The Influence of Provisioning on the Intergroup Relationships of Rhesus Macaque in Hainan, China
ABSTRACT
Intergroup competition for limited resources is a significant selection pressure that drives the evolution of animal society. The rhesus macaque (Macaca Mulatta) is the most widely distributed nonhuman primate in the world and can adapt well to environments disturbed by humans. In some areas, human provisioning provides ample food resources for rhesus macaques, leading to an increase in their population size, inevitably affecting competition patterns within and between groups. In this study, we focused on seven provisioned groups of rhesus macaque in an eco-tourism park in Hainan, China, to verify how provisioning impacted their intergroup relationships. The results showed that: (1) Peaceful coexistence was the most common form of Intergroup contacts; (2) Provisioning led to an increase in intergroup contact and conflicts, but monkeys tended to avoid direct contact with other groups at main-provisioned sites with high conflict risk. (3) Larger groups did not interfere with each other's space use in the park, but smaller groups were more easily tolerated by other groups. (4) There were no strict linear dominance relationships among monkey groups. Overall, intensive provisioning satisfied the energy requirement of all monkeys in our study site, leading to a reduction in the relative benefit of intergroup conflict. Consequently, monkeys have adopted an intergroup contact strategy that avoids direct conflicts and prevents conflict escalation. We should pay more attention to the behavior patterns of provisioned animal populations, which will help us better understand how resources such as food have influenced the evolution of social strategies of animal groups, as well as how to manage such human disturbed animal populations in the future.
Summary
-
We analyzed the intergroup relationships among seven provisioned rhesus macaque groups.
-
The energy in the anthropogenic food could satisfy the requirements of all monkeys in our study sites.
-
Our results confirm that ample food resources could reduce interference competition among monkey groups, leading to a peaceful intergroup contact pattern.
Abbreviation
-
- GLMM
-
- generalized linear mixed model
1 Introduction
Intergroup competition originates from the disagreement of interest over food, territories and other limited resources (Radford, Majolo, and Aureli 2016). It has significant impacts on social relationships, social strategies among individuals within social animal groups, ultimately influencing individual fitness (Beehner and Kitchen 2007; Christensen and Radford 2018). Therefore, Intergroup competition serves as an important selective pressure that drives the evolution of individual social behavior and group social structure (Braga Goncalves et al. 2022; Grueter 2015).
According to the socio-ecological model, the pressure of predation and competition with other animal groups for resources are the main reasons why diurnal primates gather into groups for activities (Clutton-Brock and Lukas 2012; Sterck, Watts, and van Schaik 1997). Based on this, the distribution pattern of limited resources such as food in the environment determines both the intra- and Intergroup competition patterns (Clutton-Brock and Janson 2012; Isbell and Young 2002). The competition for food resources can be classified into two forms: interference competition and exploitation competition (Van Schaik and Janson 1988). Interference competition typically targets concentrated and monopolizable food resources, resulting in direct conflicts and displacement between competitors which can lead to hierarchical relationships among competitors (Thierry 2008). In contrast, exploitation competition arises from the utilization and depletion of the same resources, with its intensity influenced by the total amount of available resources and the number of users (Thierry 2008). When food resources are evenly distributed and non-monopolizable, both intra- and Intergroup competition primarily take the form of exploitation competition, resulting in peaceful and fair social relationships (Koenig 2002). Conversely, when food resources are distributed in patches, interference competition prevails between groups, leading to additional conflicts (Koenig 2002).
Group size is a key factor in determining the outcome of intergroup competition, as large groups always hold a dominant position (Verreaux's Sifaka, Propithecus verreauxi: Benadi, Fichtel, and Kappeler 2008; guerezas, Colobus guereza: Harris 2010). First, larger groups have more individuals that can directly engage in Intergroup conflicts. Second, due to their numerical advantage and dilution effect, individuals in large groups face lower risks of injury during intergroup conflicts and demonstrate greater willingness to participate compared to individuals in small groups (chimpanzee, Pan troglodytes: Wrangham 1999). These two factors contribute to the superior resource-holding potential of large groups and consequently give them an edge in intergroup competition (Majolo et al. 2020). In addition to group size, the number of male primates - typically larger and stronger than females - could significantly impact the fighting capacity of a group (vervet monkeys, Chlorocebus aethiops pygerythrus: Arseneau-Robar et al. 2016; Bshary, Richter, and van Schaik 2022). The intensity of intergroup conflict is affected by the combination of resource-holding potential and the value of resources. When the value of resources is high and group sizes are similar, there is typically an increase in aggression during intergroup conflicts (Majolo et al. 2020; mountain gorillas, Gorilla beringei beringei: Mirville et al. 2018).
