Cortisol and Catecholamine Concentrations Are Affected by Repeated Relocations of Adult Female Rhesus Monkeys (Macaca mulatta)
ABSTRACT
In captive primate facilities, relocations—moves, within the facility, from one cage to another—can be common events. On the one hand, relocations are generally regarded as relatively benign events, as past studies have generally shown only transient elevations in cortisol concentrations following relocation. On the other hand, the frequency of relocations has been associated with adverse health and behavioral outcomes. As part of a larger project examining the effects of stress on follicular development, we relocated adult female rhesus monkeys on a weekly basis for several months in each of 3 years, and measured concentrations of urinary cortisol, epinephrine, and norepinephrine, as well as hair cortisol. Results for urinary cortisol and epinephrine were similar: significant elevations immediately following initiation of relocations during Years 1 and 3, and reductions in concentrations by the end of the relocation sequences in Year 1. No changes were seen for these two measures in Year 2. In contrast, elevated norepinephrine concentrations were found for all 3 years. Significant elevations in hair cortisol concentrations were found for Years 2 and 3, and suggested persisting and cumulative effects of relocations on the hypothalamic−pituitary−adrenal axis. Together, these results suggest that relocations may not be the benign events suggested by earlier studies. Given that all organs of the body are innervated by the sympathetic nervous system (the principal source of norepinephrine in blood and urine) and that cells of the body have glucocorticoid and catecholamine receptors, our results suggest possible mechanisms by which repeated relocations may result in adverse health outcomes. Repeated relocations may be a valuable model for experimentally generating moderate stress; however, we encourage colony managers and scientists to minimize such events to enhance the welfare of the animals.
1 Introduction
In a nonhuman primate facility, animals may be relocated—moved, within the facility, from one cage to another—for a number of reasons, including hospitalization, disbanding and reforming cages, assignment to a project, resolving social issues (e.g., removing troublesome animals), or healthcare. Relocation is not an infrequent activity. One study (Vandeleest et al. 2011) of 202 rhesus monkeys that were up to 4.7 years of age revealed the number of relocations ranged from 3 to 62. Nor is relocation a benign activity. While there are some conflicting reports, numerous studies have demonstrated that the number of relocations that an animal experiences is associated with a variety of adverse health and behavioral outcomes, including a greater likelihood of showing stereotypic motor activity (Gottlieb et al. 2013; though see Vandeleest et al. 2011; Lutz et al. 2014); greater risk for developing and displaying self-abusive behaviors (Rommeck et al. 2009); and a greater likelihood of developing diarrhea (Elfenbein et al. 2016; Gottlieb et al. 2018). Relocations of pregnant females can have a lasting impact on the offspring, including an increased risk for diarrhea (Elfenbein et al. 2016). Finally, careful study of a single relocation also revealed that monkeys that displayed self-injurious behavior (SIB) increased their rate of SIB, and also showed sleep disturbances, in response to a relocation (Davenport et al. 2008).
The impact of a relocation can be influenced by many factors. For example, the timing of a relocation can be an important factor: One study found that the number of relocations in the 90-day period preceding inoculation with the simian immunodeficiency virus (SIV), but not the entire pre-inoculation period, was associated with shorter survival (Capitanio and Lerche 1998). That study also showed that, for animals that experienced a relocation *after* SIV inoculation, shorter survival was associated with the relocation occurring closer in time to the inoculation itself. Similarly, many rhesus macaques are latently infected with Cercopithecine herpesvirus 1 (B-virus); viral DNA (suggesting reactivation of the latent virus) was detected in mucosal fluids from animals relocated during the breeding season but not from animals moved at other times of the year (Huff et al. 2003). In addition to the timing of a relocation, Gottlieb et al. (2018) and Maninger et al. (2003) showed that relocation is especially problematic for animals with particular personality characteristics. Finally, a relocation's impact can be influenced by its being accompanied by a number of other events: pharmacologic interventions (e.g., ketamine administration), social separations, changes in available space, exposure to a novel environment and novel animals (e.g., in opposite cages), and changes in zeitgebers (e.g., moving from an outdoor cage to an indoor cage where the light cycle, temperature, and humidity are all controlled). For example, studies have suggested that relocating an animal from a large, outdoor corral containing dozens of conspecifics to an individual cage indoors (involving all of the above factors except pharmacological interventions) can result in altered antibody responses and depressive behavior for some animals (Hennessy et al. 2017; Maninger et al. 2003). Such a relocation could require up to 5 months for behavior and physiology to stabilize, given the number of changes involved (Capitanio, Mendoza, and Lerche 1998; Van Scott et al. 2013).
