Sexual Signaling and Sociosexual Behaviors in Relation to Rank, Parasites, Hormones, and Age in Male Vervet Monkeys (Chlorocebus pygerythrus) in Uganda
ABSTRACT
Secondary sexual characteristics, and the extent to which they are expressed, can convey information about the signaller. The blue scrotum and red penis of male vervet monkeys (Chlorocebus pygerythrus) make them a good species in which to examine inter- and intramale variation in signal expression. We quantified genital hue and luminance of male vervets at Lake Nabugabo, Uganda from standardized photos of male genitalia taken in May to June 2016, January to March 2019, and April to June 2019 to examine how dominance rank, fecal androgens (fARMs), fecal glucocorticoids (fGCMs), and parasitism related to achromatic (i.e., luminance) and chromatic (i.e., hue) aspects of scrotal and penile coloration, as well as how genital color related to sociosexual behaviors. We examined 182 photoshoots, 214 fecal samples for hormone analyses, and 152 for parasite analyses. Linear models indicate that genital color is linked to male dominance rank; high-ranking males had a more luminant (i.e., brighter) scrotum and a redder penis. Within males, color characteristics remained relatively stable over the short-term and changed moderately over the long-term. The direction of change was inconsistent for all color characteristics except scrotal luminance, which increased in all males over the long-term. Males with a darker penis received more mating presentations, while higher-ranking males received more mating refusals than low-ranking males, suggesting that females pay attention to penile color. We did not find support for any parasite or hormone mediation of color, and while there was a correlation between fGCM and fARMs, this was positive rather than negative as predicted by the stress-linked immunocompetence handicap hypothesis. Overall, our results indicate that the production of genital color may serve as an intra- and/or intersexual signal of male dominance rank and age in vervets.
1 Introduction
Visual, olfactory, and auditory signals can relay information about the signaller, such as physical strength, reproductive status, hormonal status, health, genetics, and relatedness (Holekamp and Strauss 2016), and may be subject to natural and sexual selection. Ornamentation and weaponry are secondary sexual characteristics (SSCs) that likely play an important role in communication and may have evolved through intra- and/or intersexual selection (Clutton-Brock and Huchard 2013). Weaponry (e.g., large canines, body size, antlers) is thought to have primarily evolved through intrasexual selection because of its advantageous effect on the outcome of same-sex combat or minimizing potentially risky contests between mismatched males (Andersson and Andersson 1994; Andersson and Iwasa 1996). Meanwhile, ornamentation (e.g., coloration, exaggerated plumage) is hypothesized to have primarily evolved through intersexual selection, and likely communicates individual quality to potential mates (Andersson and Andersson 1994). It is thus likely that signals are useful in communicating condition-dependent information about the signaller, which may be used to assess unknown individuals (e.g., immigrants, residents of neighboring groups) or to supplement existing but incomplete knowledge of groupmates (Setchell and Jean Wickings 2005; Bergman and Sheehan 2013; Seyfarth and Cheney 2017) who may be potential mates or competitors. It is therefore important to better understand interindividual variation in signaling, but also how signaling may be dynamic within an individual.
The “good genes” hypothesis suggests that individuals select mates based on honest indicators of mate quality, and that these qualities are heritable and therefore passed on to offspring (Trivers and Campbell 1972). For SSCs to be useful signals to potential competitors or mates, the signals must convey honest information about the signaller while excluding cheaters. The Handicap Principle states that only traits that are energetically expensive to produce honestly represent signaller quality (Zahavi 1975). If no underlying costs are associated with producing or increasing signal expression, the signaller will “cheat” (Higham 2016; Számadó 2011). Within males, signal honesty is maintained by costs associated with elevating their signal (Higham 2016). Therefore, individual variation in the tolerance of costs paid to produce a signal, or potential costs of producing an elevated signal, may lead to individual variation in signal expression.
The parasite-mediated sexual selection hypothesis (or Hamilton–Zuk hypothesis), an extension of the Handicap Principle (Zahavi 1975), proposed that ornaments could have evolved as honest indicators of parasite resistance (Hamilton and Zuk 1982). They propose that only individuals free from parasites could produce exaggerated signals. Ornamented individuals would benefit from being able to advertise their parasite resistance, and mates would benefit by choosing less parasitized individuals because they are (1) benefitting from a lower risk of parasite transmission while mating, and/or (2) more likely to pass on genes for parasite resistance to their offspring (Hamilton and Zuk 1982). Support for this hypothesis has been equivocal because if a negative relationship between signal expression and parasites is not found, it can be argued that this is due to the poor selection of parasites examined (Balenger and Zuk 2014).
The immunocompetence handicap hypothesis, an extension of the parasite-mediated sexual selection hypothesis, proposes testosterone as the regulatory mechanism linking parasites and SSCs (Folstad and Karter 1992). Testosterone is the major circulating androgen (AR) in primates; ARs are sex steroids that contribute to the development and maintenance of SSCs, sexual behavior and motivation, facilitation of reproductive aggression in males, and are required for spermatogenesis (Dixson 2012). Consistently maintaining high testosterone levels to produce elevated signals may incur costs (e.g., suppressed immune response, increased risk of injury due to higher levels of aggression) (Wingfield et al. 1990; Beehner et al. 2006). According to the immunocompetence handicap hypothesis, only healthy males can withstand the immunosuppressive effects of elevated testosterone levels required to develop exaggerated SSCs; therefore, only healthy males can afford to express or elevate signals (Folstad and Karter 1992) without paying substantial health costs. Thus, this immunocompetence handicap hypothesis predicts a positive correlation between ARs and signal expression, but a negative relationship between ARs and some measure of costs (e.g., parasitism, immune function). However, others have hypothesized that immune response is not suppressed in response to increased AR levels, but rather redistributed to areas that may require more immediate attention (Braude, Tang-Martinez, and Taylor 1999).
The stress-linked immunocompetence handicap hypothesis is another iteration of the immunocompetence handicap hypothesis and proposes a potential trade-off between signal production and long-term physiological stress or immune response (Rantala et al. 2012). This hypothesis proposes that a trade-off may be involved in maintaining honest signals, wherein elevated glucocorticoid (GC) levels may indicate or result in impaired immune function (reviewed in Buchanan 2000). GCs are an important group of hormones owing to their role in the stress response (Higham 2016; MacDougall-Shackleton et al. 2019), with cortisol being the predominant circulating GC in primates (Abbott et al. 2003). GCs facilitate appropriate physiological and behavioral responses to stressors to maximize individual fitness (Sapolsky, Romero, and Munck 2000). Chronically elevated GC levels (e.g., exceeding normal reactivity) can lead to physiological and behavioral costs, such as immune, reproductive, and growth suppression (Cavigelli and Caruso 2015; Sapolsky, Romero, and Munck 2000; Romero, Dickens, and Cyr 2009), and high cortisol has been correlated with mortality (Pride 2005). Therefore, while both the immunocompetence handicap hypothesis and stress-linked immunocompetence handicap hypothesis predict that signal expression positively correlates with testosterone, the immunocompetence handicap hypothesis predicts these will be negatively associated with measures of costs such as parasitism or immune function, while the stress-linked immunocompetence handicap hypothesis predicts a negative relationship with GCs. In other words, both hypotheses propose that only quality males can avoid or tolerate the costs of high testosterone and associated signal production, but they suggest different mechanisms. However, a meta-analysis of the vertebrate stress response and signal expression found no relationship between ARs and GCs (Moore, Shuker, and Dougherty 2015).
