Integration of Spatially-Explicit Behavioral Data and Drone-Based Lidar Mapping Reveals Divergent Microhabitats in Sympatric Tamarins
ABSTRACT
Tamarins (Saguinus spp., Leontocebus spp.) have been characterized as tolerating or even preferring secondary growth and anthropogenically disturbed areas, and as performing critical seed dispersal in these areas. To test the hypothesis that tamarins prefer secondary growth, we segregated animal presence records by behavior and then used niche modeling to quantify the suitability of various microhabitats for emperor tamarins (Saguinus imperator) and saddleback tamarins (Leontocebus weddelli) over a 315 ha area in the southeastern Peruvian Amazon. Our analysis combines fine-scale maps of key environmental parameters derived from drone-borne lidar data with a behaviorally-sensitive niche modeling of animal movement data measured in the field. This combination allows us to define critical and non-critical areas and gain a new and detailed understanding of microhabitat choice. In saddleback tamarins, we find higher-than-expected use of primary forest for foraging activity. In emperor tamarins, conversely, we find a significant preference for secondary forest in sleeping and unexpectedly high presence in anthropogenically disturbed areas. More broadly, we show that behavioral data lends important nuance to niche modeling methods and that, in combination with fine-scale environmental data, this kind of modeling reveals forms of niche segregation not visible when studying presence alone.
Summary
-
Behaviorally sensitive maxent analysis identifies suitable habitat for critical behaviors such as foraging and sleeping.
-
Analyzed at landscape scale, two sympatric tamarin species are shown to have substantial niche overlap in presence (Schoener's D = 0.91 ± 0.01) but less in feeding (D = 0.68 ± 0.04) and sleeping (0.64 ± 0.04).
-
Saddleback tamarins use primary forest more than expected for sleeping and hunting invertebrates; emperor tamarins use less primary and more secondary forest for sleeping.
1 Introduction
Niche modeling (Boubli and De Lima 2009; Chetan et al. 2014; Chiou and Blair 2020; Tran. 2018; Vidal-García and Serio-Silva 2011) is a powerful tool for understanding the habitat needs of primate species. In its typical form, it involves measuring environmental variables and species presence records, then analyzing which environmental variables are most predictive of species presence. Often, the environmental variables are coarse measurements derived from satellite imagery, global-scale bioclimatic measurements, and categorical maps of forest type (e.g., Boubli and De Lima 2009; Condro et al. 2021; Hansen et al. 2020; Moraes et al. 2020). This paper demonstrates how niche modeling can use more fine-grained environmental data, in combination with spatially explicit behavioral data, to yield insights about microhabitat that would not be visible through traditional approaches. Because of the extensive behavioral data available through full-day follows, this approach is especially well-suited to analyzing the ecological niches of primates.
Although much has been learned from niche modeling with coarse environmental predictors, the details of small-scale vegetation structure are critical for understanding landscape-scale microhabitat use. In arboreal animals such as squirrels and woodland birds, forest structure unsurprisingly has a powerful influence on habitat suitability (Flaherty et al. 2014; Hinsley et al. 2006; Johnston et al. 2020; Nelson et al. 2005). Similar effects have been shown in several species of primates: Callithrix argentata melaneura (Wallace et al. 1998), Pithecia irrorata (Palminteri et al. 2012), Pongo pygmaea (Davies et al. 2017). When possible, therefore, niche models should include high-resolution and spatially explicit maps of environmental predictors, drone-based lidar being the highest currently available (Geue and Thomassen 2020; Palminteri et al. 2012; Rosencranz et al. 2018).
In addition to the use of fine-grained environmental variables, niche modeling can be improved by the inclusion of spatially explicit behavioral data. Niche modeling has been used to draw inferences about behavior, for example about seasonal activity (Encarnación-Luévano et al. 2013; Marini et al. 2013) or migration patterns (Menchetti et al. 2019). Furthermore, modeling of animal movement behavior has made important refinements in ecological niche modeling methodology (King 2012; Michelot et al. 2019; Potts et al. 2014; Royle and Kéry 2007). The present study uses behavioral data in a slightly different way: in our analysis, the spatial points are disaggregated by behavioral state such that suitable habitat for foraging and/or sleeping can be distinguished from suitable habitat as predicted by presence records. To our knowledge, this has been done only once before, by Singh et al. (2018). In that study, the feeding, sleeping, and ranging behaviors of red langurs (Presbytis rubicunda) and Bornean agile gibbons (Hylobates albibarbis) were geotagged and analyzed using LiDAR data and a MaxEnt modeling approach. Schoener's D index, a standard measure of habitat overlap (Rödder and Engler 2011) was used to measure the degree of overlap between the two species in different behavioral states.