Rhesus macaque (Macaca mulatta) is the most widely distributed nonhuman primate species, living in mixed-sex groups ranging from fewer than 10 to up to 250 individuals (Fooden 2000; Southwick et al. 1996). Male rhesus macaques emigrate between groups, while females remain in their natal group and establish a dominance hierarchy based on maternal kinship (Fooden 2000). Rhesus macaques are general omnivores with highly diverse and flexible diets (Cooper et al. 2022), which primarily consist of plant-based foods and vary depending on the availability of different food resources in their environment (Ding et al. 2020; Tang et al. 2016; Zhou et al. 2014). They have remarkable ability to coexist with humans as they can obtain up to 93% of their food from humans through handouts or by raiding crops (Southwick and Farooq Siddiqi 1994), and their populations thrive in rural and town areas adjacent to human settlements (Richard, Goldstein, and Dewar 1989). Human activities, which have led to the local extinction of natural predators of rhesus macaques in many areas and created concentrated high-quality food patches for macaques through food provisioning, discarded garbage and agricultural planting, have contributed to the thriving populations of rhesus macaque (Gilchrist and Otali 2002; McKinney 2011; Saraswat, Sinha, and Radhakrishna 2015). Provisioning can lead to the earlier sexual maturity of female macaques, an increase in birth rate and infant survival, a decrease in mortality, the clustering and growth of macaque populations (Shutt and Lees 2021; Zhang 2008), ultimately resulting in excessively high population densities in provisioned areas (Kurita et al. 2008; Zhu et al. 2019). This high density of population could lead to increased levels of competition both between and within groups, resulting in more intra- and intergroup conflicts (Hill 1999; Sterck, Watts, and van Schaik 1997).
- 1.
Macaques would be more likely to use areas with more anthropogenic food, and they would compete for high-value food resources (Sugiyama 2015). Therefore, we predicted that there would be more Intergroup contacts in sites with greater provisioning compared to those with less provisioning.
- 2.
The difference in resource-holding potential, along with the value of resources, affects the intensity of Intergroup competition (Majolo et al. 2020). Therefore, we predicted that there would be a higher likelihood of conflict in Intergroup contact when the difference in group size between the two groups was smaller, or when the total size of these two groups was larger, or when the contact occurred in sites with greater provisioning.
- 3.
High-level intra-group competition would lead to strict dominance hierarchy within groups (Thierry 2008), which might also apply to Intergroup relationships with high level competition under high population density (Hill 1999; Sterck, Watts, and van Schaik 1997). Therefore, we predicted that there will be strict linear dominance relationships between provisioned groups of rhesus macaques, with larger groups in a more dominant position. (Benadi, Fichtel, and Kappeler 2008; Harris 2010)
2 Methods
2.1 Ethics
All research practices in this study adhered to Chinese legal requirements and the American Society of Primatologists Principles for the Ethical Treatment of Nonhuman Primates and the American Society of Primatologists Code of Best Practices for Field Primatology.
2.2 Study Site and Subjects
We conducted this study at an eco-tourism park (109°59′ E, 18°24′ N) called “Nanwan Monkey Islet“ on the southeast coast of Hainan Island, China. The park was opened in 1985 with an area of less than 10 ha in the periphery area of Nanwan Nature Reserve. The reserve has an area of 10.2 km2 on a peninsula surrounded by sea water with a hill attaining an elevation of 255 m, and there are about 1500 ~ 2000 rhesus macaques living in it. The climate is tropical monsoon, with monthly mean temperatures range from 22.2°C in January to 28.1°C in July (Jiang et al. 1991; Zhang et al. 2016). The vegetation in the reserve is dominated by secondary evergreen monsoon rainforest (Jiang et al. 1988). A previous study found there were 388 species of vascular plants in the reserve, and that 36% of these species provided food for rhesus macaques (Jiang et al. 1991). The nature forest of the reserve could provide enough food for the rhesus macaques as both the provisioned and non-provisioned populations increased continuously since the foundation of the reserve (Jiang et al. 1998; Jiang et al. 1991).
Rhesus macaques can move freely between the park and the forest of reserve, and several groups of them regularly visit the park during the day. The park's staff feed monkeys several times a day with raw rice and sweet potatoes to keep them in the park for tourists to observe and interact with. They also sell peanuts, bananas, and other foods to tourists for feeding the monkeys at two fixed locations (GHT and YKZX). Despite constant supervision by park staff and security guards to prevent tourists from feeding monkeys with their own food, many tourists still do so privately or have their food passively stolen by the monkeys (Zhang et al. 2018).