Because most studies have found relocations to be associated with poor health and behavioral outcomes, there has been considerable interest in how relocations might affect underlying physiological processes that could mediate these relationships. A principal focus has been on cortisol, with mixed results. A modest relocation—that is, from an individual cage in one room to an individual cage in another room—affected urinary cortisol excretion, but the effect lasted only the first day after relocation (Crockett et al. 1993; see also Phoenix and Chambers 1984). In contrast, Davenport et al. (2008) reported that a similarly modest relocation (animals were singly housed in both locations) resulted in elevated hair cortisol concentrations for 4 months post-move, while Broche et al. (2023) showed no elevations in fecal cortisol concentrations in the first 7 days following relocation. More dramatic relocations, such as from a large outdoor cage to individual housing indoors, resulted in elevated cortisol concentrations for up to 8 weeks and persistently reduced lymphocyte numbers (Gordon et al. 1992; O'Connor et al. 2011).
We were interested in studying the effects of a mild stressor on reproductive outcomes, such as follicular development, and we chose as our stressor repeated relocation. Moreover, we were interested not just in how relocation affected the hypothalamic−pituitary−adrenal system, but also how it might impact the sympathetic nervous system and the adrenomedullary system, additional major stress response systems that, to our knowledge, have never been studied in the context of relocation. Singly housed females from our time-mate breeding colony were relocated weekly for a period of months, and overnight urines were assayed for cortisol, epinephrine, and norepinephrine. In addition, we assessed hair cortisol concentrations to determine whether there may have been a persisting impact of repeated relocations on the HPA system.
2 Methods
2.1 Subjects
Subjects were adult female rhesus monkeys, ranging in age from 4.3 to 11.1 years (mean = 7.4 years), and ranging in weight from 6.21 to 10.36 kg (mean = 8.16 kg). This age range is well within the peak fertility for this species in the laboratory setting. Animals were selected based on normal weight and body condition score, proven fertility, normal menses, cycle lengths of 28−32 days, and no previous ovarian hyperstimulation. Data were collected from 10 animals in Years 1 and 2 (one animal was dropped in Year 1 due to poor appetite and weight loss, even after intervention, and a new animal was substituted for Year 2), and nine animals in Year 3 (one died of left ventricular hypertrophy at the end of Year 2).
2.2 Experimental Design
Our design utilized repeated housing relocation as a potential stressor. (We note that our subjects were not relocation-naïve: the number of previous relocations ranged from 10 to 35, with a mean of 21.1 relocations.) Three housing rooms (each housing 28−68 animals), with which the animals were initially unfamiliar, were utilized in each of the 3 years, with the same three rooms used in Years 1 and 2, and a separate set of three rooms used in Year 3 (see below). Across the three rooms, 10 cages were identified; within a room, the three or four cages were selected to minimize or eliminate visual contact between the subjects, although visual contact with other animals in the room was possible. In Years 1 and 2, consecutive relocations were always to a cage in a different room. This was mostly true in Year 3, as well, but because one room in Year 3 was larger than the others, consecutive moves were occasionally to a different cage within the same room.