Our niche modeling approach uses behavioral data from full-day and half-day follows on primate groups, and derives its environmental predictors from a variety of sources including fine-grained versions of variables frequently used in more coarse analyses (e.g., elevation, forest type) and aerial LiDAR scans that provide measurements only available at this fine level of analysis (e.g., kurtosis in canopy height, leaf area density). By integrating these two datasets, we demonstrate how behaviorally-sensitive niche modeling can reveal differences in the habitat needs of two sympatric tamarin species: emperor tamarins (Saguinus imperator) and saddleback tamarins (Leontocebus weddelli). These and related species are known to play an important role in forest regeneration following anthropogenic disturbance (Culot 2009; Heymann et al. 2019). The extent of their resilience to such disturbance, however, is not entirely clear.
Saddleback tamarins have been characterized as disturbance-tolerant due to their extensive use of treefall sites (Soini 1987) and some evidence for a preference for secondary over mature primary forest (Rylands 1996; Yoneda 1984). Less work has been done on the habitats of emperor tamarins, but they have been described as exhibiting a preference for secondary forest (Terborgh 1983). More recently, however, behavioral evidence has revealed that tamarins’ tolerance for disturbed habitat may have been overestimated. Porter (2004) found a significant preference for mature forest over secondary growth in Saguinus labiatus and Leontocebus sp. In S. geoffroyi, even suburban populations rely on nearby mature forest fragments for feeding and social behavior (Díaz-Muñoz 2010), and S. nigrifrons forage less successfully in secondary than primary forest (Kupsch et al. 2014). Once behavior is brought into the analysis, tamarins’ resilience starts to seem less robust: perhaps tamarins persist in disturbed areas only if those areas are near relatively mature forest. If so, small fragments of undisturbed forest could be important targets for conservation, provided those fragments support tamarins.
The present study aims to demonstrate how behavioral data can be integrated into niche modeling to reveal greater detail about primates use of space. As a secondary aim, we apply this methodology to the question of how to characterize tamarin species’ resilience to anthropogenic disturbance. By modeling the niches of two sympatric species, our approach enables us to look for evidence of competitive exclusion. Specifically, it allows us to demonstrate that evidence for competitive exclusion is apparent when our datapoints are disaggregated by behavior but not when the model is built on presence points irrespective of behavior.
The study draws on 483 h of behavioral observation made during the dry seasons of 2017, 2018, and 2019, totaling 2907 spatial points representing individual behaviors. The behaviors of interest in this study are foraging and sleeping, behaviors chosen because of their importance in primate life history and their sensitivity to local vegetation (Anderson 1998; Chapman et al. 2012; Hankerson et al. 2007; Peres 1996). We then combine this behavioral dataset with environmental predictors derived from aerial LiDAR scans in a maximum entropy (MaxEnt) model to identify the most important environmental variables for predicting microhabitat use, and we find that our two study species respond to anthropogenic disturbance in species-dependent ways that align with the predictions of competitive exclusion theory. These nuances can be revealed only by distinguishing the spatial distribution of sleeping and foraging behaviors from that of presence records.
2 Methods
2.1 Study Area
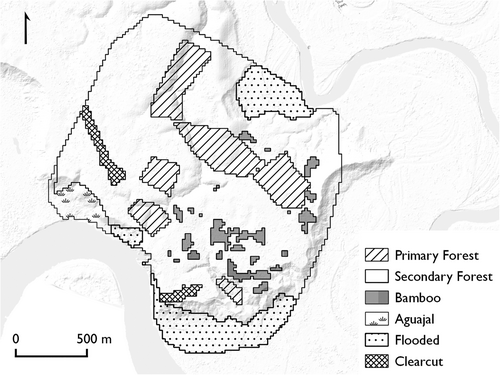
The study took place at Estación Biológica Los Amigos (EBLA, formerly known as El Centro de Investigacíon y Capacitción Río Los Amigos or CICRA), located at 12.569°S 70.100°W in the southeastern Peruvian department of Madre de Dios. EBLA provides a natural experiment for the study of anthropogenic disturbance because of its prior use as a small farm, a center of operations for a gold mining operation, and a logging camp (Pitman 2008). Clearcutting has taken place in two areas: ~2 ha of land around the station and a 5-ha landing strip 750 m to the northwest (Figure 1). The original clearcutting, around the area that is now the station, took place sometime in the 1990s and has been kept up since then as the station has gradually expanded. The landing strip was originally cleared in the early 2000s, and was maintained until 2010. A small section in the middle, approximately 120 × 25m, was maintained until 2014, at which time the entire landing strip began to regenerate.