We recognized all adult monkeys in the park by their facial features and morphological characteristics, such as body size, hair color and scars (Liu et al. 2018). Once we had determined that the entire group had arrived at the provisioned site by counting the number of recognized adults present, we proceeded to directly count the total number of monkeys in the group. We conducted this census for each group at least once during every study period. According to our census of monkeys in the park, there were 306 monkeys in six groups in 2014, then 433 monkeys in seven groups in 2019, and finally increased to 731 monkeys in ten groups in 2023. The number of groups increased due to several fission events of the two largest groups with more than 100 individuals (HL and SJB group). Most of the new groups resulting from fission remained in the park, with only two migrating away. For this study, we selected seven groups (HL, SJB, AC, HLG, SJBQ, GS, HZ) of monkeys that had been stable in the park for more than 3 years as our study groups. The largest group (HL) consisted of 140 ± 24 (mean ± SD, hereinafter the same) individuals, whereas the smallest one (HZ) had only 28 ± 7 individuals. Details about the group size of these seven groups can be found in Table S1.
The HLG group separated from the HL group in 2018, and the SJBQ group separated from the SJB group in 2019. Because experience in the same group may influence Intergroup contacts, we would not consider the HL-HLG pair and SJB-SJBQ pair in the following analysis. In 2023, the SJB group split into two new groups of the same size, so its data in 2023 was excluded from the analysis.
2.3 Energy in the Anthropogenic Food
The total energy requirement for all monkeys Etotal was calculated as, where i represented the age-sex classes of monkeys, N is for the number of monkeys, W is for the average body weight, and E is for the daily energy requirement. According to records from the tourist park in 2016 and 2020, the food provided by them could satisfy nearly half of the monkeys' energy requirements, which did not include food from tourists (see the supplement material for more details).
In the winter of 2023, the park staff roughly estimated that the total amount of anthropogenic food received by the monkeys was about 150 kg per day, including those from tourists. The food from tourists mainly consisted of peanuts, as well as bread, cookies, nuts and candies, which contained similar and even more energy than raw rice (Yang 2018). We used the energy in the raw rice to estimate the total energy contained in the 150 kg anthropogenic food, which was 519,000 kcal. In conclusion, the daily anthropogenic food in the winter of 2023 could provide an excess of 1.44 times more energy than required by all monkeys (Table 1).
| Age-sex classes | Number | Body weighta | Daily energy requirementb | Total daily energy requirement |
|---|---|---|---|---|
| Adult male | 22 | 6.33 kg | 100 kcal/kg | 13,926 kcal |
| Adult female | ||||
| Lactating | 163 | 5.24 kg | 150 kcal/kg | 128,118 kcal |
| Nonlactating | 66 | 5.24 kg | 100 kcal/kg | 34,584 kcal |
| Juveniles | 317 | 2.90 kg | 200 kcal/kg | 183,860 kcal |
| Sum | 360,488 kcal | |||
2.4 Study Periods
We completed individual recognition of all adult rhesus macaques present in the park in 2015 and started to record group locations in the park and their Intergroup contacts for at least 1 month per year during 2015 ~ 2023, except for 2022 due to the lockdown by Covid-19 in China. The total number of observation days was 518. We observed the monkeys from 8:00 to 11:30 and 13:30 to 17:30 daily. Details of the study periods can be found in Supporting Information S1: Table S2. All the group locations and intergroup contact events were recorded by Cheng-feng Wu with consistent definition across different study periods.
2.5 Different Provisioned Sites
We focused on 21 landmarks in the park (Figure 1) to study the impact of provisioning on Intergroup contacts. When at least one adult member of a group appeared within 20 m of a landmark, the group was recorded as being present at the location. One adult was needed to confirm the identity of group, and previous studies confirmed that one adult individual was enough to study group space use of rhesus macaques (Fan et al. 2024). 20 m was used because we could clearly locate and recognize the identity of monkeys within this distance at the landmarks. The distance between the landmarks could be less than 50 m. However, our observation found that the distance between monkeys from the same groups could be more than 100 m. Thus, a monkey group could be located and recorded at multiple landmarks simultaneously.