1.1 Color Signaling in Primates
Primates are good subjects for studying sexual signals because they are the most colorful mammalian order (Bradley and Mundy 2008; Setchell 2015). The males of some species exhibit conspicuous secondary sex characteristics in terms of coloration, such as the red and blue face and rump of mandrills (Mandrillus sphinx; Renoult et al. 2011), red chest patch of geladas (Theropithecus gelada; Bergman, Ho, and Beehner 2009), red face and scrotum of rhesus macaques (Macaca mulatta; Dubuc et al. 2014; Sobral et al. 2024), and blue scrotum, white fur, and red penis and perianal region of savanna monkeys (genus Chlorocebus; Hill 1966; Struhsaker 1967). Furthermore, not all individuals express skin coloration equally, and may signal inconsistently across their lifetime (Bradley and Mundy 2008). Research on color signaling in primates generally supports the hypothesis that interindividual variation is driven by intra- and/or intersexual selection (Bradley and Mundy 2008; Higham and Winters 2015; Setchell 2015). For example, male rhesus macaque facial color may be subject to intrasexual selection because males with faces of similar color were more likely to interact aggressively during the mating season than males with mismatched facial coloration (Petersdorf et al. 2017), as well as under intersexual selection since males with redder faces received more sexual solicitations from females (Dubuc et al. 2014).
Both age and rank have been variably related to color as signals of reproductive maturity and competitive ability in primates. This is likely because age and rank are often related in primates, following either an increasing, decreasing, or inverse-U shaped relationship (Machanda and Rosati 2020). Indeed, in geladas, chest patch color and age were both closely tied to dominance rank, suggesting that both reproductive maturity and dominance are conveyed in the sexual signal (Bergman, Ho, and Beehner 2009). Male mandrills of similar colors were more likely to engage in threats and fights than dissimilar males, which the authors interpret as evidence that color serves as a “badge of status,” communicating male competitive ability (Setchell and Jean Wickings 2005). In male rhesus macaques, red skin darkens during the mating season (Higham et al. 2013) and is correlated with dominance rank on days males are observed copulating (Petersdorf et al. 2017). SSCs may also reflect health status, such as in the bald uakari, where red facial skin is a good indicator of individual health status (Mayor et al. 2015).
In primates, variation in ARs and GCs has been the subject of intense research in the last two decades, although few tests of the handicap-based hypotheses have been conducted, likely because of the challenges associated with simultaneously collecting color, hormonal, and health data from wild primates. ARs and GCs vary between and within individuals in response to a variety of individual, social, and ecological variables, such as age, dominance status, directed and received aggression, coloration, parasitism, mating season or presence of ovulatory females, ecological season, and dehydration or nutritional stress (reviewed in Higham 2016). Research on mandrills has attempted to test several Handicap Principle-based hypotheses. Red color production appears to be regulated by testosterone itself, since male mandrills experiencing changes in testosterone following an agonistic encounter also experienced changes in red facial coloration (Setchell et al. 2008). While red facial coloration was linked to both fecal AR levels and male dominance rank (Setchell et al. 2008) and could indicate support for several of the hypotheses extending the Handicap Principle, no measure of costs was assessed in the study. A later study found that individuals with higher GC levels were more parasitized, which is consistent with the parasite-mediated hypothesis that parasites are costly (Setchell et al. 2010). However, neither GC levels (Setchell et al. 2010) nor parasites (Setchell et al. 2009) were related to signal expression and there was a positive relationship between ARs and GCs, rather than a negative one as predicted by the stress-linked immunocompetence handicap hypothesis.
In the present study, we examine male genital color signaling in the Lake Nabugabo population of vervet monkeys (Chlorocebus pygerythrus [Ch. pygerythrus]) in southwestern Uganda. Vervet monkeys (Ch. pygerythrus) live in multimale multifemale groups generally comprised of natal females and immigrant males (Struhsaker 1967). Females have a reddish perineal area and blueish vulva that becomes more intense during oestrus (Wickler 2017), and males exhibit a conspicuous blue scrotum, red penis, and red perianal area, which they may display by lifting their tail, revealing the red perianal area, a white patch of hair, and blue scrotum (i.e., red–white–blue display) (Struhsaker 1967). Genital coloration in the Chlorocebus genus has the potential to be involved in both intrasexual competition and intersexual preference. Penile coloration has not been the subject of much interest compared to scrotal coloration, which varies both across species and within populations (Hill 1966; Cramer et al. 2013). In the Nabugabo study population, scrotal coloration ranges from dark blue to bright aquamarine and penile coloration ranges from light pink to deep red (Figure1). Previous qualitative color assessments indicate a relationship between vervet scrotal color and male age (Henzi 1985), male–male agonism (Henzi 1985), individual health (Isbell 1995), dehydration or nutritional stress (Isbell 1995), dominance rank, and exposure to social stress (Brain 1965). Quantitative research found that in the wild, blue scrotal color was related to age, weight, as well as body and canine length (Cramer et al. 2013), but there was no relationship to dominance rank (Cramer et al. 2014; Young et al. 2020). A more recent study also reported that dominance rank, age, tenure length, injuries, or GCs were not predictors of scrotal and perianal color (Young et al. 2020). However, dominant males were more likely to present the red-white-and-blue display (Young et al. 2020), which may suggest that genital color is related to dominance, or it may simply be that flashing genitals, regardless of color, serves as a signal of dominance.

Source: Photos by Karin Snyder.
