The Influence of Age, Sex, and Season on Hematological Parameters in a Captive Population of Former Laboratory Chimpanzees (Pan troglodytes)
ABSTRACT
This study aimed to establish a baseline hematological profile and examine the influence of age, sex, and season on hematological parameters in captive chimpanzees (Pan troglodytes) living in a humid tropical climate. Hematological parameters are a useful tool for assessing health status and diagnosing diseases in animals. We analyzed 473 blood samples collected from 84 chimpanzees (43 females and 41 males) during annual health checks, conducted under anesthesia for a routine physical examination. The main findings revealed significant sex differences in some hematological parameters: males had higher hematocrit and red blood cell counts than females. Age-related variations have also been noted, with adolescents and adults having lower lymphocyte counts but higher neutrophil and monocyte counts than infants. Adults, in particular, had significantly lower platelet counts compared to other age classes. Seasonal fluctuations were also observed: lymphocyte counts were higher in the rainy season, while neutrophil counts were higher in the dry season. In addition, the general trends in hematological parameters for this captive population of chimpanzees were similar to those reported in captive chimpanzees living in the United States. These results should provide animal health professionals, particularly those working with nonhuman primates, with data to compare and interpret. They will help improve practices for monitoring and managing the health of nonhuman primates in captivity.
Summary
-
The study establishes the baseline profile of hematological parameters in former research chimpanzees in a seasonal environment.
-
The study highlights significant differences in certain hematological parameters (lymphocytes, hematocrit, white blood cells, neutrophils, and eosinophils) between the period before the widespread use of chimpanzees as experimental models and after 2012 when the CIRMF banned invasive procedures.
-
The study found significant age-related variations in some hematological parameters (lymphocytes, neutrophils, monocytes and platelets).
-
The study shows significant sex differences in some hematological parameters (hematocrit and red blood cells).
-
The study highlights seasonal variations in certain hematological parameters (lymphocytes and neutrophils).
1 Introduction
Over the past few decades, the field of hematology, which focuses on the study of blood and its related diseases, has been seen significant growth. This expansion has led to various studies in hematology, including in primatology, to assess the health status of nonhuman primates and their physiological adaptation to the environment (Koo et al. 2019; Shah et al. 2022). Hematological parameters, such as red blood cell (RBC) counts, white blood cell (WBC) counts, hemoglobin (HGB), hematocrit (HCT), platelets (PLT), and various indices like mean corpuscular volume (MCV), mean corpuscular hemoglobin (MCH) and mean corpuscular hemoglobin concentration (MCHC), serve as biological markers that provide information on the general physiological state (Koo et al. 2019; Shah et al. 2022). Studies have shown that hematological values can vary based on factors such as species, sex, age, physiological status, diet, stress, diseases, drug administration, genetics, blood collection techniques, sample handling, and laboratory procedures (Harewood et al. 1999; Kessler and Rawlins 1983). For example, in Cynomolgus monkeys (Macaca fascicularis), males have higher HCT and MCV values, whereas in rhesus monkeys, MCV values are lower in males, and WBC counts tend to be higher in females (Koo et al. 2019). Additionally, wild-born rhesus monkeys (Macaca mulatta) exhibit higher WBC, platelets, neutrophils, RBC, hemoglobin, HCT, MCV compared to their captivity counterparts, differences attributed to stress and exposure to environmental pathogens (Shah et al. 2022).
Chimpanzees (Pan troglodytes), which share approximately 98% of their DNA with humans, are valuable models for biomedical research due to this genetic similarity. This proximity has made them indispensable in studies related to behavior, physiology, virology, immunology, and neurobiology (VandeBerg, Williams-Blangero, and Tardif 2009). Chimpanzees have contributed significantly to research on infectious diseases such as HIV/AIDS and hepatitis C (Bailey 2010; Choo, Leong, and Rogers 2015; Lanford, Walker, and Lemon 2017). However, this genetic proximity also increases the risk of human pathogen transmission to wild animals (anthropozoonotic transmission), especially in captivity (Wolfe et al. 2005). Ethical concerns and the development of alternative research methods have led to a significant reduction in the use of chimpanzees in research (Council, Sciences, and Research, I. for L. A., & Chimpanzees, C. on L.-T. C. of 1997), resulting in many laboratory chimpanzees being placed in sanctuaries where their health and welfare remain a priority. Hematological assessments are crucial for monitoring their health, providing information on infections and physiological stress, as well as helping detect diseases (Obanda, Omondi, and Chiyo 2014).