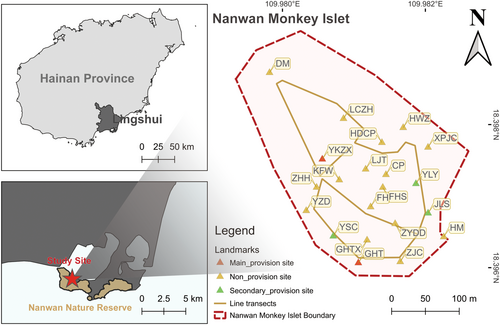
- a.
Main-provisioned site: the location where park staff fed the monkeys regularly to keep them in the park and also sell food for tourists to feed monkeys, including two locations (YKZX, GHT).
- b.
Secondary-provisioned site: the location where only park staff would feed the monkeys regularly, including three sites (YSC, JLS, YLY).
- c.
Non-provisioned site: all other locations where monkeys were not allowed to feed, including 16 sites.
In 2015 and 2016, we used Ad libitum sampling to record the location of monkey groups whenever we encountered them in the park (Altmann 1974). This data set provided us with a general distribution of monkey groups in the park but was not used in our analysis. We began using transect method to record the sightings of monkey groups at the landmarks in 2017. This transect (Green line in Figure 1) was approximately one kilometer long, and we were able to check all 21 landmarks within 30 ~ 60 min. On each observation day, we walked through the transect twice – once in the early morning upon arrival and again in the late afternoon before departure - and recorded locations of monkey groups based on the landmark system. We used the transect data set only in our analysis related to group's location.
2.6 Intergroup Contacts
Since 2015, we have been using Ad libitum sampling to record all Intergroup contacts that we directly observed in the park (Altmann 1974). For each contact event, we recorded the date, time, landmark location, initiator group, receiver group, and the identity of participants if possible. Referring to a previous study of bonnet macaques (Macaca radiata, Cooper, Aureli, and Singh 2004), we used a distance threshold of 40 m (taking the landmark as the center, within a radius of 20 m) for intergroup contact as it was the distance at our study site that monkeys from different groups could establish visual contact and confirm the identity of other group. We use one adult member as participant threshold for several reasons. First, we needed at least one adult to identify the group. Second, our records on the number of participants in the intergroup conflict showed an average of only two participants (see Results), so too high a standard would result in the loss of details of the intergroup contact. Finally, previous studies have confirmed that this standard could be used to study intergroup competition (Majolo, de Bortoli Vizioli, and Lehmann 2016; Robbins and Sawyer 2007).
- 1
Non-conflict contact:
Coexistence: Two monkey groups stay at the same location without conflicts for at least 5 min.
Avoidance: After group A approaches group B, group B leaves the location without conflict within 5 min.
- 2
Conflict contact:
Expulsion: Group A approaches and expels group B from the location with aggressive behavior (threat, chase, attack…) within 5 min.
Draw: Two monkey groups are in conflict (threat, chase, attack…) at the location, but no group leaves for at least 5 min after conflict.
- 3
Location contact (overlap):
Two groups were recorded at the same landmark location within 5 min. This definition was derived from location data, without specific details regarding the behavior of the monkeys in contact.
The conflict and non-conflict contacts would be used to analyze the influence of provisioning and group size differences on the intensity of intergroup competition. The location contacts would be used to compare with the theoretical distribution of intergroup contact under the null hypothesis that all groups move independently (described later). Expulsion and avoidance contacts would be used to analysis intergroup dominance relationships.
2.7 Data Analysis
We converted our Ad libitum sampling Intergroup contact and transect group sightings records at different landmarks to daily frequency for each year by the study days of that year. We tested the homogeneity of variance between groups, but found a significant difference (Levene Test, N = 75, F value = 13.437, p < 0.001). Then we conducted two sets of Kruskal-Wallis' test and Dunn's test: one to test whether groups of monkeys were more likely to be present at the main-provisioned site and another to test whether there were more Intergroup contacts there (prediction 1). Dunn's test is a statistical technique utilized for comparing the means or medians of several samples when significant differences have been detected in an initial omnibus test, such as the Kruskal-Wallis' test for non-parametric data (Dunn 1964). This analysis was performed with "dunn. test”package in R (Dinno 2024).
With the Ad libitum sampling data set, we built a generalized linear mixed model (GLMM) with binomial distribution with “lme4” package (Bates et al. 2015) in R to analyze the influence of provisioning and differences in group size on the probability of conflict during Intergroup contacts (prediction 2). In the model, the response variable was categorized as either conflict or non-conflict for Intergroup contacts. The fixed factors included the location types (main-, secondary-, nonprovisioned site) as indicators of provisioning strength, and the absolute values of group size differences and total group size for the two groups in contact. The random factor was the ID of group-pair and the month of records, to deal with the bias in the observation period of different years. We set “nonconflict” as the reference in the model with a significance level of 0.05. We did not include differences in number of adult females or males in this analysis due to a significant correlation between the number of adult females and group size (Pearson's r = 0.932, N = 863, p < 0.001), as well as the low participation rates of adult males in Intergroup conflicts (expulsion and draw contacts: 7/54, 13.0%).