Most studies on vervet signaling focus on blue scrotal color, and studies on the red penis aspect have largely been overlooked, despite data on mandrills indicating that both red and blue facial colors are important in signaling (Renoult et al. 2011). Another limitation of previous quantifications of vervet genital coloration is that these studies only considered the “primary” color channel for scrotal and perianal color, namely the blue–yellow (B/Y) and red–green (R/G) channels, respectively (Cramer et al. 2013, 2014; Young et al. 2020). However, the perception of any hue, whether red or blue, involves information from both the B/Y and R/G channels. Therefore, we investigated whether inter- and intramale variation in scrotal and penile hue (i.e., the blue and red components of color) and luminance (i.e., how bright or dark a hue appears) are associated with age, dominance rank, hormones (androgens, glucocorticoids), and parasitism. While we cannot explicitly test predictions of the various ultimate and proximate models explaining color signaling described above because color is perceived holistically, and we cannot know which hue and luminance components represent the exaggerated signal, we did consider these hypotheses and predictions in building our statistical models (Table 1). We further examine these variables, including genital hue and luminance, as predictors of sociosexual behavior.
| Hypothesis | Prediction | Relevant analyses | Predictor variables | Outcome variables | Results |
|---|---|---|---|---|---|
| Intrasexual selection | Male rank predicts color characteristics | Model 1 | Male rank | Color characteristics | High rank = brighter scrotum, redder penis |
| Color varies between the low-conception and high-conception seasons | Season | Color characteristics | No | ||
| Individual color characteristics change in consistent direction between seasons | Short-term RII | N/A | N/A | No | |
| Intersexual selection | If females prefer exaggerated genital signals and/or higher-ranking males, color characteristics and/or rank will predict sociosexual behavior | Model 2 | Color characteristics Male rank | Sociosexual behaviors | Dark penis = more mating presentations |
| Age-related | Individual color characteristics change in consistent direction with male age over the long-term | Long-term RII (qualitative observation) | N/A | N/A | ↑ Long-term scrotal luminance |
| Parasite-mediated handicap hypothesis | Parasite metrics predict color characteristics | Model 1 | Parasites | Color characteristics | No |
| Females prefer males with color characteristics associated with fewer parasites, if any | Model 2 | Parasite-associated color characteristics | Sociosexual behaviors | N/A, no color characteristics predicted by parasites | |
| fARMs predict color characteristics | Model 1 | fARMs | Color characteristics | No | |
| Stress-mediated handicap hypothesis | Parasites predict color characteristics | Model 1 | Parasites | Color characteristics | No |
| fARMs predict color characteristics | Model 1 | fARMs | Color characteristics | No | |
| fGCMs predict color characteristics | Model 1 | fGCMs | Color characteristics | No | |
| fGCMs correlate negatively with fARMs | Spearman's rank | fGCMs | fARMs | ARs +ve with GCs |
- Note: “Color characteristics” includes all scrotal and penile color variables (luminance, blue–yellow opponency, red–green opponency) while “sociosexual behaviors” includes mating presentations, mating refusals, and copulations.
- Abbreviations: fARM, fecal androgen metabolites; fGCM, fecal glucocorticoid metabolites; RII, Relative Interaction Index.
2 Methods
2.1 Study Site and Subjects
Data for this research were collected by field assistants, K. S., and V. A. M. S., at the Nabugabo Research Site at Lake Nabugabo, Uganda. The habitat is composed of wetlands, grasslands, patches of swamp forest, farmers' fields, degraded forests, and a few buildings (Chapman et al. 2016). The wet seasons occur from March to mid-May, and November to December and the drier seasons from December to late February and mid-May to October (Chapman et al. 2016). Data were collected on all adult and subadult male (> 4 years) vervets over two field seasons: May to June 2016 and January to June 2019. Although the Nabugabo vervets copulate and conceive throughout the year, there is a conceptive peak from April to July (Schwegel et al. 2023). Therefore, the 2019 data set was split into two separate observation periods according to conception seasonality to examine short-term intramale variation, resulting in a total of three study periods (P1: May to June 2016 “high conception season,” P2: January to March 2019 “low conception season,” and P3: April to June 2019 “high conception season”). During the 2016 field season, two neighboring groups of habituated vervet monkeys were observed: M group (N = 33 adults and subadults) and HC group (N = 22 adults and subadults). During the 2019 field season, three neighboring groups of habituated vervet monkeys were observed: M group (N = 24 adults and subadults), KS group (N = 23 adults and subadults), and HC group (N = 10 adults and subadults). M group has been habituated since 2012, while KS and HC have been habituated since 2016.
This research was approved by York University's Animal Care Committee, Uganda Wildlife Authority, and Uganda National Committee for Science and Technology, and adhered to the legal requirements of Uganda. The research also adhered to the American Society of Primatologists (ASP) Principles for the Ethical Treatment of Non-Human Primates and ASP's Code of Best Practices for Field Primatology.
2.2 Color Quantification and Analysis
Photographs were captured during each of the three study phases. Using the sequential method, multiple digital images of each subject were collected, followed immediately by an image of a color standard (X-rite ColorChecker Passport) under the same environmental conditions (Bergman and Beehner 2008; Dubuc et al. 2014; Higham et al. 2013). All images were taken from a distance of 2–10 m in RAW format (Dubuc et al. 2009, 2014; Higham et al. 2013) using a Canon EOS Rebel T4i DSLR camera with an 18-megapixel APS-C Hybrid CMOS sensor and an EF-S 55-250 mm f/4–5.6 IS lens. White balance was set manually with a white standard and was recalibrated throughout the day as needed when lighting conditions changed. The aperture was set to the smallest setting to avoid spherical aberration and photos were slightly underexposed (based on visual examination of the histogram) to avoid clipping (Stevens et al. 2007). Linearization of the sensor and equalization of red, green, and blue (RGB) values were assessed using gray card standards as per Stevens et al. (2007) and Stevens, Stoddard, and Higham (2009).
We collected one or more photoshoots for each individual photographed, defined as a series of photos of the same individual at the same time, location, and environmental conditions (e.g., lighting) with only one color standard image. We used as many photoshoots per individual as possible, selecting for ones based on the criteria that the signal was not over- or underexposed, was not obstructed by anything in the foreground, and was in focus. The best photo was selected from each photoshoot based on the focus and visibility of the genital signal and was used in subsequent color analyses. All photographs were standardized and quantified using Image J software (Schindelin et al. 2012), with the Multispectral Image Calibration and Analysis tool (MICA) plug-in (Troscianko and Stevens 2015). We measured each associated color chart photo (X-rite ColorChecker Passport) to create a cone mapping model using D65 irradiance spectrum with an R2 value no less than 0.98, indicating reliable conversion of camera measurements to cone-catch values (Troscianko and Stevens 2015). Since vervet spectral sensitivity measurements are not available in the published literature, we generated cone mapping models using human spectral sensitivity in Image J, as it is both similar to that of vervet monkeys and sufficient for detecting color differences in images (Jacobs, pers. comm.; Jacobs and Deegan 1999). Regions of interest (i.e., scrotum and penis) were selected on each vervet photo and measured in accordance with the respective cone mapping model, generating LMS quantal catches (Longwave, Mediumwave, and Shortwave) and luminance values (Troscianko and Stevens 2015), being careful to exclude any areas that were shaded.