2.2 Habitat Modeling
The majority of our predictor variables were derived from an aerial lidar scan taken in June 2019. Lidar (light detection and ranging) is a powerful tool for measuring forest structure. Based on the same principle as radar, lidar uses laser pulses to create a three-dimensional point cloud representing aboveground structures (Collis 1970; Davies and Asner 2014; Wandinger 2005). When deployed from low-flying flight platforms such as drones or small manned aircraft, lidar scans provide a high-resolution structural image that accurately replicates traditional forestry metrics such as canopy height, aboveground biomass, and leaf area (Dubayah and Drake 2000; Sabol et al. 2014). Here, we used data acquired by the GatorEye Unoccupied Flying Laboratory in June 2019. Detailed system specifications and workflow descriptions are provided at www.gatoreye.org, as well as data download links. We flew at 100 m above ground level (approx. 40–80 m above canopy height), at 10 m/s velocity, and with a spacing of 50 m between flight lines. This flight path allowed the GatorEye platform to cover the full study area while achieving the highest possible resolution. Data were preprocessed to .las (Laser) format lidar point clouds and then post-processed to topographic maps and canopy height models using the GatorEye multi-scalar post-processing workflow. Lidar data were used to derive 21 of our 35 environmental predictor variables (Table 1). Lidar point clouds were processed using ArcMap 10.8.1 (ESRI 2020). LiDAR anomalies were removed using an isolation method and by removing any point above the elevation of the highest emergent trees in the study area (360 masl). Height rasters were created at 1 and 20 m resolution, and 1 m rasters were resampled to 20 m to produce canopy variation and gap percentage rasters. In the creation of each 20 m raster, the value for each cell is based on a statistical description of all 1 m cells within that cell (see Table 1 for details). A 20 m cell size was chosen because it affords reasonable confidence that our GPS points, taken at an accuracy of ~10 m under the canopy, would be assigned to the correct cell.
| Variable | Definition | Raw data | References |
|---|---|---|---|
| Canopy variation | SD of 95th percentile values (1 m grid) in each 20 m cell | GatorEye | Rechsteiner et al. (2017) |
| Gaps | % of 1 m grid cells in which the 95th percentile value is < 5 | GatorEye | Rechsteiner et al. (2017) |
| Height Kurtosis | “Heaviness” of tails in height distribution | GatorEye | Singh et al. (2018) |
| Height MAD | Median absolute deviation in height distribution | GatorEye | |
| Height maximum | Maximum height value of all points in cell | GatorEye | |
| Height mean, max, SD | Statistical measurements on all height values within each cell | GatorEye | |
| Height skewness | Asymmetry in height distribution | GatorEye | Singh et al. (2017) |
| LAI: mean, SD | Leaf area per unit ground area | GatorEye | |
| Mean elevation | Average absolute elevation of points within the cell, measured in masl | GatorEye | |
| Number of crowns | Number of tree crowns in each cell | GatorEye | Li et al. (2012) |
| Percentiles: 5, 10, 25, 50, 75, 90, 95, 99 | Percentile distribution of height values for points within each cell | GatorEye | |
| Distance from clearcutting | Linear distance from either of the two clearcut areas | Hand-drawn polygons | |
| Distance thresholds: 50, 100, 200, and 400 m | Threshold distances from clearcutting, derived from raw distance raster | Hand-drawn polygons | |
| Forest type | Categorical: primary, secondary, bamboo, aguajal, flooded, chacra, purma | 2017–2019 ground data; canopy height model; hand-drawn polygons | |
| NDVI: max, mean, min, SD | Spectral index: | PlanetScope | Boubli and De Lima (2009); Goswami et al. (2010); Widyastuti et al. (2020) |
| SR: max, mean, min, SD | Spectral index: | PlanetScope |
We also created rasters of leaf area index (LAI), a measure of total leaf surface area per unit ground area (Monteith and Unsworth 2013). LAI tends to be low in littoral areas and in Cecropia-dominated purma forest, but is high in secondary forest, mature forest, and bamboo patches (Emmons and Dubois 2003). The primary biological significance of LAI is as an indirect measure of photosynthetic activity per unit area (Lindroth et al. 2008). Because initial analysis of LAI should use the smallest grid possible while still minimizing not-available (NA) values (Sabol et al. 2014), we calculated LAI on a 5 m grid, then resampled to 20 m. All rasters were masked to remove cells outside the study area and those within 20 m of water.
In addition to the lidar data, we used three additional sets of environmental information: spectral imagery, ground observation, and distance rasters. Using PlanetScope multispectral imagery (Planet Team 2017), we calculated rasters of simple ratio (SR) and normalized difference vegetation index (NDVI). Both of these ratios are general indicators of vegetation density in the canopy, though they are also sensitive to atmospheric disturbance and to interspecific and phenological differences in infrared reflectance (Jensen 2015). We downloaded a series of six cloud-free images dated between May 10 and July 30, 2018, averaged them to compensate for the effects of sun angle, and performed standard band calculations for SR and NDVI (Table 1) using QGIS (QGIS.org 2021). We also created distance rasters to evaluate the distance from clearcutting. We drew polygons by hand over the two clearcut areas, then calculated a minimum distance raster over the full study area using the st_distance function in the R package sf (Pebesma et al. 2018). We also calculated binary threshold rasters at 50, 100, 200, and 400 m from clearcutting (Broadbent et al. 2008). Finally, we derived categorical maps of forest type from ground observations made during behavior scans. We used seven categories of forest type: primary forest, secondary forest, bamboo, aguajal (palm swamp), floodplain forest, chacra (abandoned banana plantation, found near the present site of EBLA), and purma (low, dense forest with a high proportion of Cecropia spp.). These observations were used in combination with lidar data to manually identify bamboo patches and patches of primary forest (Figure 1).