Previous studies have highlighted significant variations in hematological parameters in chimpanzees that have participated as a biomedical model due to the implemented experimental protocols and environmental factors. For instance, changes in hemoglobin, RBC counts and WBC profiles due to stress and environmental conditions have been reported (Nehete et al. 2017; Schapiro et al. 2012). Other studies have shown that environmental enrichment of animals is associated with improved hematological health, highlighting the impact of housing and care conditions on these animals (Herndon and Tigges 2001; Lambeth et al. 2006; McClure, Keeling, and Guilloud 1972).
In the present study, we examined the hematological parameters of former laboratory chimpanzees housed at the Centre International de Recherches Médicales de Franceville (CIRMF). Established in the 1970s, CIRMF has played a pivotal role in biomedical research, contributing significantly to understanding infectious diseases, including hepatitis and HIV/AIDS, through studies on their chimpanzee population (Barré-Sinoussi et al. 1997; Georges-Courbot et al. 1996; Poaty-Mavoungou et al. 2001). This research has led to critical advancements in vaccine development and disease prevention strategies, highlighting the value of chimpanzees in translational research. The aim of our study is to establish baseline hematological profiles and to examine the influence of age, sex, and season on these parameters in a captive population of chimpanzees over 24 years. In addition, we aim to compare hematological parameters before 2012, when chimpanzees were extensively employed as experimental models, with those collected after 2012, when the CIRMF banned invasive procedures.
2 Materials and Methods
2.1 Ethical Statement
No experiments were performed on humans or animals for this study. The authors declare having followed their institutional (CIRMF) animal health protocols, as recommended by the Pan African Sanctuary Alliance.
2.2 Study Population and Study Site
This 24-year study (January 1996–July 2020) was carried out on a captive population of chimpanzees living at the CIRMF, located in Southeast Gabon (1°37'59” N, 13°34'59” E; Figure 1). The Primate Centre is home to nearly 350 primates, most of which are native to Gabon, such as chimpanzees, mandrills (Mandrillus sphinx), sun-tailed monkeys (Allochrocebus solatus), white-collared mangabeys (Cercocebus torquatus), mustached guenon (Cercopithecus cephus), and greater spot-nosed monkeys (Cercopithecus nictitans). The other species of primates come from Africa (vervet monkeys Chlorocebus aethiops) and Asia (rhesus macaques Macaca mulatta and cynomolgus macaques Macaca fascicularis). The Primate Center features both indoor and external aviaries (Figure 2). The chimpanzees are housed in two types of buildings. The first building (15 x 7 m), referred to as the retirement home, accommodates a group of older chimpanzees who are no longer able to participate in feeding competitions (Figure 2). The other chimpanzees are housed in larger enclosures (30 m x 15 and 20 m high) with access to an outdoor terrace (Figures 2 and 3).
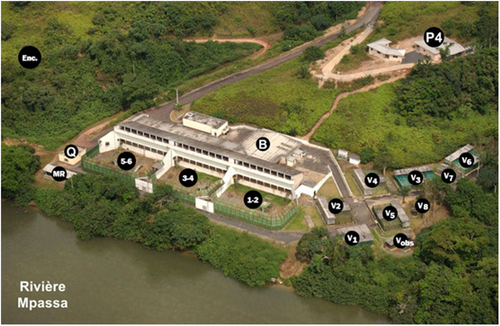
The study group was founded shortly after the creation of the CIRMF in 1979 and is monitored and fed daily by expert staff (surgical assistants, animal technicians and researchers). Their diet consists mostly of bananas, wild fruits, vegetables and soya-based feed made on-site. The environment is enriched daily with further food and positive reinforcement is used to encourage animals to cooperate and to enable noninvasive ethological studies (Figure 3). During the study period, chimpanzees have been extensively employed as experimental models. Since 2012, the CIRMF banned intrusive invasive protocols such as drug testing or deliberate exposure to substances that may pose risks to their health or psychological well-being.