In our examination of color, we looked at its achromatic (luminance) and chromatic (hue) components. To model color, we calculated LMS values of the scrotum and penis for each male per study phase (range: 1–9 photos) and converted the LMS values to reflect color opponent processes, from which the mean was calculated. To make our results comparable to Young et al. (2020), we used the same formulas to calculate luminance as well as R/G and B/Y opponency values. Luminance was calculated using the formula (M+L)/2, where higher values represent a more luminant or “brighter” signal. Red–green (R/G) opponency values were calculated using the Michelson contrast (L−M)/(L+M), in which positive values are red and negative values are green. Blue–yellow (B/Y) opponency values were calculated using the formula (S−(L+M)/2)/(S+(L+M)/2), in which yellow is composed of long and medium wavelengths (L+M) and is divided by two in order to be equally weighted with S (i.e., blue); positive values are blue and negative values are yellow.
2.3 Behavioral Data
Between 2012 and July 2019, ad libitum and scan data collection (Altmann 1974) was carried out by trained field assistants. For ad lib, any behavior of interest was recorded, including all occurrences of male–male agonistic interactions. Scan data were collected every 30 min (2012–2015) and every 15 min (2016–present) on five haphazardly selected individuals, during which behavior, interactant, and nearest-neighbor were recorded. In addition, we carried out 15-min focal follows on all adult and subadult male study participants during the 2016 (V. A. M. S.) and 2019 (K. S.) study phases.
To determine the male dominance hierarchy, we extracted all dyadic observations of male–male submission (e.g., submissive vocalizations, avoidance, cowering, fear grimace) and aggression (e.g., bite, hit, chase, lunge) from the ad lib, scan, and focal data collected since 2012. All instances of male-male agonism were cross-checked to ensure duplicates were removed (e.g., if an interaction was recorded in both focal and ad lib data) and no third parties were involved. Because of the sequential and interconnected nature of behaviors during agonistic interactions, we defined bouts of agonism between a given male–male dyad as behaviors that occurred within 15 min of one another. We identified the winner and loser from each bout, and in the case of no clear winner, the bout was recorded as a draw and subsequently removed from the analyses. To quantify male dominance rank, we calculated individual Elo scores using the “EloRating” package in R (Neumann et al. 2011, R Development Core Team 2023). Males were given an initial Elo rating of 1000 for the first data point or when they entered a new group/hierarchy, and a “burn-in period” of at least five previous interactions for each male was required to determine the males' rank (Neumann et al. 2011). Following L'Allier et al. (2022), we used the optimized k function to determine the most suitable k-value (i.e., the amount by which each agonistic encounter influences the Elo score) for each study group (Newton-Fisher 2017). Finally, Elo scores were extracted on the last day of each study phase, and subsequently transformed to ordinal ranks (1 indicating the highest ranking male), which has been identified as the best metric for density-dependent competition (Levy et al. 2020).
Sociosexual behaviors of interest were extracted from the 2019 focal data only (red–white–and blue display, mating presentations, mating refusals, and mounts with or without thrusting). Copulation was defined as a sum of all mounts with or without thrusting. The red–white-and-blue display could not be analyzed due to low sample size in each phase (nP2 = 0; nP3 = 3). Adult males LPA, PFY, and WTN were excluded from the 2019 behavioral data set since they were observed for 0 and 60 min, respectively, compared to 360–1005 min for all other 2019 study subjects.
2.4 Minimum Age Determination
For males born within our study groups and for whom we had dates of birth (DOB), age was calculated in months by subtracting DOB from the last month of each study phase. However, it was not possible to determine the exact ages of males (N = 16) who had newly immigrated into a study group or those who had not been present when data collection began. We calculated minimum age (Young et al. 2020) at the end of each phase by adding the earliest male tenure length in any of our study groups to 55 months, which is the mean age of natal dispersal in our study population (L'Allier et al. 2022). It should be noted that this metric likely underestimates the age of non-natal males, especially older ones, and is therefore only a proxy of male age.
2.5 Parasites and Hormones
We aimed to collect fecal samples from all study males twice per month before noon, to avoid any natural circadian hormone variation (Behringer and Deschner 2017). Samples were collected within 10 min of defecation and kept cool in the field inside an insulated bottle cover with an ice pack. At the end of the field day, where sufficient feces were collected, samples with 1.0 g of fecal matter reserved for parasite analyses and the remainder frozen for hormone extraction (Gilardi et al. 1999). Samples collected in 2016 were solely examined for hormones.
Parasite analysis was only conducted for samples collected between January and July 2019. Samples were stored at the Nabugabo field lab, where they were kept in 2.0 mL 10% formalin solution (for helminth parasite fixation). Samples were then taken in batches to the Central Diagnostic Laboratory in the College of Veterinary Medicine at Makerere University in Kampala, where they were examined for parasite eggs and larvae by modified ethyl acetate sedimentation method (Valenta et al. 2017). Sedimented feces were prepared as slides and examined under a light microscope at ×10 magnification. Parasite taxa were identified to the lowest possible taxonomic level and photographed for further identification and documentation (Chapman et al. 2016). We calculated two measures of parasitism at the host or individual level (rather than at the population level): (1) proportion of samples infected from a given male in each study phase and (2) index of host parasite diversity, calculated as the total number of parasite taxa per male in a given study phase (Muehlenbein 2005).
Fecal hormone samples underwent preliminary field processing, normally within 1 month of collection. Samples were thawed and 5 mL of a 50:50 ethanol:water mixture was added to 0.5 g of feces. After vortexing the sample for 10 min, we separated the hormone-containing supernatant from the fecal pellet by centrifuging for 20 min, and 2 mL of the supernatant was processed through a Maxi-Clean 300 mg Prevail C-18 solid phase extraction (SPE) cartridge (S*Pure Pte. Ltd.), followed by a wash with 2 mL water. Cartridges were labeled, capped, and stored in a cool, dry place until transported to the Glendon Primate Behavioral Endocrinology Lab at York University, Toronto. Following a 1 mL 5% methanol wash, samples were eluted from the cartridges using 2 mL 100% methanol with an Alltech vacuum manifold.
Extracted hormone metabolites were then taken to the Toronto Zoo's Reproductive Sciences Lab for analyses. Both fecal glucocorticoid metabolites (fGCMs) and fecal androgen metabolites (fARMs) were quantified using enzyme immunoassay (EIA) protocols modified from Terwissen, Mastromonaco, and Murray (2014) and Majchrzak et al. (2015). The hormone–methanol solution was dried down and reconstituted in EIA buffer to remove the methanol. For fGCMs, the extracted hormone dilution range was fourfold diluted to threefold concentrated. For fARMs, the sample dilution range was twofold to fourfold diluted. Microtiter plates were coated with 50 μL of hormone-specific antibody diluted in coating buffer and incubated overnight. Testosterone antibody (R156/R157) and cortisol antibody (R4866) were previously developed by C. Munro (UC Davis). Following incubation, plates were washed with 0.15 M NaCl solution containing 0.05% Tween 20, and reconstituted hormone extracts (50 μL) were plated along with 50 μL horseradish peroxidase diluted in EIA buffer. After a 2-h incubation at room temperature, plates were washed and 100 μL substrate solution (i.e., color reaction solution ABTS) was added. Absorbance was measured at 405 nm using a spectrophotometer (MRX microplate reader, Dynex Technologies). All samples and standards were run in duplicate and reported as nanograms per gram of wet fecal weight (ng/g). Mean fGCMs and mean fARMs metabolite levels were calculated for each male in each study phase, and fARMs were log transformed to account for high skew (3.6227) and kurtosis (1.8099).