2.3 Animal Handling and Behavioral Observations
To facilitate behavioral observations, tamarin groups were captured and fitted with radio telemetry collars following the protocol of Watsa et al. (2015). We captured each group in its entirety using a multicompartment trap baited with banana slices. Animals were then anesthetized with ketamine hydrochloride at a dosage of 10–20 mg/kg, fitted with unique beaded collars and given bleached tail patterns to facilitate individual identification, then released at the trap site on the same day. Each group's primary breeding female was fitted with a radio telemetry collar, which was used in the identification of sleep sites. In the 2017, 2018, and 2019 research seasons, a total of 220 individual captures were performed, totaling 58 unique saddleback tamarins and 62 emperor tamarins. Follows were performed on 4 groups of saddleback tamarins, totaling 19 individuals, and on 4 groups of emperor tamarins, totaling 26 individuals.
Behavioral observations were recorded from June-August in 2017 and 2018 and from June-July in 2019. Generally, animals were followed from ~0600, when they left their sleep tree, unti1 ~1400–1600 when we left them to ensure that the presence of observers did not influence their choice of a sleep site. Some follows, however, were conducted over only a half-day due to personnel constraints.
Observers carried a Garmin GPSMAP 78 unit, which recorded a GPS point from underneath the group every 30 s during the follow. Tests at the study site indicated a horizontal accuracy of ~10 m under the canopy. To identify sleep sites, we located the group by radio telemetry after it had chosen a sleep site, then confirmed the specific tree using triangulation from four cardinal directions. We then took an average waypoint at the base of the tree to mark its location.
Scan samples were taken every 10 min, including all group members visible during a 2-min window (Altmann 1974). Behaviors were coded to a nine-part ethogram: moving, resting, foraging, self-grooming, other-grooming, mating, scent-marking, aggression, or other. Geotags for scans were defined as the centroid of the four GPS fixes taken throughout the duration of the 2-min scan period. During the 2018 season, random individuals were selected for 20-min focal animal sampling (Altmann 1974). For the MaxEnt analysis, we took all foraging records ad-lib from focals and included them in the behavioral database to have a more complete picture of food sources and locations. Although this inclusion would invalidate a traditional analysis of activity budgets as a function of time, it is analogous to including ad-lib presence records for a cryptic species, a practice that is considered valid for MaxEnt spatial analysis (Elith* et al. 2006). Food items were noted only when they could be unambiguously identified; in many instances, this was not the case, so most foraging records do not include food items. Before running the model, all points were rarefied so that only one record was retained from each 20 m grid cell.
2.4 Statistical Analysis
Niche modeling frequently uses the Maximum Entropy algorithm or MaxEnt (Phillips et al. 2006). MaxEnt is a machine learning algorithm that can analyze the predictive value of a large number of environmental variables based on a relatively small sample of presence-only data, and that has been shown to outperform other similar models, especially for cryptic or low-prevalence species (Elith* et al. 2006; van Proosdij et al. 2016). Though traditionally used to model species distributions (Chiou and Blair 2020), MaxEnt can model any point-based distribution, including a distribution of discrete behaviors within a species (Singh et al. 2018).
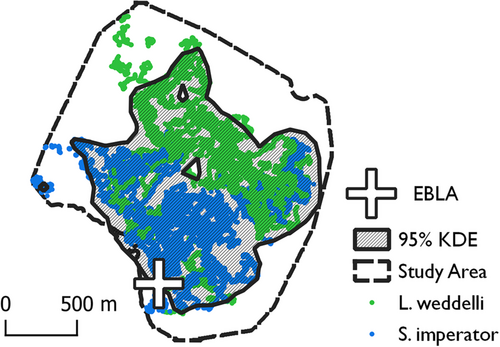
MaxEnt models are robust to small sample sizes and collinearity in environmental predictors but are susceptible to sampling bias, especially when background points are drawn from too broad a geographic area (Anderson and Raza 2010; Elith et al. 2011; van Proosdij et al. 2016). The most reliable way to avoid sampling bias in MaxEnt is to subsample presence records so that they are not as highly clustered in areas of intensive sampling (Fourcade et al. 2014). By default, MaxEnt achieves this by using only one presence point per grid cell. Sampling bias is further reduced when pseudo-absence points are drawn from well-sampled areas rather than distributed evenly across the landscape (Elith et al. 2011). For this reason, we drew 500 pseudo-absence points from the most well-sampled areas of the study site, namely those within the 95% contour of a kernel density estimation for all observations (Figure 2). The results of the model, therefore, indicate the probability of a particular behavior by a particular species given a high probability of tamarin presence (either species). Our MaxEnt models do not indicate the probability that some kind of tamarin will be present since our analysis is restricted to an area where such presence is highly likely.