2.3 Blood Collection
During the 24-year study period, 473 blood samples were collected from 84 anesthetized animals (43 females and 41 males) during annual health checks as part of routine physical examinations. Chimpanzee samples were collected at least once a year for a health check. The anesthesia protocol followed was in accordance with the procedures described by Ngoubangoye et al. (2021).
2.4 Hematological Analysis
Hematological analysis were performed at the Laboratoire des Analyses Médicales (LAM), CIRMF, using fresh blood treated with EDTA using a Roche Hematology ABX Cobas Micros. A total of 10 hematological parameters were determined (Table 1). The quality of the analyses is controlled internally and the LAM is also affiliated to the French national quality control system. However, the fact that this study was conducted over 24 years and by several observers increases the risk of error. We therefore statistically controlled for the year in which the sample was acquired in all analyses.
| Hematological parameter | Units | Mean ± SD | Median | Range |
|---|---|---|---|---|
| Hemoglobin | g/dL | 12.6 ± 2.5 | 12.7 | 3.3–35 |
| Platelets | 10^9/L | 276.9 ± 114.9 | 259 | 4–801 |
| White blood cells | 10^9/L | 9.9 ± 5.4 | 8.7 | 1–45.8 |
| Red blood cells | 10^12/L | 4.8 ± 0.8 | 4.8 | 1.3–46 |
| Hematocrit | % | 26.2 ± 19.6 | 34.9 | 0.2–107 |
| Mean cell hemoglobin concentration | g/dL | 32.6 ± 2.7 | 33 | 16.9–40.5 |
| Lymphocytes | % | 30.0 ± 16.6 | 28 | 1– 80 |
| Neutrophils | % | 53.3 ± 20.9 | 58 | 0–93 |
| Eosinophils | % | 4.2 ± 6.7 | 4 | 0–79 |
| Monocytes | % | 11.5 ± 8.9 | 9 | 0–85.7 |
2.5 Statistical Analysis
A total of 473 hematological profiles from 84 individuals (43 females and 41 males; 1 to 12 observations per individual) from 1996 to 2020 were analyzed. We initially assessed the normality of all hematological parameters using quantile plots of standard residuals. For parameters with non-normal distributions (hematocrit, monocytes, platelets and mean corpuscular hemoglobin concentrations), logarithmic transformation was applied until the residuals achieved normality.
To summarize our data, we categorized chimpanzees by age at the time of sample collection. The four age categories were defined as infant (0 to 3 years), juvenile (> 3 to 6 years), adolescent (> 6 to 10 years), and adult (> 10 years), consistent with previous studies on chimpanzees in captivity (Howell et al. 2003; Ihrig et al. 2001).
Statistical analyses were performed using R. We considered the ecological season as a categorical variable with two categories: rainy and dry. In Gabon, there are four climatic seasons characterized by a long rainy season (Feb–May), a long dry season (Jun–Sept), a short rainy season (Oct–Nov), and a short dry season (Dec–Jan) (Nsi Akoue et al. 2017). Finally, the two short seasons were combined with their respective long seasons due to the limited number of samples available from these periods. The Wilcoxon signed-rank test was used to compare data collected before 2012, when chimpanzees were extensively employed as experimental models to data collected after 2012, when the CIRMF banned intrusive invasive protocols. Linear mixed models were used to analyze the data with repeated measures, with individuals and years included as random effects. The effect of sex, age class and season were tested as well as their interactions. All the interactions were not significant (p > 0.05) and so were eliminated. Values for each age-sex class and season were reported as the mean ± SD, and p-value < 0.05 was considered statistically significant.
