longer-term part of our study.
A second study, by Coelho et al. (1991) examined four adult male baboons under three conditions, each lasting 2 weeks: individual housing, housing with a familiar companion (males), and housing with a social stranger (sex unspecified). Both social conditions utilized mesh dividers between the animals, which permitted visual, tactile, and auditory contact. BP was significantly lower when housed with familiar companions, compared to individual housing or pairing with unfamiliar companions, and the greatest difference was in the evening and nighttime. This result is consistent with our finding of a longer-term BP reduction at night-time compared to when the animals were unpaired. In contrast to our results for HR (in which we found a significant elevation on the first day of pairing, but no difference from the unpaired condition at the later time points), Coelho et al. (1991) found that HR was lowest when males were housed alone. They attributed the elevated HR in the two social conditions to activity. We, however, did not find a corresponding elevation in PA at the time we saw the elevated HR.
Overall, our results show some consistencies with published results, but also show important differences. In addition, our data are the first, we believe, to demonstrate BT changes following pairing. Moreover, despite a small sample size, results from Experiment 2 suggest that the effects of social companionship on cardiovascular measures and BT may not be the consequence of the increased cage size necessary when animals are paired. Those results also extend those of others, who reported no differences in HR (Line et al. 1989), urinary cortisol excretion (Crockett et al. 1993, 2000), or behavior (Crockett et al. 1995, 2000) in relation to cage size (although it is important to note that the largest cages examined in those studies were smaller than our social cages). We recognize that in Experiment 2, our measures were only recorded for the first day following the cage size change, and that longer recording might have revealed effects of the greater cage sizes. However, our strongest results in Experiment 1 were found on D45, the first day of pairing and exposure to the larger cages; this suggests that the single day of recording in the larger cage in Experiment 2 was very relevant in suggesting the immediate results of pairing were due to social companionship, and not just the larger cage.
8.2 Clinical Significance of Our Results
To get a sense of the clinical significance of our data, we compared our results to those of a large-scale study of M. fascicularis by Fang and Tichenor (2023), who recorded telemetered data from n = 130 animals, using hardware and software very similar to those used in the present study. The animals in their study were somewhat younger (1.8–8.2 years of age, compared to 4.8–6.0 years of age in our study), of mixed sex (although the authors showed no significant sex differences in their measures), and were socially housed (making their data most comparable to data from our study when animals were socially housed). While Fang and Tichenor (2023) reported no age differences in BT and HR, they did find that BP was higher in animals 6-8 years of age, compared to animals 2–6 years of age. Because of these differences, comparisons are made cautiously, particularly with the BP data. Our comparisons are based on Table 3 in their study. (We note that, as in our study, Fang and Tichenor (2023) found that BP, BT, and HR were significantly lower in the night-time compared to the daytime.)
In our study, compared to when the animals were unpaired, the immediate BP response to pairing was an 11 mmHg increase during the afternoon. The unpaired mean value for the afternoon in our study (94.8 mmHg) was very similar to the 96.22 daytime value in Fang and Tichenor (2023) and fell within the 95% Confidence Interval (CI) of that study. In contrast, the value for the afternoon on the first day of pairing in our study (105.69 mmHg), which reflects an 11.5% increase, was well outside the CI. The longer-term responses for BP in our study were found during the night-time, where BP declined by 8.3 mmHg, reflecting a 9.3% decline; means were 91.02 (unpaired) versus 82.55 (paired). The unpaired mean is slightly above the 95% CI, whereas the paired mean is substantially below the 95% CI. These comparisons suggest to us that the value on the first day of pairing was very high, probably representing a stressed value. In addition, the longer-term comparison, both values of which extended beyond the 95% CI, suggest a strong beneficial effect of pairing on BP.
In Experiment 1, BT was elevated by a mean of 0.56°C in the afternoon of the first day of pairing compared to the unpaired period. Mean values were 37.74°C versus 38.30℃, representing a 1.5% increase. Both means were outside the 95% CI. Analysis of the longer-term data in our study indicated a 0.36℃ increase in BT in the afternoon during the paired condition (means were 37.74℃ and 38.10℃, respectively, reflecting a 0.5% increase). The unpaired value fell just below the lower end of the 95% CI, while the paired value was at the upper limit of the 95% CI. Taken together, these comparisons suggest that the BT elevations we saw, particularly in the short-term, may be clinically significant.
Finally, we found an 11.2 bpm increase in HR immediately upon pairing. Afternoon mean values were 137.41 bpm versus 148.61. Both values were below the daytime 95% CI reported by Fang and Tichenor (2023), which ranged from 150.1 to 157.9 bpm. Nevertheless, we consider this 8.2% increase in HR on the first day of pairing as substantial.
8.3 Implications for Welfare
We believe our data have important implications for the welfare of captive macaques. First, we note that the immediate response to pairing involved substantial elevations in BP, BT, and HR. As noted above, Doyle et al. (2008) did not see elevated HR upon pairing. We believe our results show that our animals may have been somewhat stressed by the initial pairing. The failure to find such an effect by Doyle et al. may have been due to the protected contact for 24 h that was permitted before full contact. While it may be premature to make a strong recommendation without comparable BP and BT data from Doyle et al. (2008), we believe the HR data suggest that extended protected contact (i.e., longer than the hour of protected contact in our study) before full contact may be a useful way to minimize the stress of partner introductions. If only minimal protected contact is provided, it may take a few days for physiology to stabilize and for any data collection to reflect the variables of interest, and not the stress of pairing.