Some analyses have suggested that collinearity in predictor variables is less of a problem for MaxEnt than for other niche modeling algorithms, but it is still non-trivial (Elith and Leathwick 2009; Merow et al. 2013; van Proosdij et al. 2016). In addition, the inclusion of too many predictor variables may introduce too much complexity to the model and cause overfitting (Phillips and Dudík 2008). Both reduction in collinearity and reduction in total number of variables can be achieved by filtering out the most highly correlated variables. Although there is a lack of consensus in the MaxEnt literature regarding how this should be done (Feng et al. 2019), one of the most common approaches is to filter out highly correlated variables until a predefined threshold has been met, such as |r| < 0.8 (Moraes et al. 2020) or |r| < 0.7 (Dormann et al. 2013).
To reduce the number of variables and the collinearity among them, we ran a correlation matrix of all predictors, then removed the one with the highest marginal (total) collinearity until the value for any combination of predictors was |r| < 0.6. Following this process, we were left with 18 environmental predictors (Table 1).
We performed 100 replications of the MaxEnt model for each of 5 behavior categories: presence, foraging, sleeping, consuming fruit/flowers, and consuming invertebrates. To model species presence, we used all observations regardless of behavior. This included observations where no behavior was recorded, either because the animal was temporarily out of sight or because the observer was not recording behavior. Models were created using MaxEnt version 3.4.1 as implemented by the dismo package in R 4.0.2 (R. Hijmans et al. 2020; R Core Team 2020). All variables were treated as linear except forest type and 50 m/100 m/200 m/400 m from disturbance, which were treated as categorical. To account for GPS error, we randomly moved each behavioral observation within a 10 m radius on each run of the model. Points were rarefied to 1 per grid cell (1 per 400 m2) before each run (Table 2). Area under curve (AUC), correlation, and p values were calculated using a fivefold cross-validation (Hijmans and Elith 2013).
| Species | Behavior | AUC (mean ± SD) | n (raw) | n (rarefied) |
|---|---|---|---|---|
| Saddleback | Presence | 0.67 ± 0.01** | 21,817 | 1836 |
| Foraging | 0.82 ± 0.03** | 135 | 62 | |
| Fruit/flowers | 0.91 ± 0.03** | 51 | 25 | |
| Invertebrate | 0.91 ± 0.02* | 31 | 18 | |
| Sleep tree | 0.86 ± 0.03* | 39 | 37 | |
| Emperor | Presence | 0.67 ± 0.01** | 36,206 | 1773 |
| Foraging | 0.8 ± 0.03** | 209 | 81 | |
| Fruit/flowers | 0.85 ± 0.07 | 10 | 9 | |
| Invertebrate | 0.88 ± 0.03** | 67 | 39 | |
| Sleep tree | 0.85 ± 0.03** | 54 | 48 |
- * p < 0.05
- ** p < 0.01.
In addition to the MaxEnt analysis, we performed a proportional analysis of habitat type by behavior category. In each of 100 replications, behavior points were moved randomly within a 10 m radius to accommodate uncertainty in the GPS points, and a breakdown of different forest types was computed for each behavior category. We then compared the resulting proportions against randomly distributed points to simulate the null hypothesis.
Model performance was evaluated using both AUC and p value. AUC cannot be compared with frequentist measures of significance, and even direct comparison of AUC values among different models can be problematic (R. J. Hijmans and Elith 2013; Jiménez-Valverde. 2012; Lobo et al. 2008). Some authors have suggested a threshold-based approach, such as using values of 0.7 and 0.9 for “good” and “very good” model performance, respectively (see Manel et al. 2001; Pearce and Ferrier 2000; van Proosdij et al. 2016), but a p statistic can also be calculated for AUC values using a simulated null hypothesis (Raes and ter Steege 2007). Because of the lack of consensus regarding how model performance should be evaluated, we report both p values and AUC values.
2.5 Proportional Analysis
To calculate significance in the distribution of behavior by forest type, we followed Kulp and Heymann (2015) in using a χ2 test to compare the proportion of each activity observed in each forest type. Points were rarefied to no more than 1 per 20 m grid cell to minimize the effects of spatial autocorrelation. To simulate a null hypothesis, we used a random walk model with a step size equal to the median distance between consecutive scan locations (59.7 m for saddleback tamarins; 32.7 m for emperor tamarins) and a step counts drawn at random from the total number of scans per follow in our dataset. The random walk was repeated 1000 times and rarefied using the same method as observed datapoints. A null contingency table representing forest type by species was then calculated based on 1000 runs of the random walk model and compared against a similar contingency table of observed proportions for each behavioral category. We then compared observed vs expected proportions using a χ2 test.