Relocation occurred weekly on Tuesdays, and overnight urine collection (see below) occurred on Wednesday mornings (unless a holiday conflicted with this schedule, in which case the relocation and urine collection were delayed by a day). Urine collection occurred approximately 1 week before the first relocation (Time 1: baseline), then on the mornings following the first relocation (Time 2: Week 1) and the second relocation (Time 3: Week 2). The final sample (Time 4) was taken weeks later, following the final relocation of the year. Animals experienced a total of 18, 39, and 38 relocations for Years 1, 2, and 3, respectively. Hair was collected (see below) on three occasions: before the first relocation, approximately 3 months into the sequence of weekly rotations (except for Year 1, in which this sample was not obtained), and following the final relocation. After the final relocation each year, the animals were returned to their pre-experiment housing cages and were left undisturbed. Table 1 shows the dates for each urine sampling period, and Table 2 shows the same information for the hair collection. At the end of each year, urine samples were sent to the two laboratories for analysis (see below). All hair samples were assayed together at the end of Year 3.
| Year | Time 1 | Time 2 | Time 3 | Time 4 | |
|---|---|---|---|---|---|
| 1 | 10/29/20-11/12/20 | 11/17/20 | 11/24/20 | 3/23/21 | |
| 2 | 8/23/21 | 8/31/21-9/1/21 | 9/7/21 | 5/2/22 | |
| 3 | 8/22/22 | 8/30/22a | 11/21/22 | 11/28/22 | 5/10/23-5/25/23 |
- a Indicates date for first relocation in the same locations used in Years 1 and 2. Subsequent dates for Year 3 reflect relocations in the new locations.
| Year | Time 1 | Time 2 | Time 3 |
|---|---|---|---|
| 1 | 10/26/20-11/12/20 | 3/23/21 | |
| 2 | 8/23/21 | 11/23/21 | 5/3/22-5/10/22 |
| 3 | 8/23/22 | 11/16/22 | 5/10/23-5/25/23 |
During the Year 1 study, we discovered that a new feeding policy for indoor housed animals at our facility, designed to reduce obesity, was applied to our animals, thereby confounding the study with a change in feeding. This occurred after seven of the 10 animals had experienced their first relocation, and just before the remaining three animals experienced their first relocation. The old feeding regimen provided seven biscuits in the morning and seven in the afternoon; the new policy reduced the number of biscuits to five and five. We replicated the study in Year 2 using the same rooms and cage positions for the relocations; no potential food stress was involved. In Year 3, we planned to repeat the study using the same rooms and cage positions. After the first relocation, however, we received the cortisol data from Year 2, which revealed that the cortisol elevation effects found in Year 1 had attenuated in Year 2. Because, as described above, our goal was to develop a model of moderate stress to study reproductive outcomes, we then identified new rooms and cage positions and restarted the relocations for Year 3. Table 1 indicates a urine sample collection on 8/30/2022, which was from the first relocation in the old locations. These data are not considered here; rather, the samples from Time 2, on 11/21/22, reflect the first relocation in the new locations.
2.3 Husbandry
Animals were housed in standard cages (30” height, 4.3 square feet floor area) on a 12 h L/D cycle (0600 h to 1800 h). Animals were fed twice daily, before 0900 and after 1300 h with monkey chow (Lab Diet #5047). They received vegetable/fruit enrichment once per week and a seed mix daily.
2.4 Urine Collection and Processing
A pan was placed under each animal's cage at approximately 1600 h and urine was collected before 0800 the following day. Samples were immediately put on ice and centrifuged at 2000g for 5 min to remove any food or fecal particles. For the cortisol assays, 2.5 mL supernatant were placed in cryo tubes and stored at −80°C until delivery to the lab for assay. For the catecholamine assays, 1.5 mL supernatant were placed in cryotubes, and 6 N HCl was used to correct the ph to 2–4. Tubes were then stored at −80°C until shipment to the lab.
2.5 Urinary Cortisol and Creatinine Assay
Samples were delivered frozen to the Endocrine Core Laboratory, Center for Health and the Environment at the UC Davis Institute of the Environment after each round of sample collection. Assays were performed according to previously published methods (Hannibal et al. 2018).