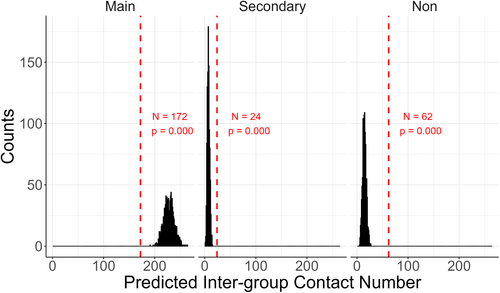
Small groups may choose to stagger their use of provisioned sites to avoid direct contact with large groups (Okamoto and Matsumura 2002), which could not be confirmed by directed observation. To test whether small groups avoided direct contact with large groups (prediction 3) and whether this avoidance led to less contact at the provisioned sites (prediction 1), we compared the actual location contacts (overlap) records with a theoretical distribution under the null hypothesis that all groups move independently regardless of other groups' positions (Alba-Mejia et al. 2013). We excluded the Ad libitum sampling data set due to its lack of records for groups without Intergroup contacts. Based on the group sightings records at different landmarks, we calculated the frequency of location contacts for each pair of groups at each location. To obtain the distribution of the null hypothesis, we repeatedly shuffled the pairing relationships between date and location within each group and each month, and then recalculated the frequency of Intergroup contacts. In more detail, the location records of HL group on May 1st could be exchanged with the records on May 31st without changing its time series of the day. The location records of HL group in May would neither be shuffled with those in other months or other years, nor with the records of other groups. This random approach altered the sequence of site utilization by monkeys on a monthly basis while maintaining consistent usage intensity, thereby eliminating the impact of Intergroup dynamics on site selection.
We repeated the random model 1000 times to obtain a distribution of the random intergroup contacts and compared it with the real data. The two-sided p-value was calculated as the probability that the observed number of intergroup contacts was as or more extreme (within the top or bottom 2.5%) than the expected distribution (Alba-Mejia et al. 2013). For example, when we found that the observed value was larger than 99.0% of expected value or lower than 99.0% of expected value, the p-value should be 0.02 (Christensen and Zabriskie 2021; Peskun 2018). If more than 97.5% of random models showed a higher frequency of Intergroup contact than the observed ones, we considered there to be a significant avoidance trend in Intergroup contact. Conversely, lower contacts in the random models suggest a significant attraction trend. We made this comparison for each group-pair and each type of location separately to see how group size and provisioning could affect avoidance or attraction tendencies.
To analyze the dominance relationship, we calculated the Elo-rating score for the seven groups based on intergroup contacts with clear winner-loser relations (expulsion and avoidance) from the Ad libitum sampling data set (Neumann et al. 2011; Newton-Fisher 2017). Elo-rating is based on the time serials of agonistic interaction. Each individual starts with an arbitrary rating of 1000 and then increases or decreases after each won or lost agonistic interaction (Neumann et al. 2011). We also used the “h” index to test the linearity of the dominance hierarchy. This measure ranges from zero to one, where one means highest linearity (de Vries 1995). All this analysis related to dominance relationships was finished by ‘EloRating’ package (Neumann and Kulik 2020).
Unless otherwise mentioned, all the analyses was finished with R 4.4.1 (R Core Team 2024), and the significant value was set to 0.05.
3 Results
3.1 Intergroup Contacts at Different Locations
From 2015 to 2023, we collected 863 intergroup contacts using Ad libitum sampling methods, including 86 expulsion, 201 avoidance, 512 coexistence and 64 draw records. According to Kruskal-Wallis' test and Dunn's test, there was significant difference of the daily Intergroup contact frequency among different types of locations (chi-squared = 32.22, N = 75, p < 0.001). The daily contact frequency was significantly higher at main-provisioned sites than at secondary- (Z = 3.14, N = 75, p < 0.001) and non-provisioned sites (Z = 5.66, N = 75, p < 0.001). There were also significant more contacts at secondary- than non-provisioned sites (Z = 1.74, N = 75, p = 0.040).