Rather than reporting activity budgets for each forest type, which would not be comparable to MaxEnt results, we report forest type choices for each activity. This strategy has the additional advantage of allowing us to include ad-lib foraging records, which would not be usable in an analysis of activity budgets.
3 Results
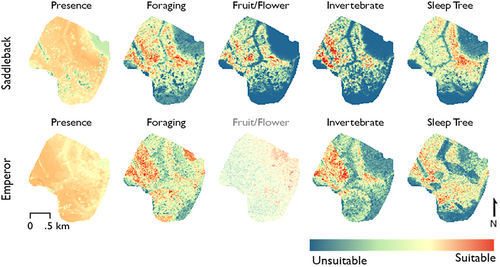
Our results show the value of combining MaxEnt modeling with fine-scale spatial data for revealing nuances of niche in rainforest species. We find conspicuous differences both within species when comparing different behavior states, and across species when comparing the same behavior state (Figure 3). Specifically, elevation is an important predictor of critical behaviors but is less important for presence: tamarin species are observed in low-elevation forest but forage and sleep exclusively in terra firme. In addition, tamarins generally used secondary forest more for sleeping and less for foraging than would be expected by chance. Between species, we found evidence for minimal niche segregation in presence but much more pronounced niche segregation in sleeping and especially foraging (Table 3).
| Saddleback | |||||
|---|---|---|---|---|---|
| Presence | Foraging | Fruit/flower | Invert. | Sleep tree | |
| Emperor | |||||
| Presence | 0.91 ± 0.01 | 0.68 ± 0.04 | 0.52 ± 0.03 | 0.50 ± 0.04 | 0.62 ± 0.03 |
| Foraging | 0.76 ± 0.02 | 0.68 ± 0.04 | 0.56 ± 0.04 | 0.53 ± 0.05 | 0.63 ± 0.04 |
| Fruit/flower | 0.76 ± 0.10 | 0.59 ± 0.09 | 0.44 ± 0.07 | 0.42 ± 0.08 | 0.56 ± 0.07 |
| Invert. | 0.67 ± 0.05 | 0.71 ± 0.04 | 0.64 ± 0.04 | 0.61 ± 0.04 | 0.63 ± 0.04 |
| Sleep | 0.65 ± 0.04 | 0.64 ± 0.04 | 0.54 ± 0.05 | 0.53 ± 0.05 | 0.64 ± 0.04 |
3.1 Behavioral Observations
Across the three research seasons, we conducted a total of 483 h of behavioral observations, identified 107 sleep trees, and recorded 2801 non-sleep behaviors through scan sampling. Observations of saddleback and emperor tamarins did not completely overlap in geographic space (Figure 2). Still, we found a niche overlap value of 0.91 ± 0.01 for presence points, which indicates substantial overlap in covariate space (Table 3). Both species, therefore, are found in similar forest types, and differences in microhabitat are unlikely to be due to the non-overlapping spatial extents of our observations.
3.2 MaxEnt Results
As measured by AUC, model performance varied from 0.67 ± 0.01 (presence models) to 0.91 ± 0.03 (saddleback fruit/flower consumption). However, when analyzed for significance from a frequentist perspective, the AUC values proved to be a poor measure of model performance: models with low AUC often proved highly significant, and one model (emperor tamarin fruit/flower consumption) had high AUC but was not significant (Table 2). The overall correlation between AUC and p value was near zero (Pearson product-moment correlation, R2 = 0.04, t = 0.57, df = 8). As measured by statistical significance, the strongest models were presence and foraging for both species, fruit/flower consumption for saddlebacks, and sleeping and invertebrate consumption for emperor tamarins. The only statistically non-significant model (p > 0.05) was emperor tamarin fruit/flower consumption. Across all species and behaviors, the strongest contributors were elevation and forest type, with gaps and distance to clearcutting as important secondary factors (Supporting Information S1: Table S1).
Elevation was the strongest predictor of all behavior categories in saddlebacks, though it was not a strong contributor to overall presence. In emperor tamarins, elevation was the second-strongest overall predictor and the strongest predictor of foraging and invertebrate consumption. In all statistically significant models, higher elevations were associated with greater habitat suitability. (Emperor tamarin fruit/flower consumption in our model was projected to increase at lower elevations, but this model was not statistically significant.) Forest type was revealed to be a highly significant predictor of behavior in both MaxEnt and proportional analyses. In MaxEnt, forest type was the strongest predictor of saddleback presence and of emperor tamarin sleep trees. It was the second- or third-strongest predictor of foraging and fruit/flower consumption in saddlebacks and of presence and fruit/flower consumption in emperor tamarins.
Forest gaps had a strong effect on saddlebacks, but less so on emperor tamarins (Supporting Information S1: Table S1). In saddleback tamarins, gap percentage was negatively associated with all behavior categories and was a strong predictor of overall presence. Emperor tamarins showed less sensitivity to gaps. Although gaps were the second-strongest predictor of sleep tree distribution in emperor tamarins, the correlation value was nearly zero, indicating that this species prefers a moderate level of canopy gaps for its sleep sites. In other behavior categories, gap percentage made a relatively minor contribution.