2.6 Urinary Catecholamine and Creatinine Assay
Frozen urine samples for the entire year were shipped overnight to the Wisconsin National Primate Research Center's Assay Laboratory at the end of each Year's sample collection. High-pressure liquid chromatography with electrochemical detection (HPLC-ECD) was used for the measurement of catecholamines. Briefly, 1 mL of urine was mixed with 50 mg of alumina, 0.5% sodium metabisulphite, and 3 M Tris Buffer. Samples were vortexed, washed three times, and then catecholamines were eluted with 0.2 N perchloric acid. The eluate was then run through a filter vial before HPLC-ECD analysis as described in Campi et al. (2014). Quality control was assessed using two pools of acidified human urine. Intra-assay coefficient of variation (CV) ranged from 7.36% to 13.47% and inter-assay CV ranged from 10.27% to 19.42%. Creatinine was measured using the Jaffe reaction as previously described in Reyes et al. (2014). Quality control was measured using one pool of human urine. Intra- and inter-assay CVs were 3.38% and 13.46%, respectively.
2.7 Hair Collection
At baseline, after approximately 3 months of relocations, and following the final relocation each year (approximately 5.5 months later), animals were given ketamine (10 mg/kg) and hair was shaved from the upper back in an area approximately 2 inches x 2 inches and carefully handled to maintain order of hair alignment at enrollment. The second and third hair samples were taken from the same location. Once collected, hair was wrapped in tin foil and stored at −80° until processing. Table 2 shows the dates of the hair collection.
2.8 Hair Cortisol Assay
Hair cortisol was assessed using previously described methods (Vandeleest et al. 2019).
2.9 Data Processing and Statistical Analysis
Ratios were constructed by dividing the values of urinary cortisol, norepinephrine, and epinephrine by their respective creatinine values. Ratios were then natural log transformed, and these served as the outcome measures. Creatinine values for seven time points were missing (two animals for Year 1/Time 4; two animals for Year 2/Time 1; and one animal each for Year 2/Time 2, Year 2/Time 3, and Year 3/Time 3), due to the threshold of sensitivity of the platform used for the cortisol assays. Creatinine values for all seven time points were available from the catecholamine assays, however. Consequently, for the missing values of log-transformed creatinine for the cortisol assays, we imputed the value from the log-transformed creatinine value from the catecholamine assay for that animal at that timepoint. We then corrected the imputed value, taking into account the strong correlation between the measures (r = 0.875) and the slightly but significantly greater mean values for the catecholamine creatinine values compared to the cortisol creatinine measure: mean difference = 0.22 mg/mL (95% CI: 0.16, 0.28).
We fit linear mixed-effects models in SAS (version 9.4), using the GLIMMIX procedure with robust standard errors with a small-sample adjustment based on first-order residuals. Year and Time (and their interaction) were considered fixed effects, and a random intercept for animals was included. For each year, planned comparisons contrasted mean outcome values for Time 2 (first overnight following the first relocation), Time 3 (first overnight following the second relocation), and Time 4 (overnight following the final relocation) with Time 1 (baseline). A further comparison contrasted Time 4 with Time 2. Because we planned on using year-specific between-timepoint comparisons, given the known year-specific features in the experimental context, we opted to report the F-tests associated for the three terms in our model, but not to let nonsignificant test statistics for the interaction term result in our summarizing between-timepoint comparisons by pooling across the 3 study years.
Hair cortisol measures were obtained at three time points: baseline (hTime 1), after approximately 3 months of weekly relocations (hTime 2), and following the final relocation (hTime 3). During Year 1, however, no hair collection occurred at hTime 2. Cortisol values were expressed as picograms of cortisol per milligram of hair. Analysis proceeded as with the urinary measures, with comparisons between all time points. Values were natural-log transformed before analysis. The plate number was a covariate.
3 Results
3.1 Urinary Cortisol
We found a significant effect of Time (F(3, 94) = 9.82, p < 0.0001), but no significant overall effects for Year or the Year x Time interaction (both p > 0.27).
Year 1: Planned comparisons for each year showed that, for Year 1, cortisol concentrations were significantly elevated at Time 2 compared to Time 1 (p < 0.0001) and at Time 3 compared to Time 1 (p = 0.0074). Concentrations for Time 4 were not significantly different from Time 1 (p = 0.3858) but were significantly lower than at Time 2 (p < 0.0001) (see Figure 1).