Biological validation of fGCMs has been previously conducted using an adrenocorticotropic hormone (ACTH) challenge in captive vervet monkeys (Young et al. 2017). To test for parallelism, serial dilutions in EIA buffer of the vervet hormone extract were compared against a 9-point standard curve (cortisol standard: Sigma H0135, 78–20,000 pg/mL; testosterone standard: Steraloids Inc., A6950, 48–125,000 pg/mL). Linear regression indicates a high degree of parallelism between the standard curves and the serially diluted samples. For fGCMs, inter-assay CVs were 7.1% (high pool) and 7.2% (low pool), and intra-assay CV was 7.4%. For fARMs, inter-assay CVs were 12.9% (high pool) and 8.9% (low pool), and intra-assay CV was 6.0%.
2.6 Statistical Analyses
We used data from P2 and P3 in 2019 to test predictor variables in relation to intermale color variation (luminance and hue components) and sociosexual behaviors. Because rank and age are correlated in many primate species (Machanda and Rosati 2020), we first tested for a correlation between male ordinal rank and minimum age variables. Male ordinal rank was negatively correlated with minimum male age (ρ = −0.7188, p < 0.0001), indicating that higher-ranking males were likely older. Given the strong correlation between these two predictors and the likely underestimation of age for several males (especially older ones), we opted to exclude minimum male age from all models. As expected, our two metrics of parasitism—proportion infected samples and parasite diversity—were moderately positively correlated (ρ = 0.4815, p = 0.0039); given that they were only moderately correlated, we opted to include both predictor variables in our models and test for multicollinearity (see below). To test some predictions of the stress-linked immunocompetence handicap hypothesis, we ran Spearman's rank correlations between fGCM and fARM, proportion of samples infected, and parasite diversity.
For intermale color variation (i.e., Model 1: scrotal luminance, scrotal B/Y, scrotal R/G, penile luminance, penile B/Y, and penile R/G), we used the 2019 data to run linear models with the predictor variables ordinal rank, mean fGCM, log mean fARM, proportion samples infected, parasite diversity, and season (P2: low-conception season vs. P3: high conception season). For sociosexual behavior (i.e., Model 2), we used generalized linear models (glm) with a Poisson distribution for mating presentations and a negative binomial distribution for copulations (to account for overdispersion). Mating refusals were extremely zero-inflated and even models and packages developed to account for zero-inflation (e.g., function glmmTMB in package “glmmTMB“) would not converge; we therefore did not analyze these data. We included the predictor variables scrotal luminance, scrotal B/Y, scrotal R/G, penile luminance, penile B/Y, penile R/G, ordinal rank, and season, and we used an offset to account for the total focal time for each male in a given phase. All models initially included male ID and group as random effects, though in all cases, they had to be removed to resolve model convergence issues. We tested both sets of models for multicollinearity and model 2 for overdispersion. We ran automated model selection (function: dredge, package: MuMIn; Bartoń 2023) and conducted model averaging if there was more than one model < Δ7 AICc (Burnham, Anderson, and Huyvaert 2011) and reported the confidence intervals (95% CI). Due to our small sample size, we also conducted power analyses using the pwr.f2.test function in the pwr package (Champely et al. 2022) of all predictor–outcome variable combinations for models 1 (n = 36) and 2 (n = 16).
To understand intramale variation, we used data from all three study phases (P1: May to June 2016 high conception season, P2: January to March 2019 low-conception season, P3: April to June 2019 high conception season) to calculate a Relative Interaction Index (RII) using the formula RII = (future value − past value)/(future value + past value). Values close to zero (e.g., −0.1 < RII < 0.1) indicate stability over time, and values closer to −1 and +1 indicate a high degree of decrease or increase, respectively (Armas, Ordiales, and Pugnaire 2004). For each male, we calculated RII values for each variable in the short-term (i.e., 3 months: P2 vs. P3 in 2019), and long-term (i.e., 3 years: P1 vs. P3, except for male PFY for whom we used P1 vs. P2 because he had emigrated by P3). We conducted Spearman's rank correlations based on short-term and long-term RII values for scrotal and penile luminance, B/Y, and R/G as well as ordinal rank, mean fGCM, log mean fARM; we adjusted p values using the Benjamini–Hochberg correction for multiple comparisons (herein pBH) with α = 0.05. All statistical analyses were done in R (R Development Core Team 2023). Data and code availability: dataset and code are available at https://doi.org/10.6084/m9.figshare.27135186 and https://karinsnyder.github.io/VervetColour2024/, respectively.
3 Results
We analyzed photos from 182 photoshoots (NP1 = 8, NP2 = 78, NP3 = 96) from 18 unique males, with the number of photoshoots per male in each separate study phase ranging from 1 to 9 (see Table S1). We included P1 scrotal and penile color data for the five males who were also present in P3; and in P2 and P3 we analyzed scrotal and penile coloration for 17 males. Descriptive statistics for color characteristics are presented in Table S1.
We collected 214 fecal samples for hormone analyses (NP1 = 62, NP2 = 73, NP3 = 80) from 18 unique males. Spearman rank correlation between fARM and fGCM found a moderately strong positive correlation (ϱ = 0.7257, p < 0.0001). We identified six parasite taxa from 152 fecal samples collected in 2019, including unidentified cestodes, the nematodes Strongyloides spp. and Trichuris spp., an unknown trematode, the trematode Schistostoma mansoni, and the protozoan Eimeria spp. Descriptive statistics, including range, mean and standard deviation of male rank, age, fARM, fGCM, proportion infected samples, and parasite diversity are presented in Table S2.
3.1 Intermale Variation in Genital Color Characteristics
The model selection procedure identified 28 models for scrotal luminance with ΔAICc < 7. Model averaging found that scrotal luminance was negatively associated with male ordinal rank, indicating that high-ranking males (with ordinal ranks closer to 1) had a more luminant or brighter scrotum (Figure 2a). The model selection procedure identified 48 and 40 models with ΔAICc < 7 for scrotal B/Y and scrotal R/G, respectively. Model averaging yielded null models with no significant predictors (Figure 2b,c). Results of power analyses are available in Table S3 and indicate limited power for several predictor–outcome pairs.
