How Do Common Marmosets Maintain the Balance Between Response Execution and Action Inhibition?
Marcello G.P. Rosa and Farshad A. Mansouri had equal contributions.
ABSTRACT
Socio-dynamic situations require a balance between response execution and action inhibition. Nonadaptive imbalance between response inhibition and execution exists in neurodevelopmental and neuropsychological disorders. To investigate the underlying neural mechanisms of cognitive control and the related deficits, comparative studies in human and nonhuman primates are crucial. Previous stop-signal tasks in humans and macaque monkeys have examined response execution (response time (RT) and accuracy in Go trials) and action inhibition (stop-signal reaction time (SSRT)). Even though marmosets are generally considered suitable translational animal models for research on social and cognitive deficits, their ability to inhibit behavior remains poorly characterized. We developed a marmoset stop-signal task, in which RT could be measured at millisecond resolution. All four marmosets performed many repeated Go trials with high accuracy (≥ 70%). Additionally, all marmosets successfully performed Stop trials. Using a performance-dependent tracking procedure, the accuracy in Stop trials was maintained around 50%, which enabled reliable SSRT estimates in marmosets for the first time. The mean SSRT values across sessions ranged between 677 and 1464 ms across the four marmosets. We also validated the suitability and practicality of this novel task for examining executive functions by testing the effects of a natural hormone, oxytocin, on response execution and action inhibition in marmosets. This marmoset model, for reliable (millisecond resolution) assessment of the balance between response execution and inhibition, will further facilitate studying the developmental alterations in inhibition ability and examining the effects of various contextual and environmental factors on marmosets' executive functions.
Summary
-
Established a marmoset model of response inhibition employing a Stop-signal task (similar to those employed in humans and macaque monkey studies) and measured response time and stop signal reaction time at millisecond resolution.
-
Marmosets performed many repeated Go and Stop trials at a consistent level with high accuracy in the computerized stop-signal task.
-
Demonstrated the utility of a marmoset Stop-signal task to study the effects of contextual and environmental factors, such as oxytocin, on marmosets' response execution and action inhibition.
Abbreviations
-
- ADHD
-
- attention-deficit and hyperactivity disorders
-
- DLPFC
-
- dorsolateral prefrontal cortex
-
- GoRT
-
- response time in Go trials
-
- NHP
-
- nonhuman primate
-
- RT
-
- response time
-
- SSD
-
- stop signal delay: the delay between the presentation of the Stop-cue following the onset of the Go-cue
-
- TI
-
- trial initialization
-
- VLPFC
-
- ventrolateral prefrontal cortex
1 Introduction
Our engagement in daily activities requires various facets of cognitive control, including regulating the balance between response execution and action inhibition (Wade 1978; Miller and Cohen 2001; Mansouri, Tanaka, and Buckley 2009; Mansouri, Freedman, and Buckley 2020). Action inhibition is the ability to stop no-longer relevant actions (Mostofsky and Simmonds 2008; Verbruggen and Logan 2008), and therefore requires adapting new behavioral strategies while inhibiting previously applied response rules or strategies (Hrubesch, Preuschoft, and Van Schaik 2009; Manrique, Völter, and Call 2013). Executive control of action execution and inhibition may evolve in a context that includes various external and internal contextual factors, such as audio-visual information, emotional-social state, and hormonal fluctuations. That is, both internal and external factors may impact our ability to cease a pre-potent action appropriately and promptly.
Disturbances in the balance between response execution and action inhibition have been consistently reported in neurodevelopmental and neuropsychological disorders, including autism spectrum disorders and schizophrenia (Kiehl et al. 2000; Seidman, Valera, and Bush 2004; Brunet-Gouet and Decety 2006; Dumontheil, Burgess, and Blakemore 2008; Enticott, Ogloff, and Bradshaw 2008b; Agam et al. 2010; Hughes et al. 2012; Xiao et al. 2012; Pooragha, Kafi, and Sotodeh 2013; Vara et al. 2014). Neuroimaging studies suggest alterations in activation of dorsolateral prefrontal cortex (DLPFC) and ventrolateral prefrontal cortex (VLPFC) in these patients in the context of tasks involving inhibition (Aron et al. 2003; Aron, Robbins, and Poldrack 2004; Chikazoe et al. 2007; Dumontheil, Burgess, and Blakemore 2008; Swick, Ashley, and Turken 2008; Hampshire et al. 2010; Swann et al. 2012; Steele et al. 2013; Zhang and Iwaki 2019). These prefrontal regions are crucial in executive control of goal-directed behavior in humans and nonhuman primates (NHP) (Gilbert and Burgess 2008; Mansouri, Tanaka, and Buckley 2009; Passingham 2009; Levy and Wagner 2011; Shamay-Tsoory and Abu-Akel 2015; Mansouri, Egner, and Buckley 2017b; Mansouri et al. 2017c; Mansouri, Freedman, and Buckley 2020). However, it remains unclear when and how these neural alterations develop in these patients, leading to deficits in executive control. The lack of suitable animal models to address these questions has been a hindering factor.