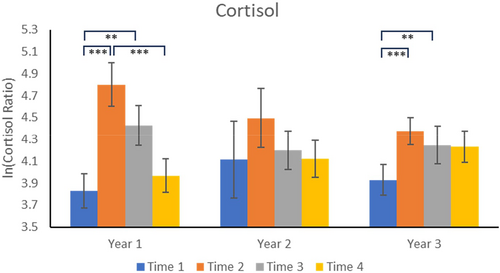
Year 2: No significant effects were found.
Year 3: Following the change in locations for the animal relocations, we found that cortisol concentrations were significantly higher at Times 2 and 3 compared to the Time 1 baseline (p < 0.0001 and p = 0.0043, respectively). Concentrations at Time 4 were not different from those at Time 1 (p = 0.1451) or Time 2 (p = 0.5002).
3.2 Urinary Epinephrine
No significant effects were found for Time (p = 0.0785), Year (p = 0.1423), or for the interaction of Year x Time (p = 0.3433).
Year 1: Epinephrine concentrations were significantly elevated at Time 2 compared to Time 1 (p = 0.0100), but not at Time 3 (p = 0.1586) or Time 4 (p = 0.7650). Concentrations at Time 4 were significantly lower than at Time 2 (p = 0.0270) (see Figure 2).
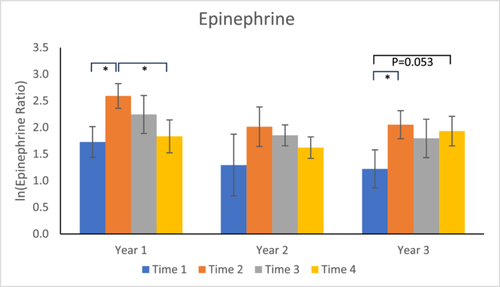
Year 2: No significant effects were found.
Year 3: Time 2 concentrations were significantly elevated over Time 1 (p = 0.0130). Time 3 concentrations were not significantly different from those at Time 1 (p = 0.1787), and a trend was seen for Time 4 compared to Time 1 (p = 0.0533). There was no significant difference between Time 4 and Time 2 (p = 0.5731).
3.3 Urinary Norepinephrine
Significant effects were found for Time (F(3, 94) = 6.84, p = 0.0003) and for the Year x Time interaction (F(6, 94) = 12.72, p < 0.0001).
Year 1: Norepinephrine concentrations were significantly higher at Times 2 and 3 compared to Time 1 (p = 0.0008 and p = 0.0035, respectively), but concentrations at Time 4 were not significantly different from Time 1 (p = 0.1948). In addition, there was a trend for lower concentrations at Time 4 compared to Time 2 (p = 0.0712) (see Figure 3).
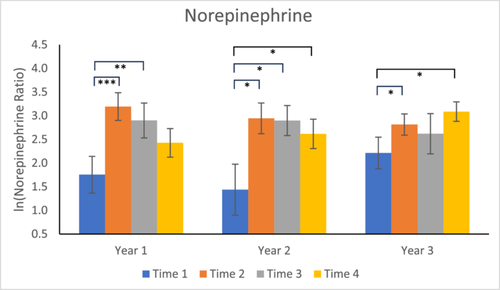
Year 2: Norepinephrine levels were significantly higher at Times 2, 3, and 4 compared to Time 1 (p = 0.0355, p = 0.0287, p = 0.0400, respectively). Time 4 was not significantly different from Time 2 (p = 0.3637).
Year 3: After the change in locations in Year 3, norepinephrine levels were significantly higher at Time 2 compared to Time 1 (p = 0.0495), but Time 3 levels were not significantly different from Time 1 (p = 0.2577). Time 4 levels were significantly higher than at Time 1 (p = 0.0216), but not Time 2 (p = 0.3110).