For emperor tamarins, suitability models were highly responsive to distance from clearcutting, especially at the < 400 m threshold. The 400 m threshold was the strongest contributor to presence and the second strongest contributor to foraging and invertebrate consumption. It was not, however, a strong contributor to the sleep tree model. The association was positive in all statistically significant models. Presence models showed a small but significant difference between the habitats used by the two tamarins species. More substantial differences were observed in individual behavior categories. Fruit/flower foraging, in particular, showed marked contrast between the types of forest used by the different species. Invertebrate and fruit/flower consumption by saddleback tamarins had relatively little overlap with any emperor tamarin behaviors.
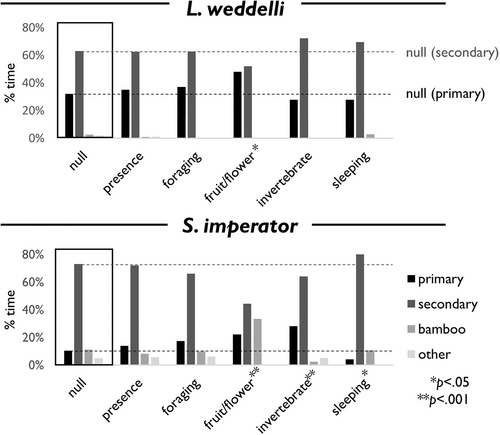
3.3 Proportional Analysis
Proportional analysis revealed no significant deviation from a random walker in presence (saddleback tamarin: χ2 = 1.18, df = 3, p > 0.1; emperor tamarin: χ2 = 1.09, df = 3, p > 0.1) but significant differences were found in several behaviorally explicit models. For saddleback tamarins, fruit/flower consumption occurred significantly more often in primary forest and less in secondary forest than expected (χ2 = 8.99, df = 3, p < 0.05). For emperor tamarins, sleep sites were significantly more likely to be found in secondary forest and less likely to be found in primary forest when compared with a random walker (χ2 = 8.55, df = 3, p < 0.05). Although foraging in general did not differ significantly from the null, both fruit/flower consumption and invertebrate consumption did, with invertebrates consumed more often in primary and less in secondary forest (χ2 = 14.23, df = 3, p < 0.01) and fruit/flowers consumed more often in primary forest/bamboo and less in secondary forest (χ2 = 27.09, df = 3, p < 0.01).
4 Discussion
Our results show that both saddleback and emperor tamarins distribute their behaviors into different forest types, complicating the idea of tamarins as exhibiting a preference for second-growth forest (Wolfheim 1983) and suggesting that presence-based models obscure important information about how animals use their environment. Saddleback tamarins, in particular, were frequently observed in secondary forest but nonetheless found to be highly dependent on primary forest for fruit/flower consumption (Figure 4 and Table 4). Although saddleback tamarins were found to avoid bamboo patches and other forest gaps, their behavior seems to be unaffected by distance to areas of anthropogenic disturbance. Whatever the edge effects of such disturbance may be, they appear to have minimal impact on the spatial distribution of behavior in saddleback tamarins.
| Species | Behavior | Primary | Secondary | Bamboo | Other |
|---|---|---|---|---|---|
| Saddleback | presence | 0.03 | 0.00 | −0.02 | −0.01 |
| foraging | 0.05 | 0.00 | −0.03 | −0.02 | |
| fruit/flower* | 0.16 | −0.11 | −0.03 | −0.02 | |
| invertebrate | −0.04 | 0.09 | −0.03 | −0.02 | |
| sleeping | −0.04 | 0.06 | 0.00 | −0.02 | |
| Emperor | presence | 0.04 | −0.01 | −0.03 | 0.01 |
| foraging | 0.07 | −0.07 | −0.01 | 0.01 | |
| fruit/flower** | 0.12 | −0.29 | 0.22 | −0.05 | |
| invertebrate** | 0.18 | −0.09 | −0.09 | 0.00 | |
| sleeping* | −0.06 | 0.12 | −0.01 | −0.05 |
- * p < 0.05
- ** p < 0.001.
Emperor tamarins show more evidence of secondary growth preference with a high tolerance for anthropogenic disturbance. They use secondary forest significantly more than would be expected by chance and are more likely to forage within 400 m of clearcutting. This is in line with the general view of tamarins as tolerating or preferring secondary growth (Rylands 1996) and with previous descriptions of emperor tamarin ecology specifically (Terborgh 1983). Emperor tamarins in our study used more primary and less secondary forest for invertebrate foraging, which corroborates the findings of Kupsch et al. (2014) and complicates our understanding of how tamarins use secondary growth.