NHPs are ideal animal models to examine the neural substrate of cognitive control and its dysfunction underlying neurodevelopmental disorders because of similarities to humans in sensory processing, social behavior and overall brain structure, including clear homologies in organization of the frontal cortical areas (Cheney, Seyfarth, and Smuts 1986; Burman and Rosa 2009; Mansouri, Tanaka, and Buckley 2009; Byrne and Bates 2010; Le Roux et al. 2013; Solomon and Rosa 2014; Mansouri, Egner, and Buckley 2017b; Mansouri, Freedman, and Buckley 2020). Until recently, the majority of studies have used various species of genus Macaca (Passingham 2009; Mansouri et al. 2017c; Mansouri et al. 2019; Preuss 2019; Mansouri, Freedman, and Buckley 2020). However, common marmosets (Callithrix jacchus) are gaining traction as a complementary nonhuman primate model for examining cognitive and social deficits underlying neurodevelopmental disorders (Takemoto et al. 2011; Takemoto et al. 2015a; Nakamura et al. 2018b; Pomberger et al. 2019; Ash, Ziegler, and Colman 2020; Axelsson et al. 2021; Ash et al. 2022; Samandra et al. 2022). The choice of marmosets is justified, among other reasons, by this species having homologous prefrontal cortical regions to those in humans and macaques (Roberts 2000; Price 2007; Burman and Rosa 2009; Burman et al. 2011; Vogt et al. 2013; Reser et al. 2017; Fukushima, Ichinohe, and Okano 2019; Ma et al. 2022), demonstrated by well-developed social behaviors (Varella and Ghazanfar 2021) which enable testing in social/group environment (Abbott et al. 2003; Mansfield 2003; Miller et al. 2016), as well as smaller size, shorter gestation and developmental periods in comparison to human and macaques (Missler et al. 1992; Tardif et al. 2003; Sawiak et al. 2018). However, executive control of action inhibition, which is required in the context of a Stop-signal task, has rarely been investigated in marmosets.
2 Description
This study aimed at establishing a marmoset model to closely examine regulation of response execution and action inhibition by employing a Stop-signal task. Then, to demonstrate and gain insights on how a specifically designed Stop-signal task can be utilized in marmoset behavioral studies, in the Example study, we examined the cognitive effects of intranasally-administered oxytocin in marmosets.
2.1 Response Inhibition in Marmosets: What Do We Know?
A number of studies, using an array of cognitive tasks, have examined cognitive abilities in marmosets, including object-reward association, attentional set-shifting, reversal learning, goal-directed and habit-based behavior, habitual control and response monitoring (Roberts, Robbins, and Everitt 1988; Dias, Robbins, and Roberts 1996; Dias, Robbins, and Roberts 1997; Rygula et al. 2010; Takemoto et al. 2011; Takemoto et al. 2015a; Nakamura et al. 2018b; Laclair et al. 2019; Pomberger et al. 2019; Ash, Ziegler, and Colman 2020; Axelsson et al. 2021; Ash et al. 2022; Calapai et al. 2022; Samandra et al. 2022). To examine inhibitory control, a majority of these studies have employed visual discrimination and reversal learning paradigms. Reversal learning involves suppressing and disengaging from previously learnt stimulus-reward or action-reward associations (Dias, Robbins, and Roberts 1996; Izquierdo and Jentsch 2012). In these studies, the percentage of correct trials, percentage of errors and the number of trials it takes to learn the response-reversal association are typically used as the means of quantification of reversal ability. These measures are indicative of the marmosets' ability to establish stimulus-reward associations and adaptively change these associations (cognitive flexibility).
The Go/no-go task involves responding to a repeated “go” stimulus (e.g., a pink square) and refrain from responding when an assigned “no-go” stimulus is presented (e.g., a blue square) (Meule 2017; Wessel 2018). In Go/no-go paradigms, particular cues are associated with response execution or response inhibition, respectively, and are presented before the response is planned or initiated. Therefore, in the Go/no-go paradigm response inhibition is explicitly instructed before the initiation/planning of response execution. Previous studies have examined marmoset performance on Go/no-go paradigms and reported accuracy (percentage of corrects) in go trials (response execution) and no-go trials (response inhibition) (Pomberger et al. 2019; Ash, Ziegler, and Colman 2020; Ash et al. 2022).
2.2 Advantages of Stop-Signal Task Paradigm
In many daily experiences, the demand for inhibition arises when the response has already been planned or initiated; thus, action inhibition processes are triggered unexpectedly and compete with ongoing response execution processes. An example of a real-life situation is when a green traffic light instructs initiation of a Go response for a waiting car, but suddenly a child runs across the street. This change in the environment would require immediate inhibition of the planned or initiated action for stopping the car. In various types of social interactions, the necessity for inhibition of expressions arises unexpectedly. This entails managing the balance (competition) between response execution and action inhibition. Stop-signal task paradigms (Logan and Cowan 1984; Verbruggen and Logan 2008; Verbruggen et al. 2019) have been developed to assess this aspect of inhibition ability, and unlike the Go/no-go paradigms provide the opportunity to quantify inhibition ability by estimating the stop signal reaction time (SSRT), a measure that indicates how long it takes (in milliseconds) for completion of the action inhibition process. The SSRT can be estimated for individuals or across different conditions, with shorter SSRTs indicating a better ability to inhibit behavior.
The Stop-signal task requires a balance between response execution and action inhibition (reactive inhibition) (Logan and Cowan 1984; Verbruggen and Logan 2008; Verbruggen et al. 2019). Such a balance has been modeled as a horse race model (Logan and Cowan 1984; Band, Van Der Molen, and Logan 2003; Verbruggen and Logan 2008) in which the go (response execution) and stop (action inhibition) processes compete to determine the fate of a response. Therefore, in Stop-signal paradigms, unlike in Go/no-go tasks, response inhibition is instructed while the initiation/planning of response execution is actively occurring. Additionally, what sets Stop-signal tasks apart from other inhibitory tasks is that it uniquely measures reactive inhibition by requiring subjects to stop an already initiated response, allowing to quantify response inhibition (SSRT).
Unlike Go/no-go tasks (assessment of ability to withhold a response), and reversal tasks (assessment of cognitive flexibility in changing stimulus-reward associations and proficiency in adapting to changing reward contingencies), Stop-signal tasks focus on the temporal dynamics of stopping an ongoing action and therefore allows for estimation of a quantifiable measure of reactive inhibition in millisecond resolution (stop signal reaction time: SSRT). Moreover, the Stop-signal task incorporates an adaptive procedure (stop signal delay: SSD) that adjusts task difficulty based on individual performance.