3.4 Hair Cortisol
Significant effects were found for Year (F(2, 59) = 4.37, p = 0.0169), hTime (F(2, 59) = 6.07, p = 0.004), and the Year x hTime interaction (F(3, 59) = 29.67, p < 0.001). (Recall that for the hair cortisol measure, hTime 1 was baseline, hTime 2 was after 3 months of weekly relocations, and hTime 3 was after the final relocation, approximately 5.5 months later.)
Year 1: As no hTime 2 measures were obtained in Year 1, the only comparison was between hTime 3 and hTime 1; this comparison was not significant (p = 0.7043) (see Figure 4).
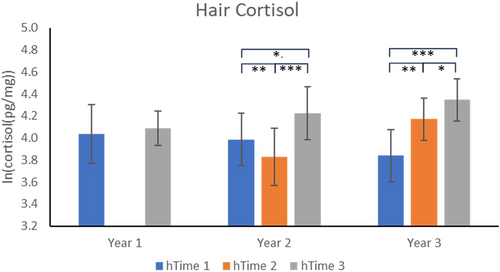
Year 2: All three hTime points were significantly different from each other. Mean cortisol concentrations were significantly higher at hTime 1 than at hTime 2 (p = 0.0014). The hTime 3 mean was significantly higher than at hTime 1 (p = 0.0117) and at hTime 2 (p = 0.0003).
Year 3: All three hTime points were significantly different from each other. Mean cortisol concentrations were significantly higher at hTimes 2 and 3 compared to hTime 1 (p = 0.0023, p < 0.001, respectively). hTime 3 concentrations were also significantly higher than at hTime 2 (p = 0.0315).
4 Discussion
Repeated relocation from an individual cage in one room to an individual cage in a second room resulted in significant changes in overnight urinary stress-related hormonal measures in our adult females. Hair cortisol data suggested persisting and cumulative effects of the repeated stressor.
4.1 Cortisol
Urinary cortisol was immediately responsive to the relocations, as others have noted (e.g., Crockett et al. 1993). In both Year 1 and Year 3, concentrations were significantly higher following the first relocation and the second relocation. Because we only measured urinary cortisol at one additional time point (i.e., Time 4), we don't know whether, or for how long, that pattern might have continued. For Year 1, cortisol levels at Time 4 were significantly lower, however, compared to Time 2, and were not different from baseline (Time 1) levels (Figure 1). In Years 2 and 3, the Time 4 time points were also not significantly different from Time 1; in contrast to Year 1, however, in Years 2 and 3, Time 4 was also not significantly different from Time 2. Although the Time 4 levels were not different from Time 1 in Year 3 (p = 0.1451), Figure 1 shows that the mean values were elevated at Time 4, suggesting there might still be an effect of relocation on the HPA axis up to 9 months after the weekly relocations began, a suggestion consistent with the hair cortisol results (below).
No significant effects for urinary cortisol were found for Year 2. As described earlier, we discovered that a change in the feeding quantity was implemented by mistake on our animals at the beginning of Year 1. We found the null result for Year 2 just as we were beginning the Year 3 relocations. Because the goal of the larger project was to develop repeated relocations as a model of moderate stress, we decided to change the relocation cage positions for Year 3. Our concern was that the results for Year 1 might have been unduly influenced by the feeding change, and not by the relocation, a concern that was supported by studies showing the cortisol levels can be affected by dieting (e.g., Tomiyama et al. 2010). In fact, the Year 3 data indicated that the relocation effect on cortisol was indeed rescued by the change in relocation cage positions. Inspection of Figure 1, however, shows that, for Year 2, Time 2 concentrations were higher than Time 1, although the effect was not statistically significant; in fact, we found no significant overall effect of Year.