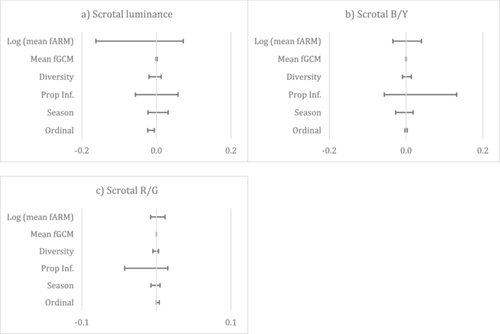
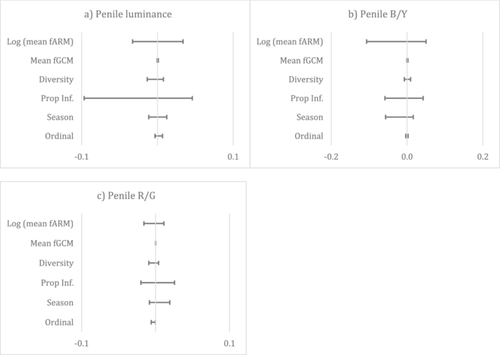
The model selection procedure identified 57 and 43 models for penile luminance and B/Y with ΔAICc < 7 respectively, but model averaging yielded null models with no significant predictors (Figure 3a,b). The model selection procedure also identified 38 models for penile R/G (Figure 3c) and found that penile redness was slightly negatively associated with male ordinal rank, indicating that high-ranking males had a redder penis. Results of power analyses are available in Table S3 and indicate limited power for several predictor–outcome pairs.
3.2 Intramale Variation in Genital Color Characteristics
We assessed short term (i.e., P2 vs. P3) variation in scrotal and penile coloration for 15 males by examining correlations among the RII values for all predictor and outcome variables (Table S4). Over the short term, the mean RII across all male traits remained relatively stable (mean RII range = −0.094 to 0.135), and the direction of change was not consistent across all males. The color variables that exhibited the highest degree of change in the short-term were scrotal B/Y (mean ± SD; RII = −0.079 ± 0.201) and penile B/Y (RII = 0.134 ± 0.186). Short-term variation in scrotal B/Y was positively correlated with changes in scrotal R/G (ϱ = 0.892, pBH = 0.0004) and there was a trend for a positive correlation between scrotal B/Y and ordinal rank (ϱ = 0.684, pBH = 0.068), indicating a trend for increases in rank to be associated with a decrease in scrotal blueness. Variation in penile B/Y and ordinal rank were negatively correlated (ϱ = −0.735, pBH = 0.033) indicating that rank increases were associated with increases in penile blueness. Finally, changes in fARM were positively correlated with changes in fGCM (ϱ = 0.829, pBH = 0.003). No other short-term RII values were significantly correlated (Table S5).
We examined long-term (i.e., P1 vs. P3) variation in scrotal (N = 5) and penile (N = 4) coloration for five males (Table S4). The average long-term RII values were less stable (mean RII range = −0.181 to 0.373) than in the short-term, indicating that these variables exhibited more change over time. Scrotal luminance increased for all five males in the long term, while the direction of change for the other color characteristics varied between males. Generally, males exhibited a moderate degree of change in scrotal variables in the long term, with an increase in scrotal luminance (RII = 0.244 ± 0.180), blueness (RII = 0.174 ± 0.321), and redness (RII = 0.373 ± 0.408). Males also generally exhibited a moderate degree of change over the long term in penile variables as well, with an increase in redness (RII = 0.299 ± 0.296). No long-term RII values were significantly correlated (Table S5).
3.3 Sociosexual Behavior
We collected 227.5 h of focal data in 2019 (P2: 129 h, P3: 98.5 h), with a mean ± SD of 8.1 ± 1.7 h/male (range: 3.5–10) in P2 and 6.2 ± 1.17 h/male (range: 2.5–8) in P3. The mean rate of mating presentations received by males was 0.05 ± 0.10 mating presentations per hour (range: 0–0.32) in P2 and 0.13 ± 0.14 (range: 0–0.38) in P3. The mean rate of mating refusals received by males was 0.05 ± 0.13 mating refusals per hour (range: 0–0.4) in P2 and 0.07 ± 0.11 (range: 0–0.34) in P3. The mean rate of copulations was 0.24 ± 0.3 copulations per hour (range: 0–1.12) in P2 and 0.41 ± 0.37 (range: 0–1.4) in P3.
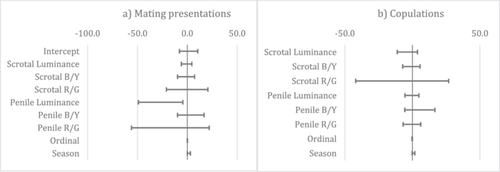
The model selection procedure identified 97 models with ΔAICc < 7 for mating presentations. Model averaging found that males received more mating presentations when they had a darker, less luminant penis as well as during the P3 high conception season compared to the P2 low conception season (Figure 4a). The model selection procedure identified 67 models with ΔAICc < 7 for copulations but model averaging yielded a null model with no significant predictors (Figure 4b). Results of power analyses are available in Table S6 and indicated limited power for several predictor–outcome pairs.
4 Discussion
Vervet male genital coloration varied by male rank and age. Intermale variation in penile redness and scrotal luminance was best described by male dominance rank. Male genital characteristics remained relatively stable in the short-term but showed moderate change over the long term, with scrotal luminance being the only variable that increased in all males over the long-term. Furthermore, sociosexual behavior was related to genital color characteristics and conceptive seasonality. Most notably, we found that penile luminance was a predictor of mating presentations received from females, with darker males receiving more presentations than lighter males. None of the color characteristics were predicted by either fecal parasite metrics or hormone levels.
We predicted that if male genital coloration evolved by intrasexual selection, it could signal male competitive ability and play a role in male–male competition. Therefore, we predicted that male color characteristics would vary by male rank as well as between low- and high-conception seasons, given that male–male competition is expected to be higher during seasonal conception peaks. We did not have any a priori predictions about which color characteristics might be important, nor in what direction the relationships might be, given that previous quantitative research has reported no relationship between male rank and scrotal coloration (Cramer et al. 2013, 2014; Young et al. 2020). Our analyses indicate that dominance rank was the best predictor of intermale penile redness and scrotal luminance, where higher-ranking male vervets had a redder penis and a brighter scrotum. However, season was not a predictor of any color characteristics, which is consistent with our finding that while color characteristics vary within males over the short-term (3 months), these changes are relatively small and not in a consistent direction. Our results are similar to those in mandrils that found adult males did not exhibit consistent changes in sexual skin coloration with mating seasonality (Setchell and Dixson 2001b), but contrast with findings in snub-nosed monkeys that do exhibit mating season changes in all males (albeit in different directions for harem-holding males and bachelors; Grueter et al. 2015). It is possible that since the Nabugabo population of vervets copulates and conceives year-round (Schwegel et al. 2023), changes in male coloration occur at a finer temporal scale (e.g., days) than used here (i.e., 3 months seasonality), as seen in rhesus macaques whose faces darken on days they are observed copulating (Petersdorf et al. 2017).