Accumulated evidence indicates significantly longer SSRT in neurodevelopmental (attention deficit activity disorders: ADHD), neuropsychological (schizophrenia) and substance use disorders (Fillmore and Rush 2002; Monterosso et al. 2005; Bellgrove et al. 2006a; Enticott, Ogloff, and Bradshaw 2008a; Hughes et al. 2012; Jentsch et al. 2014). Numerous studies in humans (Bellgrove et al. 2006b; Hughes et al. 2012; Mansouri et al. 2016; Fehring et al. 2021; Hall, Jenkinson, and Macdonald 2022; Doekemeijer et al. 2023) and macaque monkeys (Godlove et al. 2011; Middlebrooks and Schall 2014; Ghasemian et al. 2021a; Mowlavi Vardanjani et al. 2021; Ghasemian et al. 2022; Sajad, Errington, and Schall 2022) have examined the effects of various environmental and contextual factors on SSRT, using Stop-signal tasks. However, it remains unknown whether marmosets can learn the Stop-signal task, and if so what level of proficiency can be achieved.
2.3 How Can the Stop-Signal Task be Used in Marmoset Behavioral Studies?
To exemplify how the Stop-signal task may be used in marmoset behavioral studies to examine the effects of various environmental, pharmacological and hormonal factors (including oxytocin), we intranasally administered oxytocin in two marmosets and compared their performance between the oxytocin and control (saline) testing sessions. Oxytocin is a neuropeptide hormone primarily synthesized in the magnocellular neurons of the paraventricular and supraoptic nuclei of the hypothalamus. It is released directly into portal vessels, thus reaching the posterior pituitary gland (Rhodes, Morriell, and Pfaff 1981; Baribeau and Anagnostou 2015; Jones et al. 2017). Oxytocin has numerous functions within the brain (Rhodes, Morriell, and Pfaff 1981; Neumann et al. 2013; Baribeau and Anagnostou 2015; Jones et al. 2017), and its receptors are distributed within many brain regions, including the reward processing and executive control networks (Bethlehem et al. 2013; Russell and Brunton 2017). Several studies have reported critical links between oxytocin levels, increased impulsivity (Demirci, Ozmen, and Oztop 2016), and deficits in social cognition (Dessoki et al. 2020; Artık et al. 2023) and metacognition (Aydın et al. 2018). Therefore, oxytocin's potential as a therapeutic agent for impairments associated with neurobehavioral disorders has long been recognized. However, the benefits of oxytocin in human clinical and nonclinical trials remain inconclusive (Hollander et al. 2003; Guastella, Mitchell, and Dadds 2008; Andari et al. 2010; Norman et al. 2011; Parker et al. 2017; Flanagan et al. 2018; Hayashi et al. 2019; Bozorgmehr et al. 2020; Sikich et al. 2021; Martins, Paduraru, and Paloyelis 2022; Yamasue et al. 2022), partly because it remains unknown how, when and where oxytocin exerts its beneficial effects on social and cognitive functions. Addressing these critical questions demands developing suitable animal models for examining the effects of oxytocin at various stages of prenatal and postnatal development.
Oxytocin studies in marmosets have predominantly focused on social behavior and have demonstrated that exogenous oxytocin administration modulated pro-social behaviors in marmosets (i.e., food sharing, grooming, conspecific bonding, affiliation toward their mate, and social learning) in a context and sex-dependent manner (Smith et al. 2010; Cavanaugh et al. 2014; Cavanaugh et al. 2015; Mustoe et al. 2015; Taylor and French 2015; Mustoe et al. 2016; Kotani et al. 2017; Cavanaugh, Mustoe, and French 2018). While the oxytocin-related modulation of reproductive and other body systems is well documented, the neural substrate and underlying mechanisms of its cognitive effects, and particularly various facets of executive control, have not yet been extensively explored in marmosets.
2.4 Experimental Approach and Method
All experiments adhered to the Australian Code of Practice for the Care and Use of Animals for Scientific Purposes and were approved by the Monash University Animal Experimentation Ethics Committee. This research also adhered to the American Society of Primatologists Principles for Ethical Treatment of NHP. The marmosets, used in this research, were group housed in a large room with multiple home cages allowing for full visual and auditory access to other marmosets. Marmosets were mainly pair-housed in each home cage, however, in case of unpreventable and continuous fights, they had to be separated through partitions (still having visual and auditory access to other marmosets). They were provided with different daily enrichment, including tubes, ropes, foraging exposure and a variety of toys. Everyday access to a large outdoor enclosure (with enrichment and sunlight) was also provided on weekdays. Their weight and health were monitored on all training/testing days.
In this repeated-measures crossover study, four marmosets (Table 1) with no previous experience in any experimental tasks were trained to perform a computerized Stop-signal task (Figure 4), which required maintaining a balance between response execution and action inhibition. Once marmosets learnt the Stop-signal task, 15 data sessions were collected for Experiment 1 (Baseline study). Two marmosets, which had been trained earlier then moved on to Experiment 2 (Oxytocin-saline study), which is an example of how the Stop-signal task can be used in future marmoset behavioral studies.
| Marmoset | Sex | Age (at the start of training) | Weight (g) |
|---|---|---|---|
| F | M | 4 years and 7 months | 348 |
| L | F | 1 year and 11 months | 401 |
| J | M | 3 years and 7 months | 360 |
| D | M | 1 year and 10 months | 365 |
Marmosets had ad libitum access to food in their home cages within in a 12 h light–dark cycle. Their daily diet consisted of fresh fruit, vegetables, nuts, seeds, and nectar. Marmosets were trained within a specifically designed plexiglass box on weekdays (five times per week), and after each daily training/testing session, they were returned to their home cage. Liquid reward (diluted fruit juice) was provided for each correct response during task performance. Marmosets received their full daily water and food (fresh fruits/vegetables and pellets) at the end of each training/testing session. No training was done on weekends, during which they had ad libitum access to food and water.