We identified adult participants of 54 conflict cases out of the 150 conflict contacts (86 expulsion plus 64 draw), including 27 expulsion and 27 draw contacts. A total of 73 adults attended these conflicts, with six adult males and 67 adult females. The average number of participants involved in conflicts was 2 ± 1, representing a small proportion of adult group members. Of the 54 conflict contacts, adult males participated in only seven, accounting for a rate of 13.0%, whereas females participated in 52, with a rate of 96.3%. This shows that adult females tended to be the primary participants in Intergroup conflicts.
We collected 2340 group sightings records at different landmarks using the transect method, including 1570 records at main-provisioned sites, 257 at secondary-provisioned sites, and 513 at nonprovisioned sites. According to Kruskal-Wallis' test and Dunn's test, there was a significant difference in the daily frequency of presence among different types of locations (chi-squared = 34.41, N = 78, p < 0.001). Monkey groups were more likely to be present at main-provisioned sites than at secondary- (Z = 2.23, N = 78, p < 0.001) and nonprovisioned sites (Z = 5.49, N = 78, p < 0.001), and they were also more likely to be present at secondary- than nonprovisioned sites (Z = 2.93, N = 78, p = 0.002). More details on the daily records of Intergroup contacts and group locations can be found in Supporting Information S1: Table S3.
We detected 258 location contacts in which two groups were present at the same landmark location within 5 min, with 172 contacts at main-provisioned sites, 24 at secondary-provisioned sites, and 62 at nonprovisioned sites. Although monkey groups were attracted to the main-provisioned site, they tended to avoid direct contact with other groups there. When compared with the null distribution model that assumes all monkey groups moved independently, the observed value of location contacts at the main-provisioned sites was significantly lower than the predicted distribution (p < 0.001, Figure 2). Conversely, the observed value of location contacts at the secondary- (p < 0.001, Figure 2) and non-provisioned site (p < 0.001, Figure 2) was significantly higher than predicted distribution, indicating an attractive tendency for Intergroup contacts there.
3.2 Factors Influencing Intergroup Contacts
According to the GLMM results (N = 863, Table 2), when considering all locations together, the probability of nonconflict contact between monkey groups was significantly higher than the probability of conflict contact. At the main-provisioned sites, the probability of intergroup conflict was significantly higher than at non-provisioned sites, but there was no significant difference with secondary-provisioned sites. Difference in group size did not influence the probability of Intergroup conflict, nor did total group size.
| Estimate | Std. Errors | Z-score | p value | |
|---|---|---|---|---|
| Intercept | -2.084 | 0.535 | −3.896 | < 0.001* |
| Non- versus main-provisioned | -0.595 | 0.241 | −2.463 | 0.014* |
| Secondary- versus main-provisioned | -0.333 | 0.305 | −1.092 | 0.275 |
| Total group size | 0.003 | 0.004 | 0.757 | 0.449 |
| Group size difference | -0.002 | 0.006 | −0.283 | 0.777 |
The results of the comparison with the null hypothesis for each pair of groups were summarized in Table 3, and more details can be found in Supporting Information S1: Figure S1. The result showed that there was neither a tendency of avoidance nor attraction for location contacts among the largest five groups (HL, SJB, AC, HLG, SJBQ). For the two small groups (GS, HZ), their Intergroup contacts showed various patterns: The GS group had an avoidance tendency with SJB group (observed value = 31, p < 0.001), but was attracted to HLG group (observed value = 9, p < 0.001); The HZ group avoided contact with SJBQ group (observed value = 0, p < 0.001), but had an attractive tendency with four bigger groups (HL, observed value = 16, p = 0.026; AC, observed value = 14, p = 0.010; HLG, observed value = 4, p = 0.020; GS, observed value = 10, p = 0.048).
| Group Size (Mean ± SD) | HL | SJB | AC | HLG | SJBQ | GS | HZ | |
|---|---|---|---|---|---|---|---|---|
| HL | 140 ± 24 | Non | Non | Non | Non | Attract | ||
| SJB | 98 ± 13 | Non | Non | Avoid | Non | |||
| AC | 75 ± 23 | Non | Non | Non | Attract | |||
| HLG | 56 ± 21 | Non | Attract | Attract | ||||
| SJBQ | 55 ± 14 | Non | Avoid | |||||
| GS | 50 ± 19 | Attract | ||||||
| HZ | 28 ± 7 |
- Note: Attract: there was a tendency for attraction compared to null hypothesis, more than 97.5% of random models show lower Intergroup contact frequency than the observed value. Avoid: there was a tendency for avoidance compared to null hypothesis, more than 97.5% of random models show higher Intergroup contact frequency than the observed value. Non: there was neither a tendency for attraction nor avoidance compared to null hypothesis.