3 Results
We measured and analyzed 473 blood samples collected from 84 chimpanzees, including 43 females and 41 males of different ages (Table 1). Hematological parameters showed significant differences between the periods before and after 2012 (Table 2). Specifically, lymphocyte and hematocrit levels were higher after 2012, while white blood cells, neutrophils, and eosinophils were higher before 2012 (Table 2).
| Hematological parameter | Units | Before 2012 (n = 365) | After 2012 (n = 108) | P | ||
|---|---|---|---|---|---|---|
| Mean ± SD | Range | Mean ± SD | Range | |||
| Hemoglobin | g/dL | 12.65 ± 2.74 | 3.8–35 | 12.48 ± 1.90 | 6.8–20.5 | 0.72 |
| Platelets | 10^9/L | 281.54 ± 118.45 | 4–80.1 | 261.12 ± 101.13 | 22–54.8 | 0.07 |
| White blood cells | 10^9/L | 10.29 ± 5.12 | 1–33.4 | 8.55 ± 1.89 | 1.89–45.85 | < 0.0001 |
| Red blood cells | 10^12/L | 4.88 ± 2.43 | 1.32–46 | 4.87 ± 0.70 | 2.64–7.8 | 0.15 |
| Hematocrit | % | 22.72 ± 20.58 | 0.24–107 | 38.59 ± 8.11 | 21–97.4 | < 0.0001 |
| Mean cell hemoglobin concentration | g/dL | 32.64 ± 2.81 | 23–40.59 | 32.80 ± 2.73 | 16.9–37.7 | 0.64 |
| Lymphocytes | % | 28.35 ± 16.52 | 1–80 | 35.71 ± 15.65 | 9.7–73.4 | < 0.0001 |
| Neutrophils | % | 55.35 ± 19.14 | 0–93 | 41.34 ± 26.34 | 0.08–82 | < 0.0001 |
| Eosinophils | % | 5.00 ± 7.02 | 0–79 | 0.30 ± 0.44 | 0–1.42 | < 0.0001 |
| Monocytes | % | 11.20 ± 7.80 | 0–39 | 12.81 ± 12.21 | 2.7–85.7 | 0.1906 |
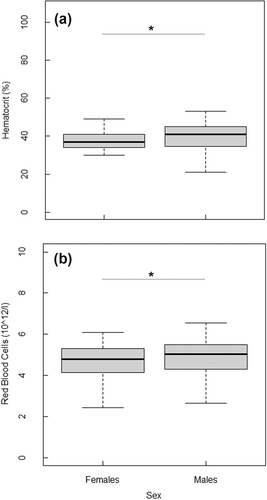
Sex significantly influenced hematocrit and red blood cell counts (Tables 3 and 4). Males had significantly higher hematocrit levels (p < 0.04, Figure 4a) and red blood cells counts (p < 0.03, Figure 4b) compared to females.