2.5 Apparatus
In each testing session, the marmosets were placed in a testing box (32 cm × 22 cm × 30 cm) and transferred by a wheeled trolley from their home cages to a sound attenuated testing room. The trolley was positioned in front of a 17″ touchscreen (3 M MicroTouch) mounted on the wall, and the front panel of the testing box was removed so that the animals could reach out to the touchscreen monitor. The CORTEX program (National Institute of Mental Health) was used for controlling Stop-signal task, registering the responses and reward delivery at a millisecond resolution. A computer-controlled precision pump (Kd Scientific) was used to deliver juice reward. Marmosets' behavior during cognitive task performance was monitored through two video cameras.
2.6 Behavioral Training and Task
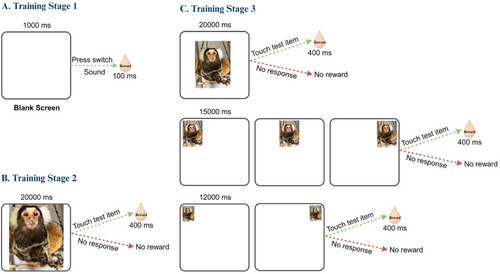
Training marmosets to perform cognitive tasks entails several shaping steps. Our training protocols were informed by previously published studies (Roberts, Robbins, and Everitt 1988; Pearce et al. 1998; Takemoto et al. 2011; Takemoto et al. 2015b; Nakamura et al. 2018a; Laclair et al. 2019; Workman et al. 2019; Rothwell et al. 2022). Once marmosets were acclimatized to the researcher and to the testing environment, they were trained to associate a sound with a reward (within a 100 ms timeframe) and take a reward (small drops of juice) from the metal tube positioned in front of their mouth (Training stage 1, Figure 1A). The marmosets were then presented with a visual stimulus that almost covered the entire extent of the screen (Training stage 2, Figure 1B). Therefore, touching anywhere on the screen, within 20 s, led to the reward. To encourage the marmosets to reach out and engage with the screen, occasionally mashed banana was placed on the monitor. Once the animals made the association that touching the screen leads to the reward, the size of the stimuli was gradually decreased, until the size required to perform the Stop-signal task was reached. The stimuli also appeared at random locations on the screen (Training stage 3, Figure 1C). The response window (allowed time to deliver the response and touch the items on touchscreen) was also decreased to 15 s.
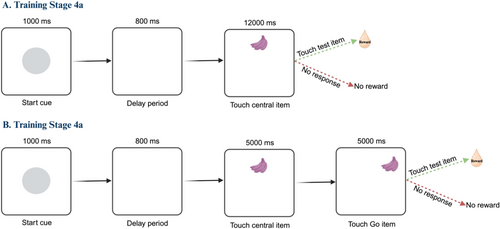
Then, in preparation for the Stop-signal task, marmosets were introduced to a start-cue (gray circle) that appeared on the screen for 1000 ms and signified the beginning of a new trial. After an 800 ms delay, a sample item appeared at the top middle panel of the screen (Training stage 4a, Figure 2A). Marmosets were required to touch the sample item (referred to as the Trial initialization (TI) target) to receive reward. The response window for TI started at 15 s and as marmosets became more proficient at initiating the trials, it was decreased to 12 s. Once marmosets consistently performed at least 30 trials in each daily testing session over a 2-week period, they progressed to Training stage 4b (Go trials) (Figure 2B). In this stage, the same sequences of events as Training stage 4a occurred; however, after touching the TI target, the Go-target stimulus (same as the TI target) appeared on the upper left or upper right side of the touchscreen. To receive a reward, marmosets were required to touch the Go-target (which was considered as response execution) (Figure 2B). To encourage marmosets to completely detach from the screen between the TI and Go trial stage, the Go-target stimulus only appeared if the monkey was not touching the screen for 200 ms. The response window for TI and Go response started at 12 s, and as marmosets became more proficient at the TI and Go response, the response window was gradually decreased (in steps of 2–3 s) until the response window became 5 s. Absence of response to TI target (timeout) terminated the trial, and an error signal was shown (purple annulus) for 500 ms. To indicate a successful touch response, the selected item flashed twice. To progress to Training stage 5 (Figure 3), marmosets were required to perform at least 30 correct trials in each daily testing session over a 2-week period.
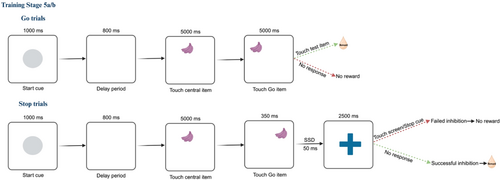
Training stage 5 consisted of three substages. All three substages consisted of Go trials (same as Training stage 4b), as well as newly introduced Stop trials. In training stage 5a, marmosets were introduced to Stop trials. In this stage, stop trials randomly occurred in 10% of trials and SSD (following the onset of the Go stimulus) was set at 50 ms. Specifically, 350 ms after the appearance of the Go stimulus, a blue cross (Stop-cue) replaced the Go stimulus and appeared at the center of the screen, upon which marmosets had to refrain from touching the screen to receive a reward (i.e., successful response inhibition) (Figure 3). Once they made the association that the Stop-cue signifies the necessity for response inhibition, they moved to Training stage 5b. The only difference between Training stage 5a and 5b was that the proportion of Stop trials increased to 20% (80% Go and 20% Stop trials). To progress to the final version of the Stop-signal task (Training stage 5c) (Figure 4), the marmosets were required to perform at least 30 correct trials in each daily testing session over a 2-week period.
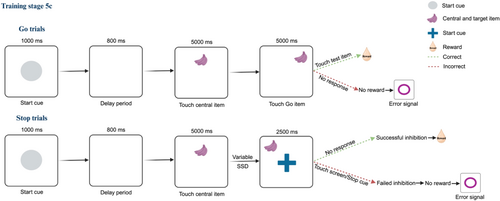
The final Stop-signal task consisted of randomly presented Go and Stop trials. The Go trials had the same sequence of events as in Training stage 5a/b. In a subset of randomly intermingled trials (approximately 30%), a Stop signal appeared (Figure 4). Unlike in Training stage 5a/b, the Go-target stayed on the screen (presumably increasing the difficulty of response inhibition). Thus, the marmosets were given a go-cue, and, after a delay period (SSD), a stop-cue. A correct response inhibition was defined as no engagement with the stimuli on the touchscreen (not touching the Go-target or the Stop-cue) for twice as long as the average RT in Go trials (specific to each animal: 2500, 2000, 4000, and 2000 ms for Marmosets F, L, J, and D, respectively), while failed response inhibition was defined as touching the screen and/or stimuli.