In both species, absolute elevation was an important predictor of habitat use. Saddleback tamarins were found at both high and low elevations but preferentially use terra firme forest for all critical behaviors. The effect of elevation on behavior is conspicuous in the suitability models for saddleback tamarin behaviors, where the lowest values are consistently found in the flooded forests to the south and northeast and in the ravine running through the center of the study area (Figures 1 and 3). The relationship is similar, but weaker, in emperor tamarins. This is surprising given that emperor tamarins have previously been described as strictly using terra firme (Wolfheim 1983). Although most of our study area was composed of high-elevation terra firme forest, most groups’ ranges include areas of low elevation in ravines. The finding that elevation was a major predictor of foraging and sleeping, but only a weak predictor of presence, indicates that the tamarins at our site behave differently in high- and low-elevation forest despite spending time in both. This difference cuts across the categorical analysis of forest type, since primary forest and especially secondary forest exist at both high and low elevations at our site. We hypothesize that distance from water may be an important variable here, since the main cause of variance in elevation at our site is the presence of large ravines cut by streams.
We found evidence of niche partitioning under behavioral analysis but not under presence-based analysis. Competitive exclusion theory predicts that selection will drive ecologically similar species into separate niches: if one species does not outcompete the other, then both species must either segregate their ranges or exploit different resources through behavioral niche partitioning (Álvarez-Yépiz et al. 2017; Conners et al. 2015).
In the case of saddleback and emperor tamarins, presence points gave an overlap index of 0.91, confirming that the two species are found in broadly similar types of forest (Table 3). A small but significant non-overlap was found, driven in part by saddleback tamarins’ avoidance of bamboo and greater use of primary forest. Under behavioral analysis, however, we found strong and significant niche segregation in all feeding categories and in the distribution of sleep sites. The two species, therefore, appear to exploit resources differently despite their presence in similar forest types. Previous research has shown that callitrichine primates exhibit vertical niche segregation (Heymann et al. 2000) and segregation in foraging strategy (Peres 1992), but to our knowledge, this is the first evidence that such segregation has a spatial component as well. We suspect that this spatial segregation is an adaptation to reduced food availability during the dry season, but our dataset does not include enough wet season data to test this hypothesis.
Our analysis addresses how the spatial distribution of behavior is affected by physical ecology. But in a territorial primate, ecology is not the only constraint on behavior: inter- and intra-group social forces also play an important role (Harvey and Clutton-Brock 1981; Mitani 2006). A social analysis might examine the distribution of behavior relative to intergroup boundaries or core areas of the group's home range. Furthermore, the social analysis could disaggregate the behavioral database, examining each group's behavior relative to the distribution of habitat types within its own territory. Territoriality is rarely considered in niche modeling analyses, presumably because the resolution of such analyses tends to be too coarse for individual group territories to be resolved. However, territoriality is commonly included in models of animal movement (Hansen et al. 2024; Lutnesky and Brown 2015), and the methods of the animal movement literature could be applied to fine-grained niche modeling. Such an analysis would require a larger dataset than the current study affords.
More broadly, our results suggest the importance of behavioral sensitivity in niche modeling. By using high-resolution structural lidar data in combination with geotagged behavioral observations, researchers can discover important nuances in ecological niche that would not be visible from a purely presence-based approach. Although full-day follows are often infeasible with non-primates, useful behavioral data may be derived from camera traps (Bridges and Noss 2011) or citizen science projects (Styring et al. 2016) and incorporated into niche models so that conservation managers can distinguish critical from non-critical habitat.
Acknowledgments
Our work is performed in collaboration with the Amazon Conservation Association, which operates the Los Amigos Biological Station, and with Field Projects International, which operates the annual mark-recapture program. We are indebted to the station staff and student volunteers. Lynne Isbell and Eileen Lacey provided guidance on study design and helped edit the manuscript. We thank the editorial staff of AJP and the two anonymous reviewers for their thoughtful comments and questions. Authors were funded by a University of California Berkeley Fellowship (RGS), USDA McIntire-Stennis grant (AMAZ, ENB), and by Field Projects International (MW, GE).
Ethics Statement
This study was conducted under University of Missouri-St. Louis IACUC# 1208181-4, and with permission of the Peruvian National Forest and Wildlife Service (SERFOR), permit #245-2018-SERFOR/DGGSPFFS. We followed the American Society of Primatologists’ “Principles for the Ethical Treatment of Non-Human Primates” and “Code of Best Practices for Field Primatology,” as well as the American Society of Mammalogists’ Guidelines for the Use of Wild Mammals in Research. Throughout the study, our highest priority was the wellbeing of our study animals, our collaborators and assistants, and the local community.
Conflicts of Interest
The authors declare no conflicts of interest.
Open Research
Data Availability Statement
Raw data and R code available at: https://github.com/fieldprojectsint/tamarin_habitat_analysis. This repository includes R Markdown files for the MaxEnt analysis along with a database of geotagged behaviors and sleep sites.