| Hematological parameter units | Sex | Age | Season | |||||
|---|---|---|---|---|---|---|---|---|
| Male | Female | Infant | Juvenile | Adolescent | Adult | Dry | Wet | |
| n | 243 | 230 | 19 | 10 | 52 | 392 | 212 | 261 |
| Hemoglobin g/dL | 12.8 ± 2.2 | 12.4 ± 3 | 12 ± 6 | 15 ± 7.4 | 12 ± 1.5 | 13 ± 2 | 13 ± 3.17 | 12.3 ± 1.9 |
| Platelets 10^9/L | 271.7 ± 117.6 | 282.4 ± 112 | 282 ± 116 | 307 ± 165.5 | 340 ± 122 | 261.4 ± 106.4 | 272.8 ± 132.7 | 280.3 ± 98.4 |
| White blood cells 10^9/L | 9.6 ± 5.4 | 10.2 ± 5.4 | 10 ± 4 | 11.3 ± 2 | 10 ± 5 | 10 ± 5.6 | 10.6 ± 6.7 | 9.4 ± 4.03 |
| Red blood cells 10^12/lL | 5.0 ± 1.4 | 4.7 ± 0.8 | 4.5 ± 0.7 | 6 ± 0.3 | 5.4 ± 6 | 5 ± 1 | 4.9 ± 0.85 | 4.8 ± 2.8 |
| Hematocrit % | 40.2 ± 11.4 | 36.9 ± 9.8 | 10.3 ± 19.4 | 17.6 ± 28.4 | 17 ± 18.5 | 29 ± 19 | 32.4 ± 18.03 | 21.3 ± 19.5 |
| Mean cell hemoglobin Concentration g/dL | 32.7 ± 2 | 32.7 ± 3 | 31 ± 3.3 | 32.2 ± 3 | 33 ± 3 | 33 ± 3 | 32.1 ± 3.09 | 33.2 ± 2.4 |
| Lymphocytes % | 30.3 ± 17 | 29.7 ± 16 | 47 ± 15 | 48 ± 21 | 31 ± 15 | 28.4 ± 16 | 26.9 ± 17.5 | 32.6 ± 15.4 |
| Neutrophils % | 52.3 ± 21 | 54.4 ± 21 | 40 ± 18 | 36.4 ± 26 | 52 ± 18 | 55 ± 21 | 58.8 ± 20.4 | 49.6 ± 20.4 |
| Eosinophils % | 4.3 ± 6.7 | 4.2 ± 6.6 | 4.8 ± 4.7 | 3.8 ± 2.4 | 4.5 ± 3 | 4.2 ± 7.2 | 4.3 ± 6.9 | 4.3 ± 6.5 |
| Monocytes % | 12 ± 8.7 | 11.2 ± 9.2 | 6 ± 2.2 | 8 ± 3.3 | 11.4 ± 8 | 12 ± 9.3 | 10.9 ± 8.2 | 12.3 ± 9.7 |
| Hematological parameter | Explanatory variable | Estimate | t | P |
|---|---|---|---|---|
| Lymphocytes | Sexa | 0.77 | −0.09 | 0.92 |
| Age classb | ||||
| Juvenile | 1.77 | −0.09 | 0.92 | |
| Adolescent | −12.63 | −3.93 | < 0.0001 | |
| Adult | −12.74 | −4.37 | < 0.0001 | |
| Seasonc | 4.78 | 3.89 | < 0.0001 | |
| Neutrophils | Sexa | −1.76 | −0.62 | 0.53 |
| Age classb | ||||
| Juvenile | −2.29 | −0.25 | 0.79 | |
| Adolescent | 11.03 | 2.09 | 0.03 | |
| Adult | 10.45 | 2.32 | 0.02 | |
| Seasonc | −10.39 | −4.56 | < 0.0001 | |
| Eosinophils | Sexa | 0.08 | 0.13 | 0.88 |
| Age classb | ||||
| Juvenile | 0.37 | −0.29 | 0.76 | |
| Adolescent | −0.17 | −0.07 | 0.94 | |
| Adult | 0.83 | −0.27 | 0.78 | |
| Seasonc | 0.34 | −0.36 | 0.71 | |
| Monocytes | Sexa | 0.41 | 0.89 | 0.37 |
| Age classb | ||||
| Juvenile | −1.53 | 0.47 | 0.63 | |
| Adolescent | 3.33 | 2.01 | 0.04 | |
| Adult | 2.80 | 2.69 | < 0.001 | |
| Seasonc | 2.05 | −0.34 | 0.73 | |
| Hemoglobin | Sexa | 0.09 | 0.31 | 0.75 |
| Age classb | ||||
| Juvenile | 1.44 | 1.89 | 0.05* | |
| Adolescent | 0.77 | 1.67 | 0.09 | |
| Adult | 0.98 | 0.28 | 0.07 | |
| Seasonc | −0.10 | −1.33 | 0.18 | |