3.3 Intergroup Dominance Relationships
The Elo-rating scores calculated from 87 expulsion contacts and 203 avoidance contacts for the seven study groups were shown in Table 4. Overall, the dominance ranking of the seven groups was nearly consistent with their group size order. However, the linearity of the dominance hierarchy was not significant (h index = 0.661, p = 0.082).
| Group size (mean ± SD) | Elo-rating | |
|---|---|---|
| HL | 140 ± 24 | 1480 |
| SJB | 98 ± 13 | 1198 |
| AC | 75 ± 23 | 1127 |
| HLG | 56 ± 21 | 960 |
| SJBQ | 55 ± 14 | 1025 |
| GS | 50 ± 19 | 722 |
| HZ | 28 ± 7 | 488 |
4 Discussion
We investigated the impact of provisioning on intergroup contacts among seven rhesus macaque groups on Nanwan Monkey Islet, Hainan, China. The total amount of food provided could exceed the energy requirements for all monkeys at our study site by 1.44 times and led to increased intergroup contacts as monkey groups were drawn to two main provisioned sites. However, they tended to avoid direct contact with other groups at these sites compared to what would be expected by independent movement. Our results indicated that non-conflict contact was the most prevalent form of intergroup interactions overall. Although high-level provisioning could increase the likelihood of conflicts during contact, only a small number of group members actually engaged in conflicts (2 ± 1 adults). Total group size and difference in group size did not affect the probability of conflict contact. Large groups neither avoided nor attracted each other, whereas small groups displayed mostly attractive contact patterns. The dominance rank order of monkey groups generally aligned with group size but did not follow a strictly linear relationship pattern.
Our result was consistent with the first prediction that there would be more intergroup contacts in more-provisioned sites than less-provisioned sites. The daily frequency of groups presented at main-provisioned sites (2.56 ± 0.99) were 16 times higher than at non-provisioned sites (0.16 ± 0.19). Compared to natural food resources, anthropogenic food contains more energy and is more digestible (Iwamoto 1987; Saj, Sicotte, and Paterson 1999). It is also more predictable in distribution and easily available (Ganguly and Chauhan 2018; Sugiyama 2015). Therefore, it was not surprising that groups of rhesus macaque were attracted to provisioned sites, resulting in a significantly higher daily frequency of intergroup contact at main-provisioned sites (0.54 ± 0.24) compared to non-provisioned sites (0.08 ± 0.08). A Study of Thomas's langurs (Presbytis thomasi) has also found that most of the group encounters took place in fruit patches (Steenbeek 1999). Similar attraction to provisioned sites or anthropogenic habitats was also found for moor macaques (Macaca maura) in Indonesia (Riley et al. 2021), rhesus macaques in India (Chaudhuri et al. 2006; Ganguly and Chauhan 2018; Sengupta and Radhakrishna 2018), and long-tailed macaques (Macaca fascicularis) in Singapore (Sha and Hanya 2013).
Our second prediction was only partly supported by our result. We found an increased probability of conflicts during contacts at the provisioned site, which was consistent with previous studies on intra-group conflicts for rhesus macaques in India (Southwick et al. 1976), and intergroup conflicts for northern pig-tailed macaques (Macaca leonina) in Thailand (José-Domínguez et al. 2015). However, only a few group members participated in conflicts, and nonconflict contact was the predominant form of intergroup contacts. A previous study of moor macaques also found a lack of female aggression in intergroup contacts, indicating that this behavior may be a result of small groups actively avoiding encounters with larger groups (Okamoto and Matsumura 2002). However, difference in group size did not influence the probability of conflict in intergroup contacts for our study groups. Our comparison analysis with independently moving model even revealed an attractive tendency between large and small groups (Table 3). This result is similar to findings from heavily provisioned Japanese macaques (Macaca fuscata) groups in Shodoshima, whose intergroup relationships were characterized by frequent neutral encounters and low intensity of aggression (Zhang and Watanabe 2012).