A second point concerns the longer-term results that we found, namely a decline in BP and a smaller elevation in BT nearly a week following the initial pairing. We note that these results are consistent with a literature on loneliness in humans: greater perceived social isolation (i.e., loneliness) is associated with elevated blood pressure (Hawkley et al. 2010; see also a review by Cacioppo et al. 2014) and reduced body temperature (Ijzerman et al. 2012). Macaques and their ancestors have been social for more than 20 million years, and humans for their entire existence. This suggests that it is the absence of social companionship (loneliness in humans, single housing in macaques) that is the aberrant condition, as regulatory agencies (described above) have noted for macaques. We believe that our results are consistent with the idea that social living returns the animals' physiological status back to its “natural” condition–lower blood pressure and slightly elevated temperature.
A final point is that the results of Experiment 2 suggest that increasing cage size, by itself, may have few benefits physiologically (although we are cautious in this conclusion, owing to the small sample size and the short duration of exposure to the larger cage). This is consistent with other studies, described above, that showed no differences in HR, urinary cortisol excretion, or behavior in relation to cage size. Unless larger enclosures contain additional enrichment, a doubling of cage size alone may not be a useful procedure to enhance the animals' welfare, at least initially.
8.4 Limitations
We recognize two major limitations of our studies. The first concerns the relatively small sample sizes: seven animals and four animals in Experiments 1 and 2, respectively. Despite the small sample sizes for Experiment 1, we did find results consistent with most of our expectations and with a previous study from another laboratory and the human literature (although we note that both primate studies described above suffered similarly with small samples, a reflection of the complexity and expense of conducting such research: Doyle et al. 2008 and Coelho et al. 1991, had sample sizes of eight and four animals, respectively.) We mitigated somewhat the small sample size for Experiment 2 by employing a crossover design, which resulted in all four animals experiencing both the small cage and the large cage conditions (in counterbalanced order). Confidence in the nonsignificant results for this study are further heightened by the consistency of our nonsignificant results with those reported in the literature (see previous paragraph). We do acknowledge, however, that our null result for Experiment 2 might be due to sample size limitations; in fact, inspection of Figure 3 suggests doubling the floor area of the cage did produce a (nonsignificant) reduction in BP. A larger sample size might demonstrate a beneficial effect on BP from being in a larger cage.
The second limitation was the brevity of our manipulations. We recorded physiological data periodically in Experiment 1 over a period of 51 days, 6 days of which the animals were paired. In contrast, recording only occurred for 2 days each for Experiment 2. Others (e.g., Doyle et al. 2008) recorded similar data over a more than 20-week period after pairing. Consequently, we are unable to speak directly to the long-term physiological benefits of social housing. Our data do, however, indicate that there are immediate physiologic consequences, and somewhat longer-term (Experiment 1) benefits of pair-housing; future studies may need to examine more closely how these effects develop and either persist or change over a longer timeframe.
8.5 Conclusions
Our data suggest that pairing of adult male long-tailed macaques with females has some physiological benefits with no significant variation in physiologic data attributable only to cage size. These data contribute to the growing number of studies indicating that social housing of laboratory macaques has beneficial effects on behavior and biology. In particular, our data suggest that data collection (of any type, but particularly in safety pharmacology studies) should probably not be undertaken on the first (and perhaps the first few) days of social housing, and that housing changes should also not occur during ongoing studies. Overall, we believe our results also suggest the importance of considering the social nature of the animals during safety studies, many of which are aimed at evaluating products that might be beneficial to humans, which are themselves a highly social species.
Author Contributions
Rosemary Santos: conceptualization (lead), investigation (lead), methodology (lead), project administration (lead), writing – original draft (lead), writing – review and editing (lead). Dong-Bin Tran: formal analysis (equal), writing – review and editing (equal). Dingzhou Li: Formal analysis (supporting); Methodology (lead). Peter Harris: formal analysis (equal), investigation (equal), methodology (equal), writing – review and editing (equal). Jan Bernal: investigation (equal), methodology (equal), writing – review and editing (supporting). Steven Kreuser: Methodology (equal). Erin Ricciardi: methodology (supporting), project administration (supporting). Siri Skowronek: methodology (supporting). Kiran Palyada: investigation (supporting), writing – review and editing (supporting). John P. Capitanio: formal analysis (lead), methodology (supporting), writing – original draft (lead), writing – review and editing (equal).
Acknowledgments
We thank the following colleagues: Laura Singer, Suzanne Murphy, Pfizer husbandry and behavior colleagues, Pfizer clinical pathology, and Dr. Stacey Hosking ACVIM-Cardiology (independent consultant). This paper grew out of conversations between the first and last author at one of the annual Primate Behavioral Management Conferences, held in Bastrop, Texas.
Open Research
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.