To ensure the requirement of the horse race model for proper estimation of SSRT, the accuracy in Stop trials was maintained at approximately 50% (Verbruggen et al. 2019). Therefore, the interval between the appearance of the Go-target and the Stop signal (SSD), was adjusted using an adaptive, staircasing procedure based on the individual performance in Stop trials. We refer to this as the variable SSD. To determine the required initial SSD for each marmoset, the starting delay was increased in 100 ms increments, until Stop trial accuracy was maintained around 50% for 5 days. Therefore, the starting SSD was specific to each individual marmoset (750 ms for Marmosets F and J, and 350 ms for Marmosets L and D). After setting the starting SSD in each session, the SSD was dynamically modulated depending on marmoset's performance: either increased (after response inhibition was successful) or decreased (after failed response inhibition) in 100 ms steps. The mean SSD across 15 sessions was 896 ms for Marmoset F (range: 534–1136 ms), 492 ms for Marmoset L (range: 298 to 689 ms), 922 ms for Marmoset J (range: 818–1028 ms), and 355 ms for Marmoset D (range: 206–641 ms).
A longer SSD between the Go-cue and Stop-cue makes successful inhibition more difficult, as subjects have more time to initiate their response to the Go-cue. Additionally, the occurrence of left and right Go-cue was counterbalanced, in both Go and Stop trials. This Stop-signal task for marmosets followed paradigms previously used in humans and macaque monkeys (Logan and Cowan 1984; Verbruggen and Logan 2008; Mansouri et al. 2016; Verbruggen et al. 2019; Fehring et al. 2021; Ghasemian et al. 2021a; Mowlavi Vardanjani et al. 2021), but was tailored for individual animal to balance the requirements for response execution and action inhibition.
2.6.1 Oxytocin Administration
Following several demonstrations that intranasal oxytocin administration elevates oxytocin levels in the cerebrospinal fluid of rodents (Neumann et al. 2013; Smith, Korgan, and Young 2019), humans (Striepens et al. 2013; Martins et al. 2020), macaque monkeys (Dal Monte et al. 2014; Modi et al. 2014; Lee et al. 2020), and marmosets (Kotani et al. 2017) and consequently influences different facets of social behavior (Bethlehem et al. 2013), we have adopted this delivery method in the present study. In a repeated-measure crossover design, two marmosets (F and L) received vehicle (saline) or oxytocin in alternative sessions. The administration of oxytocin and saline (control) sessions was counterbalanced across the two marmosets. We aimed to collect eight sessions of oxytocin and eight sessions of control in each marmoset. There was a washout period of 3–4 days between oxytocin and vehicle administrations. Either 40 International Units (IU) of oxytocin (10 IU/mL) (ilium Syntocin), or saline (4 mL) were vaporized using a child nebulizer (for 10 min) and administered through a tube connected to the marmoset box, while the marmoset was present. Task performance started 30 min after the end of oxytocin administration. The marmosets did not show any adverse reaction to the vaporized/nebulized oxytocin or saline.
2.7 Statistical Analysis
-
Initiate at least 25 trials consistently (without stopping for a long period between trials)
-
Percentage of correct in Go trials ≥ 70%
-
Percentage of correct in Stop trial between 30% and 75% (Verbruggen et al. 2019)
For Experiment 1 (Baseline study), 15 sessions were collected to examine marmosets' performance in the final version of the Stop-signal task. For Experiment 2 (Oxytocin-saline study), eight oxytocin sessions and eight saline sessions were collected from Marmoset F. For Marmoset L, however, the eighth oxytocin session did not meet the inclusion criteria; therefore, only seven oxytocin sessions were included in the analysis.
3 Example
In this study, we present the results of two experiments: Experiment 1 (Baseline study) and Experiment 2 (Oxytocin-saline study).
3.1 Experiment One: Baseline Study
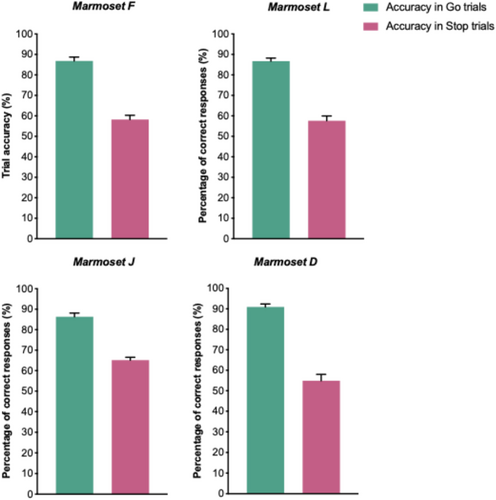
We evaluated performance of four marmosets (F, L, J and D) in the Stop-signal task. After learning the task, all four marmosets performed the Go and Stop trials at consistent level. To evaluate marmosets' ability to perform the Stop-signal task, first we report the findings from the Baseline data, focusing on four key behavioral measures of response execution (RT and accuracy in Go trials) and inhibition (accuracy in Stop trials and SSRT). RT in Go trials and SSRT are depicted in Figure 5, while accuracy in Go and Stop trials is displayed in Figure 6. Detailed information regarding descriptive statistics for these behavioral measures are provided in Table 2. Supporting Information S1: Figures 1 and 2 show the distribution of the mean response time in Go trials (S1), mean Stop signal reaction time (S1) and percentage of correct responses in Go and Stop trials (S2) in all data collection sessions for each marmoset.