Our result could be attributed to the ample food resources provided at our study site, which met the needs of all monkey groups and thereby significantly reduced the benefits of direct interference competition. Animal's behavior strategy in intergroup contacts is based on the consideration of both cost and benefit (Braga Goncalves et al. 2022; Morris-Drake et al. 2022). Individuals involved in intergroup conflicts are inevitably confronted with the risk of injury or death (Nichols, Cant, and Sanderson 2015), and injured individuals would subsequently have greater mortality and reduced reproductive capacity (Bernardo and Agosta 2005; Krause et al. 2017). Previous studies have found that valuable resources such as fruit could affect the pattern of intergroup interactions for blue monkeys (Cercopithecus mitis stuhlmanni, Thurau and Cords 2024), and black howler monkeys (Alouatta pigra, Van Belle and Estrada 2019). When the benefit of winning an intergroup conflict is outweighed by the cost of participation, animals should minimize the possibility of intense intergroup conflict. This was supported by a study on barbary macaques, which found no significant difference in intra-group aggression between provisioned and non-provisioned macaques with sufficient food (Unwin and Smith 2015), as well as by the study of Japanese macaques in Shodoshima (Zhang and Watanabe 2012). In our research, we observed this phenomenon through the avoidance of direct contact by monkey groups at high-risk main-provisioned sites, as well as the disinterest of group members in participating in intergroup conflicts.
In such a peaceful context, there was no need for a strict linear dominance hierarchy among monkey groups, which is contrary to our third prediction. Larger groups held dominant positions, but there was no clear avoidance tendency in their interactions with smaller groups (Table 3). We attributed this result to the presence of abundant food in our study site. According to the socioecological model, when the food resources can meet the needs of the whole group, the intra-group female dominance hierarchy would become absent or nonlinear (Koenig 2002). This could also work for intergroup relationships in our study site with enough food for all groups.
Another reason for the absence of a strict linear hierarchy could be attributed to the presence of universal “free riders” in intergroup conflicts. According to the study on collective action problems, individuals bear the risks during intergroup conflicts whereas the benefits are shared by the entire group. As a result, some group members may choose to be “free riders,” avoiding direct participation in conflicts but still reaping benefits (Willems and van Schaik 2015). Adequate food resources from provisioning could reduce the relative benefits of winning intergroup conflict, and accentuate the risks, giving rise to “free riders.” Study of ring-tailed lemurs (Lemur catta) have confirmed that these “free riders” introduce uncertainty into the outcome of intergroup conflicts (McGuire and Sauther 2023), thereby diminish the linearity of dominance hierarchy between groups. In our records of conflict contacts that including participant identity, there were only an average of 2 adult participants. Such a small participation rate naturally leads to uncertainty in the relationship between conflict outcome and group size.
5 Conclusion
In summary, high-level provisioning could potentially reduce intergroup competition of rhesus macaques. The high value food resources from humans increased the frequency of intergroup contacts. However, sufficient food for all groups decreased the relative benefits of intergroup conflict while highlighting the risk, leading to peaceful intergroup relationships. Future studies could focus on the relationship between intergroup contacts and intra-group social behaviors, as previous studies suggested that intergroup competitive pressure could foster intra-group affinity and cooperation (Majolo and Maréchal 2017; Puurtinen and Mappes 2009), but did not get consistent results (Cheney 1992; Grueter 2013; Majolo, de Bortoli Vizioli, and Lehmann 2016). Provisioning of wild rhesus macaques was a very common practice in China, and it was difficult to stop due to the absence of national laws prohibiting the feeding of wild animals, which might cause ecological problems in the future (Zhu et al. 2019). We should pay more attention to the behavior patterns of human provisioned animal populations, which will help us better understand how resources such as food have influenced the evolution of social strategies of animal groups, as well as how to manage such human disturbed animal populations in the future.
Author Contributions
Cheng-Feng Wu: conceptualization (lead), formal analysis (lead), investigation (lead), methodology (lead), writing–original draft (lead), writing–review and editing (lead). Zhi-Hong Xu: investigation (equal), methodology (equal), writing–review and editing (equal). Yu-Xuan Fan: methodology (equal), software (equal), visualization (equal), writing–review & editing (equal). Tao Chen: investigation (equal), writing–review and editing (equal). Pu-Zhen Xie: formal analysis (supporting), writing–original draft (supporting), writing–review and editing (equal).
Acknowledgments
We thank Bo-Jun Liu, Xiao-Chan Yan, Xiao-Chen Ma and other volunteers and colleagues for assisting with field data collection. We also thank Dr. Peng-Fei Fan and Dr. Li Yang in the discussion of the article. We are grateful to staffs from Nanwan Monkey Islet and Nanwan Nature Reserve for assisting during our field work. This study was supported by Fundamental Research Funds for the Central Universities, Sun Yat-sen University (23lgzy002).
Conflicts of Interest
The authors declare no conflicts of interest.
Open Research
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.