Our finding that penile redness was predicted by dominance rank, with high-ranking males having a redder penis, is consistent with reports from other primate species indicating that red skin coloration is associated with dominance rank and may play a role in intrasexual selection. For example, in mandrills, red sexual skin coloration is associated with dominance rank (Setchell and Wickings 2005) and androgens (Setchell et al. 2008), and males who lose their alpha status have a concomitant decrease in redness (Setchell and Dixson 2001a). In geladas, harem-holding males have the reddest chest (Bergman, Ho, and Beehner 2009). Harem-holding black-and-white snub-nosed males have redder lips than bachelor males, though this is only true in the mating season (Grueter et al. 2015). Similarly, facial redness is also associated with dominance rank in male rhesus macaques, but only on days when males copulate (Petersdorf et al. 2017).
Our analyses also indicate that dominance rank was the best predictor of intermale scrotal luminance, where higher-ranking male vervets had a brighter scrotum. Our intermale finding of a relationship between rank and scrotal coloration contrasts with previously reported quantitative color analyses in vervets, which found no relationship between male dominance rank and scrotal color characteristics (Cramer et al. 2013, 2014; Young et al. 2020). That said, our findings are somewhat consistent with the results of experimental work in Chlorocebus sabaeus (Ch. sabaeus) that found scrotal coloration predicts dominance status, though the direction of the relationship appears to differ given that we found that brighter males were higher ranking whereas males painted “darker” became dominant (Gerald 2001). Differences in results from Gerald (2001) could be due to (1) lack of clarity about which aspect of blue color was being perceived by male competitors, since that study used observer descriptions of color described by “pale” and “dark” blue color, and/or (2) use of a closely related, but different, study species. The latter is certainly likely given that with increasing age, the scrotum has been reported to lighten and become bluer in Ch. pygerythrus but lighten and become less blue in Ch. sabaeus (Cramer et al. 2013).
We opted not to include our estimate of minimum male age in our models testing predictors of scrotal and penile color characteristics because (1) knowing the exact age of males in species with male-biased dispersal is a logistical challenge associated with the movement of males between groups, such that males born in non-study groups often immigrate into study groups, (2) rank and age are strongly associated with one another in primates (Machanda and Rosati 2020), and (3) rank and minimum age were moderately correlated in our data set. We did, however, test for age by examining changes in color characteristics within males over the long-term (3 years). Color characteristics varied moderately but most color characteristics did not change in a consistent direction for all males. One exception is scrotal luminance: males experienced a moderate increase in scrotal luminance in the long-term, which suggests that at least some of the variation in scrotal luminance is associated with aging. A cross-sectional analysis indicates that variation in the luminance component of vervet scrotal coloration is related to age and further indicates that older males had a bluer scrotum, based on the blue–yellow opponency channel (Cramer et al. 2013); however, a separate analysis found no such relationships (Young et al. 2020). Our results generally suggest that scrotal luminance likely serves as a signal of male dominance and/or age, though, as noted, this is confounded by the relationship between rank and age in primates.
Overall, our findings suggest that the penile redness and scrotal brightness could be intrasexual signals of male–male competitive ability (i.e., a “badge of status”). Redness is a particularly good candidate given that in mandrills who also have both red and blue skin coloration, male redness but not blueness changes with the loss of dominance (Setchell and Dixson 2001a), and males of similar redness are more likely to engage in conflict than males with large differences in redness (Setchell and Wickings 2005). The “badge of status” for vervet male competitive ability could be further tested by examining the relationship of color in relation to actual rates of male agonism in our study population, rather than looking at rank which was calculated from the sequence of dyadic male interactions. However, previous studies from South Africa suggest that neither aggression nor rates of injury predict male genital color (Cramer et al. 2014; Young et al. 2020). Another way to test this hypothesis would be to look at patterns of agonism between males with similar or dissimilar color characteristics, as done in mandrills (Setchell and Wickings 2005) and rhesus macaques (Petersdorf et al. 2017). It would also be interesting to see how resident male color changes in response to immigrant males, though this is much more challenging to test as it requires data collection prior to an immigration event, which is a relatively rare occurrence.
It is interesting that our results differ from previous quantitative analyses of South African populations that found no effect of rank on scrotal color (Cramer et al. 2013, 2014; Young et al. 2020). These differences could be due to methodological differences (cross-sectional vs. longitudinal), examination of different color components, variation in the timing of data collection, or true interpopulation differences. One potentially important methodological difference is that Young et al. (2020) had access to vervet color space data that we did not, which may have implications for the color measurements; however, there appear to be limited differences in the spectral color sensitivities of vervets and humans (Jacobs, pers. comm.). Another methodological difference is that we examined hue along the B/Y and R/G opponency changes for both the scrotum and penis, rather than only examining color along the B/Y for blue coloration and R/G for red coloration. Although color is described through its achromatic (i.e., luminance) and chromatic components (i.e., R/G and B/Y channels), color is perceived holistically rather than through its separate components. For example, we interpret information from both color channels together, resulting in perceived hue. It is thus possible that incorporating a second opponency channel in the analyses of blue scrotal and red penile coloration accounts for some of the variation previously attributed to the “primary” opponency channel. Despite the methodological differences and limited power of some of our analyses, we should not discount the possibility that true interpopulation variation exists. The potential energetic costs of signaling may differ in the Nabugabo population given that there is very little food seasonality, and dehydration stress is not likely given that the site does not experience a true dry season but rather wet and wetter seasons (Schwegel et al. 2023). Additionally, the breeding seasonality at Nabugabo is less strict than observed in other wild populations of vervets (Schwegel et al. 2023), and a preliminary analysis suggests that copulation rates are higher at Nabugabo than elsewhere (Rademacher and Schoof, in prep), both of which are factors that could change the male competitive landscape and thus the costs and benefits of investing in color signaling. If future studies found evidence that the signals were costly, one might predict that costs could be higher in our population compared to others. Reduced mating and conceptive seasonality in our population compared to others may explain why we found no differences in coloration between low and high conceptive seasons, as it is beneficial for males to signal to both males and females year-round.
Genital color may also be used in mate choice, allowing females to glean information about new immigrant males or supplement existing social knowledge about male groupmates. While we expected that females may prefer males with certain color characteristics, we did not have any a priori predictions about what color characteristics might represent the “exaggerated” state. Our results indicate that males with a less luminant (i.e., darker) penis received more mating presentations from females than males with a more luminant (i.e., brighter or pinker) penis, suggesting that females have a preference for males with this color characteristic. This result is consistent with a recent finding that among top-ranking rhesus macaques, males with a darker red scrotum had higher reproductive success (Sobral et al. 2024). Interestingly, neither male rank, season, parasites, nor hormones were predictors of penile luminance in our intermale analyses, and our intramale analyses do not indicate that penile luminance changes in a consistent direction with male age. Therefore, it is unclear what information—if any—is contained in penile luminance that may lead to a female preference for males with a darker penis. The absence of predictors of penile luminance may be due to our small sample size and should therefore be re-examined with a larger sample size and perhaps looking at different health metrics.