The hair cortisol results are somewhat consistent with the urinary cortisol results, particularly for Years 1 and 3. In Year 1, there was no sample taken at hTime 2, but the hTimes 1 and 3 comparison was nonsignificant, similar to what the urinary results suggested for Time 1 versus Time 4. In Year 2, where none of the urinary cortisol data points were significantly different from each other, the hair cortisol data indicated a significant decline from baseline at hTime 2 (after 3 months of weekly relocations), and a significant elevation in hair concentrations following the final sample at hTime 3. The decline at hTime 2 is puzzling; because there were no effects on the urinary measures, we do not believe that this decline is reflecting a chronic stress condition, which could lead to a suppression of cortisol output, as has been found in blood measures (e.g., Capitanio, Mendoza, Lerche, and Mason 1998). The significant elevation at hTime 3 suggests, however, that there could have been small elevations in cortisol due to the weekly relocations that accumulated in the hair, but would not have been reflected in the final, Time 4, urinary cortisol result. Finally, in Year 3, we found elevations in hair cortisol at hTime 2 (after 3 months of weekly relocations) that would be consistent with the elevations in urinary cortisol seen in Weeks 1 and 2. Similarly, at the final time point of sample collection, the elevation in urinary cortisol seems consistent with the elevated hair values. It remains unclear, however, why the hair cortisol result for the final time point was significantly elevated over the hTime 2 time point, while the final urinary cortisol concentrations did not appear elevated compared to the Times 2 and 3 time points. The discrepant results could be a reflection of different dynamics of cortisol release into hair versus urine, as studies have shown only modest correlations (r = 0.3 to r = 0.4) between cortisol measured in these two tissues (van Ockenburg et al. 2016; Short et al. 2016).
Together, these results suggest that cortisol is responsive to relocations, and that with repeated, weekly relocations, the cortisol response may not attenuate completely.
4.2 Catecholamines
Urinary epinephrine and norepinephrine concentrations were also affected by the relocation stressor, though in somewhat different ways. This is not surprising, in that epinephrine is principally released by the adrenal medulla, while norepinephrine is primarily released by post-ganglionic fibers of the sympathetic nervous system, which innervate the organs of the body. Moreover, the idea of a unitary sympathetic-adrenal-medullary response system has recently given way to the idea of somewhat separate systems (adrenomedullary and sympathoneural) that are differentially responsive to stressful events, with considerable support in the literature (Goldstein 2012).
In both Years 1 and 3, the Time 2 levels of epinephrine in urine were significantly higher than baseline, and in Year 1, the Time 4 levels were significantly lower than at Time 2. For Year 2, nonsignificant elevations in epinephrine were evident for Time 2. The pattern of epinephrine results is reminiscent of our cortisol results (compare Figures 1 and 3) and is different than the pattern of results for norepinephrine (see next paragraph). As Goldstein notes (2012, 43), “Across a variety of stressors, increases in adrenomedullary activity as indicated by elevated plasma epinephrine levels correlate more closely with increases in pituitary-adrenocortical activity as indicated by elevated plasma levels of corticotropin than with increases in sympathoneural activity as indicated by elevated plasma levels of norepinephrine.” This seems to be the case, as well, for levels in urine, a fluid produced from the kidneys' filtering of wastes from blood. One important difference from the urinary cortisol results (but consistent with the hair cortisol results), however, was that in Year 3, Time 4 levels were nearly significantly higher than Time 1 levels, suggesting continued activation of the adrenal medulla.
Most interesting was the pattern of results for norepinephrine. As was the case for urinary cortisol, Time 2 and Time 3 levels were significantly higher than baseline (Time 1) for Year 1, and Time 2 levels were higher than baseline for Year 3. In Year 2, however, only norepinephrine showed a significant response to the relocations, with concentrations at Times 2, 3, and 4 significantly higher than at Time 1. Moreover, whereas in Year 1, the Time 4 levels of cortisol and epinephrine were significantly lower than at Time 2, for none of the 3 years was there a significant decline in norepinephrine levels between Times 2 and 4. This strongly suggests there was no attenuation of the immediate, overnight urinary norepinephrine response to repeated relocations, even over a 9-month period. We consider this novel result to be extremely important: while to our knowledge there have been no other studies of repeated relocations, studies involving a single relocation, which have shown that the cortisol response rapidly attenuates, have suggested that relocations are relatively benign events. Of course, we do not know the time course of attenuation following a single relocation (an important question for future research), but our data support the idea that animals show the sympathoneural response at every relocation event. Given that the sympathetic nervous system innervates every organ in the body, it's possible that adverse health effects could accrue due to repeated activation of this system from frequent relocations.