| Marmoset | Response execution | Action inhibition | |||
|---|---|---|---|---|---|
| RT in Go trials (ms) | Accuracy in Go trials (%) | Mean SSRT ± SEM (ms) | Accuracy in Stop trials (%) | ||
| Range | Mean ± SEM | ||||
| F | 1225–2037 | 1572 ± 61 | 86.86 ± 1.8 | 677 ± 72 | 58.22 ± 2.19 |
| L | 968–1707 | 1332 ± 57 | 86.72 ± 1.52 | 839 ± 53 | 57.63 ± 2.33 |
| J | 1896–2992 | 2386 ± 81 | 86.34 ± 1.76 | 1465 ± 81 | 65.15 ± 1.37 |
| D | 811– 1402 | 1076 ± 44 | 90.97 ± 1.43 | 702 ± 54 | 54.91 ± 3.11 |
3.2 Response Inhibition in Marmosets: Accuracy in Stop Trials and SSRT
As explained above, to maintain marmosets' accuracy in Stop trials between 30% and 75%, we employed an adaptive staircasing procedure whereby the difficulty of response inhibition in Stop trials was adjusted depending on the animal's performance. According to the horse race model (Logan and Cowan 1984; Eagle et al. 2008; Verbruggen and Logan 2008; Congdon et al. 2012; Verbruggen et al. 2019), the SSRT can be estimated (mean GoRT – mean SSD) when the success rate in inhibition of response ranges between 30% and 75% (Verbruggen et al. 2019; Tran et al. 2024). The accuracy in Stop trials across all four marmosets ranged between 37.5% and 75% (Table 2 and Figure 6) indicating that the tracking procedure was successful in maintaining marmosets' accuracy around the desired range.
3.3 Experiment 2: Oxytocin-Saline Study
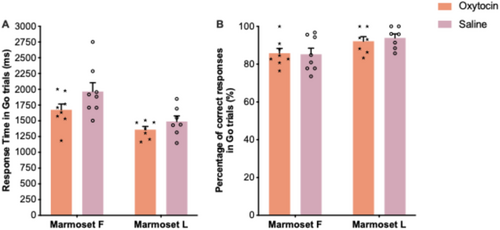
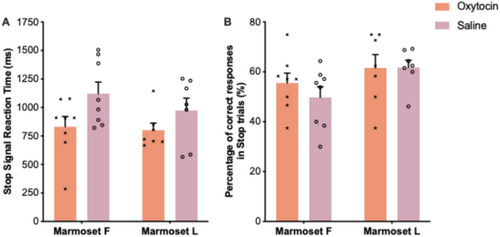
In Experiment 2, to exemplify the utility of the stop-signal task in marmosets for assessing various aspects of inhibition ability, we examined how oxytocin may influence response execution and inhibition (in two marmosets). Figures 7 and 8 show the oxytocin effects on various behavioral measures following oxytocin and saline administration.
3.4 Comparison and Critique
In this study, we successfully trained four marmosets in a Stop-signal task in which their response time was registered at a millisecond resolution. This enabled examining their ability to maintain the balance between response execution and action inhibition in the dynamic context of the stop-signal task and estimate, for the first time, marmosets' SSRT, which is the most quantitatively reliable index of inhibition ability. We then demonstrated the importance of establishing this model by examining how oxytocin may modulate response execution and inhibition in two marmosets. We discuss the significance of these findings in two aspects: developing a marmoset model of response inhibition (Baseline study) and then demonstrate how such a model can be used, by presenting an Example study (examining the potential effects of oxytocin on the balance between response execution and response inhibition). We also proceed to compare performance of marmosets with macaque monkeys and humans performing various versions of Stop-signal tasks.
3.5 Response Execution in Marmosets
Control of actions recruits neural networks involved in planning, initiating, and executing voluntary actions or movements. We found that, even though marmosets performed lesser number of trials, compared to humans and macaque monkeys (Mansouri et al. 2016; Fehring et al. 2019; Fehring et al. 2021; Ghasemian et al. 2021b), the accuracy in Go trials was high and consistent across sessions (Figure 6). It is important to note that the RT in Go trials were longer in marmosets (~1500–2500 ms) (Figure 5), compared to humans (~450 ms; (Mansouri et al. 2016; Fehring et al. 2019; Fehring et al. 2021; Ghasemian et al. 2021b)) and macaques (~800 ms; (Ghasemian et al. 2021a; Mowlavi Vardanjani et al. 2021; Ghasemian et al. 2022)). This difference can be primarily attributed to differences in the way these species deliver their responses in the Stop-signal tasks. In previous studies of human and macaque monkeys, the subjects sat at a specified distance from the screen. They were typically required to initiate a trial by pressing a switch, and to respond to the Go-cue by releasing the switch (Aron et al. 2003; Aron, Robbins, and Poldrack 2004; Eagle et al. 2008; Aron 2011; Aron, Robbins, and Poldrack 2014; Mansouri et al. 2016; Mansouri et al. 2017a; Fehring et al. 2019; Fehring et al. 2021; Ghasemian et al. 2021a; Ghasemian et al. 2021b; Mowlavi Vardanjani et al. 2021). In contrast, in the present task, marmosets had to initiate the trial by moving the entire body for touching a stimulus on the screen, disengage from the screen before the Go-cue onset, and then touch the screen again after the Go-cue onset. Therefore, for humans and macaques, the initiation of the trial and the execution of the Go response corresponded to a single coordinated action of an arm, whereas for marmosets the task entailed multiple whole-body actions coupled to arm motions. In designing the present task, we took into consideration the marmoset's less developed hand coordination, and the shorter arm length, which mandate the use of a different type of apparatus. Given the difference in RT, the marmoset task also allowed for a longer response window in Go trials (5000 ms), compared to those used in humans (e.g., 900 ms) or for macaques (e.g., between 2000 and 3000 mms). Despite these differences, we found that marmosets can perform many repeated Go trials with a high accuracy within a consistent response timeframe, a condition that is necessary for a reliable calculation of the SSRT.