We tested several predictions of the parasite-mediated handicap hypothesis (Hamilton and Zuk 1982), immunocompetence handicap hypothesis (Folstad and Karter 1992), and stress-linked immunocompetence handicap hypothesis. Contrary to predictions of the parasite-mediated handicap hypothesis, neither the proportion of samples infected with parasites nor the diversity of parasites an individual was infected with were predictors of variation in genital color characteristics between males. As such, we could not test the hypothesis that females would prefer males with color characteristics that signaled a low level of parasitism. Contrary to a prediction of the immunocompetence handicap hypothesis, fecal testosterone metabolites did not predict any of the color characteristics, and the relationship between fGCM and fARM was positive rather than negative as predicted by the stress-linked immunocompetence hypothesis. The absence of a relationship between parasites, androgens, and color characteristics should be interpreted with caution, given the limited power of our analyses. Furthermore, as Higham (2016) points out, measuring the actual “costs” that an individual pays to produce a signal is not particularly informative, given that the cost of the signal is paid if males increase their signal expression. When we examined intramale variation in color, none of this variation was associated with parasites, androgens, or glucocorticoids once we incorporated p value adjustments for multiple tests. An intramale analysis with a larger sample size may yield different results. However, a recent study found that chest redness in male geladas was associated with genes associated with vascularization but not with androgen or estrogen regulation (DeLacey et al. 2023), indicating that red sexual skin coloration is not necessarily associated with hormone activity. A potential limitation of our study may lie in its reliance on gastrointestinal parasites, as these may also not be ideal for testing the parasite-mediated sexual selection hypothesis (Balenger and Zuk 2014); Hamilton and Zuk (1982, 385) hypothesized that “suitable parasites for tests of the theory are those that debilitate their host rather than either kill it or allow total recovery after brief sickness.” It may be less costly for an individual to tolerate infection by a single parasite taxon than to mount an immune defense against that parasite (Medzhitov, Schneider, and Soares 2012). In mandrills and chimpanzees, parasite metrics were positively associated with fecal glucocorticoids (Setchell et al. 2010; Muehlenbein 2006), but this was not the case in gorillas (Shutt-Phillips et al. 2021). A gastrointestinal parasite removal experiment in vervets found that the duration of resting events decreased following deworming (Chapman et al. 2016) and while there was no decrease in fGCMs after deworming, levels did increase following natural reinfection (Upadhayay et al. accepted). Changes in behavior and fGCMs suggest there are nonlethal costs to gastrointestinal parasites in vervets, but whether the costs are sufficiently high to affect signal production is unclear. The proportion of samples that contained at least one parasite taxon may also not be an ideal metric of parasitism because an individual can have a low proportion of infected samples but multiple taxa or high prevalence of a single taxon, and it is not known how the cost of these different infection types differ (if at all). Although parasite load could be used to quantify the number of gastrointestinal parasites of a given species, this metric does not necessarily reflect actual degree of parasitizing due to temporal variation in the shedding of eggs in relation to phase of infection and individual host factors (Giver et al. 2000). Finally, our ability to determine signal elevation is complicated by the fact that little is known about the mechanisms associated with the production of luminant skin, though Wolff et al. (1976) point to a role for hydration in scrotal color, though it is unclear if they are referring to hue, saturation, or luminance.
Future studies could utilize a deworming and natural reinfection experiment to examine changes in coloration in response to changes in parasitism. As parasites may be costly and associated with elevated glucocorticoid levels, an experimental regime may also allow researchers to examine the relationship between changes in hormone levels and parasites. Furthermore, looking at color and hormones over an entire year may allow researchers to observe changes in color that are reflected with naturally occurring changes in androgens, as has been observed in many species in response to seasonal availability of fertile females.
Our aim was not to determine whether signals are honest per se, due to perceived costs of production (e.g., parasites, hormones) for males of different ages, dominance ranks, or quality. As Higham (2016) points out, there are numerous caveats to attempts to identify the cost(s) of signaling through observational studies, and costs can only be determined through experimental manipulation of signals beyond what an individual is currently producing, which is outside the scope of this study. Rather, we aimed to add to the growing literature of quantitative measures of color signaling in primates to contribute to an understanding of signal information content and production mechanisms while addressing some of the gaps of previous research. Our findings indicate that vervet scrotal and penile color characteristics are associated with and likely contain information on male dominance rank, and possibly age. Females appear to use penile luminance in mating decisions, indicating that females pay attention to the achromatic component of male penile color. Taken together, our results confirm that penile color characteristics, in addition to scrotal color characteristics, should not be ignored as a probable sexually-selected signal. Thus, this study adds to the growing body of research that quantifies male color characteristics in primates and examines the oft-neglected but striking red penile coloration seen in vervet monkeys.
Author Contributions
Karin P. Snyder: conceptualization (equal), data curation (lead), formal analysis (equal), investigation (lead), methodology (equal), writing–original draft (equal), writing–review and editing (equal). Dina Greenberg: conceptualization (supporting), formal analysis (supporting), methodology (equal), writing–review and editing (supporting). Taylor Fane: formal analysis (equal), methodology (supporting), writing–original draft (supporting), writing–review and editing (equal). Alessandro Filazzola: formal analysis (equal), writing–review and editing (supporting). Gabriela F. Mastromonaco: methodology (supporting), resources (supporting), writing–review and editing (supporting). Valérie A. M. Schoof: conceptualization (equal), data curation (supporting), funding acquisition (lead), methodology (supporting), resources (lead), supervision (lead), writing–original draft (equal), writing–review and editing (lead).
Acknowledgments
The authors thank Matovu Ponsyano, Livingstone Katwere, Hillary Tashobya, and Justine Namuyomba for their dedication collecting fecal samples and behavioral data, to Aneta Tasheva for her hard work capturing photos in 2016, and to Dr. Dennis Twinomugisha for on the ground assistance. Thanks are due to Lordrick Alinaitwe at the Central Diagnostic Laboratory in the College of Veterinary Medicine at Makerere University for examining fecal helminths, Paula Mackie and Christine Gilman at the Toronto Zoo for hormone analyses, Joylon Troscianko and Lais Pacheco for fruitful discussions of color, and Pooja Upadhayay for discussion of statistical methods. This research was funded by the Faculty of Graduate Studies at York University (K.S.), NSERC (V.A.M.S.), and York Research Chair (V.A.M.S.).
Open Research
Data Availability Statement
Full data set is available at, and code available at https://doi.org/10.6084/m9.figshare.27135186, and code available at https://karinsnyder.github.io/VervetColour2024/. Raw data may be available for collaborative research by reaching out to the corresponding author (V.A.M.S.).