4.3 Limitations
We recognize several limitations of our study. First, as described earlier, the goal of the larger study was to develop a model of repeated relocations to examine stress effects on follicular development in rhesus macaques. Consequently, our subjects were all females. It would be useful to know if males (or animals of other commonly used laboratory species such as long-tailed macaques) would respond similarly to repeated relocations. Second, our catecholamine assays measured epinephrine and norepinephrine, but not other important measures, such as catecholamine metabolites. These would have given a more thorough picture of catecholamine output. Finally, we recognize that our discussion must be tempered by the sampling scheme we employed. While we are confident in our results that relocations impact the HPA axis and adrenomedullary and sympathoneural responses, we don't have good information on the timing of the response. For example, how quickly do these systems respond to a relocation event? Similarly, in some cases, we found that Time 3 values, taken after the second relocation, were significantly different from baseline, but Time 4 values, taken months later, were not. What might be the time course of the attenuation of these effects in the intervening months? We believe our data suggest multiple avenues to study the impact of relocations on the animals in our care.
4.4 Conclusions
In a primate facility, relocations are relatively common events, and previous research has shown that they can result in somewhat transient elevations of cortisol. Our results indicate that catecholamines are also elevated in response to relocations and that, with repeated relocations, these effects may not attenuate completely. We believe there are two implications of our results. First, relocations are not the benign events that many people believe them to be, particularly if relocations are repeated. Because cells of the immune system contain receptors for both cortisol and catecholamines, relocations may have adverse impacts on a disease process, either naturally occurring or induced as part of an experimental study. The second implication is that repeated relocations are a useful procedure to model moderate stress. As indicated earlier, our larger goal for the repeated relocation study was to determine whether such stress might have an impact on ovarian function. Ovaries are, in fact, innervated by fibers of the sympathetic nervous system, as are all organs of the body. Soon, we will know whether relocations have had the hypothesized effect on ovarian function, but at this point, our recommendation is that greater attention should be paid, in primate facilities, to minimize relocations, particularly if animals are on study. Finally, we urge further study, to determine, among other things, whether repeated relocations have a direct impact on other physiological systems (e.g., the immune system) and, importantly, whether social pairing, or allowing animals to have some control over the relocations, might buffer the adverse endocrine (and possibly immune) consequences of repeated relocations.
Author Contributions
John P. Capitanio: conceptualization (equal), formal analysis (supporting), funding acquisition (supporting), methodology (equal), writing – original draft (lead). Daniel J. Tancredi: formal analysis (lead), funding acquisition (supporting), methodology (equal), writing – review and editing (equal). Jasmin Zarrabi: data curation (equal), investigation (equal), writing – review and editing (equal). Catherine VandeVoort: conceptualization (equal), funding acquisition (supporting), investigation (equal), methodology (equal), project administration (equal), writing – review and editing (equal). Cheryl K. Walker: conceptualization (equal), funding acquisition (lead), methodology (equal), project administration (equal), writing – review and editing (equal).
Acknowledgments
We thank the staff at the Endocrine Core Laboratory, Center for Health and the Environment at the UC Davis Institute of the Environment, and at the Assay Laboratory at the Wisconsin National Primate Research Center for their expert analyses of our samples. We also thank Dr. Jessica Vandeleest for training and assistance with the hair cortisol analysis, as well as the animal care and veterinary staff at the California National Primate Research Center. Finally, we thank two anonymous reviewers for helpful comments on an earlier draft of the paper. Research described in this paper adheres to the ethical requirements of the American Society of Primatologists' Principles for the Ethical Treatment of Non-Human Primates and all relevant laws of the United States.
Ethics Statement
The research was approved by the Institutional Animal Care and Use Committee of the University of California, Davis.
Conflicts of Interest
The authors declare no conflicts of interest.
Open Research
Data Availability Statement
Data are available upon reasonable request.