3.6 Response Inhibition in Marmosets
The SSRT is a reliable individualized measure of inhibition of prepotent responses, where a shorter SSRT indicates better inhibition ability. While there is a substantial number of studies reporting SSRT in humans and macaque monkeys (Aron et al. 2003; Aron, Robbins, and Poldrack 2004; Eagle et al. 2008; Lipszyc and Schachar 2010; Aron 2011; Aron, Robbins, and Poldrack 2014; Mansouri et al. 2016; Mansouri et al. 2017a; Fehring et al. 2019; Chen et al. 2020; Fehring et al. 2021; Ghasemian et al. 2021a; Ghasemian et al. 2021b; Mowlavi Vardanjani et al. 2021; Epstein et al. 2023), to the best of our knowledge, this has not been reported for marmosets. Since the accuracy in Stop trials was maintained between 55% and 65% (Figure 6), while the accuracy in Go trials was high (Figure 6), the assumptions of the race model (Band, Van Der Molen, and Logan 2003; Verbruggen et al. 2019; Tran et al. 2024) were met, enabling a reliable estimation of the SSRT. We note that the SSRT values were considerably longer in marmosets (mean SSRT: ~750–1500 ms) (Figure 5) than in humans (mean SSRT: ~200 ms) (Mansouri et al. 2016; Mansouri et al. 2017a; Fehring et al. 2019; Fehring et al. 2021) or macaque monkeys (mean SSRT: ~250 ms) (Ghasemian et al. 2021a; Mowlavi Vardanjani et al. 2021; Ghasemian et al. 2022). This difference, once again, can be attributed to the differences in the mode of response delivery dictated by the biomechanical constraints of the tasks used in the different species. The SSRT in marmosets would naturally be estimated to be longer mainly because the mean GoRT would be longer. Therefore, when assessing the inhibition ability across species we need to consider the modality and the way the response execution (GoRT) is measured. The present paradigm may be utilized to investigate inhibitory dysfunctions manifested in neurodevelopmental disorders (Oikonomidis et al. 2017; Feng et al. 2020) and to examine the effects of environmental and hormonal factors on inhibitory control.
3.7 The Marmoset-Specific Stop-Signal Task Allows for Examining the Effects of Treatments and Drugs on the Balance Between Response Execution and Action Inhibition at Millisecond Resolution
Considering that the balance between response execution and action inhibition is impaired in neurodevelopmental and neuropsychological disorders, it is essential to assess various aspects of response execution and inhibition at high temporal resolution in a controlled experimental environment. The Stop-signal task in marmosets will enable measuring the response time (as an index of response execution) and stop-signal reaction time (as an index of response inhibition) at millisecond resolution and also examine the effects of various environmental, hormonal, and pharmacological interventions in a translational animal model.
It is well established that oxytocin is critical in early development of social and cognitive behavior (Feldman et al. 2007; Galbally et al. 2011; Feldman 2012; Chang and Platt 2014; Hammock 2015; Miller and Caldwell 2015; Feldman et al. 2016). Importantly, there is a link between oxytocin and dopaminergic activity within the mesocorticolimbic network, which is imperative in reward and motivated behavior (Love 2014; Florea et al. 2022). Motivated behavior, in goal-oriented behavior, involves recruitment of an interconnected neural network that regulates and determines the significance of stimuli, ascribe motivational value, and then select an appropriate action (Salamone 1992; Kalivas and Volkow 2005; Gordon et al. 2011; Salamone and Correa 2012; Love 2014).
Our preliminary results indicated a trend suggesting that oxytocin, in comparison to saline, enhanced response execution (RT decreased in Go trials) in both Marmoset F and Marmoset L (Figure 7A). Previous macaque studies that employed a visually guided speeded response task (Ebitz, Watson, and Platt 2013) and the Stop-signal task (Mowlavi Vardanjani et al. 2021) have also reported improved response execution. In Stop-signal task paradigms, the primary task is the Go trials; therefore, a shorter RT in Go trials indicates that the response execution was enhanced. This suggests that oxytocin may heighten the motivation to complete the primary task for receiving the reward, subsequently leading to enhanced response execution. However, oxytocin might also directly affect neural networks involved in action selection and execution (regions such as anterior cingulate cortex and motor control regions). Future studies, using a larger cohort of marmosets, should examine whether the observed trends are statistically significant and assess whether oxytocin also influences the accuracy in Go trials as well.
In Stop-signal paradigms, percentage of correct responses (accuracy) in Stop trials (i.e., the percentage of successfully inhibited stop trials) is not a direct measure of response inhibition, as it is influenced by the SSD, which is dynamically adjusted according to subject's performance in Stop trials. This dynamic adjustment aims at keeping the accuracy around 50% in Stop trials. A shorter SSD generally increases Stop accuracy as there is more time for inhibition, whereas a longer SSD reduces it, as the Go response may already be initiated. On the other hand, the SSRT, provides a more precise measure of inhibitory control by estimating the latency of the stop process independently of the SSD. Therefore, when examining the effects of various treatments on action inhibition, in Stop-signal tasks, the SSRT is preferred over accuracy in Stop trials as it directly reflects the efficiency of the response inhibition mechanism (how long it takes to inhibit the prepotent response), rather than task-related success probability. Our preliminary results indicated a trend suggesting that oxytocin improved action inhibition (manifest as shorter SSRTs) in both Marmoset F and Marmoset L (Figure 8A). This is in contrast to previous findings in macaque monkeys performing a version of Stop-signal task, in which intranasal oxytocin administration did not have any significant effect on SSRT (Mowlavi Vardanjani et al. 2021). However, several studies in humans have reported significant effects of oxytocin in alleviating impulsive responses, suggesting improved inhibition ability in these patients (Hollander et al. 2003; Hayashi et al. 2019; Bozorgmehr et al. 2020). Future studies with a larger cohort of marmosets should examine whether the observed trends are statistically significant and whether there is a concordance in the effects of intranasal oxytocin on inhibition ability across different primate species.
Prominent models of oxytocin-related modulation in social and cognitive behaviors propose that the modulatory effects of oxytocin on cognitive functions (including response execution and response inhibition) depend on either the emotional content of the stimuli, or the valence of the stimuli (Savaskan et al. 2008; Maroun and Wagner 2016; Zarei et al. 2019; Mowlavi Vardanjani et al. 2021). However, the findings in relation to valence-dependent interactions between oxytocin, emotional stimuli and cognitive functions varies across the studies (Hurlemann et al. 2010; Chang et al. 2012; Ebitz, Watson, and Platt 2013; Chang and Platt 2014; Parr 2014; Taubert et al. 2019; Zarei et al. 2019; Mowlavi Vardanjani et al. 2021). In this study, as emotional stimuli were not utilized, the oxytocin-related improvements on both response execution and action inhibition may not have been dependent on social information; therefore, we should consider the possibility of oxytocin's direct inference on motivation/reward or executive control networks.
In the context of the Stop-signal task, the same reward was given for a correct response execution (in Go trials) and successful inhibition (in Stop trials); therefore, the motivation to execute the Go response as quickly as possible and inhibit the Go response in Stop trial should be equivalent. Our preliminary results suggest that oxytocin can improve both response execution and action inhibition, and therefore offering the possibility that oxytocin may be involved in allocation of cognitive resources to enhance the balance between execution and inhibition of actions in the dynamic environment of the Stop-signal task. Further examination of oxytocin's influence on cognitive control, as well as the neural mechanisms underlying this effect, is essential. Establishing a marmoset-specific Stop-signal task provides a new tool to explore the potential benefits of oxytocin for managing cognitive deficits such as impulsivity and impaired inhibition in neuropsychological and substance use disorders at a mechanistic level.
3.8 Limitations and Future Directions
Although our preliminary results indicate that effects of oxytocin on response execution and response inhibition displayed a similar trend in both marmosets, our study is limited by its sample size. Previous studies in marmosets (Smith et al. 2010; Cavanaugh et al. 2015) macaques (Simpson et al. 2017), and humans (Shi et al. 2020; Zheng et al. 2021) have demonstrated that modulation of social behaviors by oxytocin is often sex-dependent; therefore, further investigation into sex-dependent differences in oxytocin-related modulation of response execution and inhibition in a larger sample size is warranted.
Another limitation is the use of ilium Syntocin that contains the consensus mammalian oxytocin ligand (Leu8-OXT), rather than the marmoset-specific oxytocin ligand (Pro8-OXT). By using social-behavioral tasks, studies have documented ligand-specific modulation of social behavior between long-term marmoset pairs, with intranasal Pro8-OXT administration having a more potent effect than intranasal Leu8-OXT (Cavanaugh et al. 2014; Mustoe et al. 2015). However, Cavanaugh et al. (2015) reported that both intranasally administered Pro8-OXT and Leu8-OXT modulated social behavior between marmoset pair-mates. Likewise, studies have also demonstrated that intranasal and intracerebroventricular administration of Leu8-OXT facilitated affiliative behaviors in newly formed marmoset pairs and enhanced paternal tolerance for food sharing with offspring (Smith et al. 2010; Saito and Nakamura 2011).
Given that we used Leu8-OXT and observed oxytocin-induced enhancements in both response execution and action inhibition in marmosets, the discrepancies seen in various marmoset studies may not solely stem from the structural differences between the Pro8-OXT and Leu8-OXT ligands and their binding affinities (Ren et al. 2015), but also the targeted neural circuitry (and distribution of the oxytocin receptors) that support particular social behavior or cognitive functions.
Additionally, considering that the amount of intranasal oxytocin that reaches the cerebrospinal fluid might be limited (Chang et al. 2012; Striepens et al. 2013; Dal Monte et al. 2014; Modi et al. 2014; Lee et al. 2020), it is also plausible that intranasal oxytocin stimulates the release of endogenous oxytocin through a peripheral feedback mechanism (Hurlemann and Scheele 2016). However, taking into account that other studies have demonstrated that oxytocin variants may result in varying behavioral outcomes (Cavanaugh et al. 2014; Mustoe et al. 2015), assessing whether Pro8-OXT or Leu8-OXT differentially influences executive functions in a larger cohort of marmosets, and whether any potential differences are age- or sex-dependent, deserve further investigation.
4 Conclusion
We report that four marmosets could successfully perform a computerized Stop-signal task, in which marmosets' response time and SSRT could be calculated with millisecond resolution. We also demonstrated how the marmoset Stop-signal task can be employed in future studies. In our Example study, administration of intranasal oxytocin demonstrated how such factors may modulate aspects of cognitive control (e.g., response execution and action inhibition). We also elucidated how the Stop-signal task can be applied in future studies to further investigate these effects at a mechanistic level. The marmoset Stop-signal task used in this study, to assess response execution and inhibition, lays down the groundwork for utilizing this task with lager cohorts of marmosets, in conjunction with various physiological techniques to address critical questions regarding the neurodevelopmental changes in social behavior and cognitive control and their underlying neural mechanisms.
Author Contributions
Ranshikha Samandra: conceptualization (equal), data curation (equal), formal analysis (equal), investigation (equal), methodology (equal), writing–original draft (equal), writing–review and editing (equal). Marcello G P Rosa: funding acquisition (equal), investigation (equal), resources (equal), supervision (equal), writing–review and editing (equal). Farshad A Mansouri: conceptualization (equal), formal analysis (equal), funding acquisition (equal), investigation (equal), methodology (equal), resources (equal), supervision (equal), writing–original draft (equal), writing–review and editing (equal).
Conflicts of Interest
The authors declare there are no conflicts of interest.
Open Research
Data Availability Statement
Data are available upon reasonable request to the corresponding author.