| Platelets | Sexa | −18.14 | −0.92 | 0.35 |
| Age classb | ||||
| Juvenile | −28.55 | −0.80 | 0.41 | |
| Adolescent | −0.55 | −0.10 | 0.91 | |
| Adult | −76.35 | −3.03 | < 0.001 | |
| Seasonc | −6.13 | −1.14 | 0.25 | |
| Red blood cells | Sexa | 0.30 | 2.83 | 0.03 |
| Age classb | ||||
| Juvenile | 0.36 | 0.15 | 0.87 | |
| Adolescent | 0.77 | 1.24 | 0.21 | |
| Adult | 0.13 | −0.05 | 0.95 | |
| Seasonc | −0.07 | −0.88 | 0.32 | |
| White blood cells | Sexa | −0.99 | −0.88 | 0.38 |
| Age classb | ||||
| Juvenile | 0.59 | 0.45 | 0.65 | |
| Adolescent | 1.15 | 0.20 | 0.83 | |
| Adult | −0.06 | −0.81 | 0.41 | |
| Seasonc | −1.81 | −0.44 | 0.21 | |
| Hematocrit | Sexa | 0.58 | 1.94 | 0.04 |
| Age classb | ||||
| Juvenile | −0.56 | 1.13 | 0.25 | |
| Adolescent | 2.22 | 1.76 | 0.07 | |
| Adult | 1.53 | 0.78 | 0.38 | |
| Seasonc | −0.60 | −0.72 | 0.75 | |
| Mean cell hemoglobin cencentration | Sexa | −0.01 | −1.13 | 0.25 |
| Age classb | ||||
| Juvenile | 5.54 | 1.73 | 0.08 | |
| Adolescent | 8.25 | 3.43 | 0.91 | |
| Adult | 8.64 | 3.73 | 0.85 | |
| Seasonc | 2.92 | 0.44 | 0.65 |
- Note: In bold significant P-values.
- a Reference category: females.
- b Reference category: Infant.
- c Reference category: dry season.
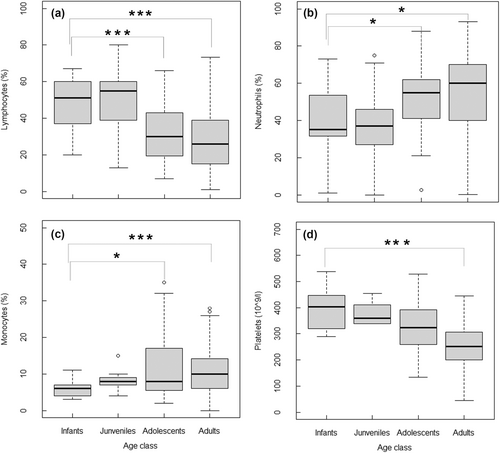
Age also had a significant impact on certain hematological parameters (Tables 3 and 4). Adolescents and adults of both sexes had significantly lower lymphocyte counts (Figure 5a) and significantly higher levels of neutrophils (Figure 5b) and monocytes (Figure 5c) than infants (Table 4). Adults had significantly lower platelet counts (p < 0.001, Figure 5d) compared to other age classes (Table 4).
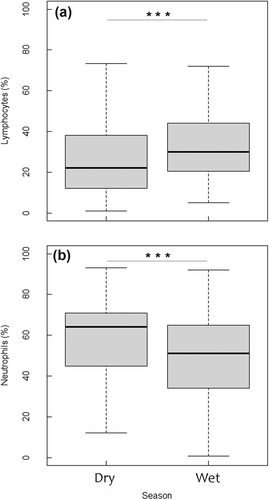
Seasonal variations were observed, with lymphocyte counts significantly higher during the rainy season than during the dry season (p < 0.001, Figure 6a), while neutrophil levels were significantly lower during the rainy season (p < 0.001, Figure 6b).
4 Discussion
The aim of this study was to establish a baseline hematological profile of former research chimpanzees living in a seasonal environment in sub-Saharan Africa and to identify variations according to age, sex, and season. Our results showed that these factors significantly affect certain hematological parameters. In addition, the results of this study are in line with the established intervals of hematological values for chimpanzees (Howell et al. 2003; Ihrig et al. 2001) and are similar to those observed in other primate species, such as mandrills (Setchell et al. 2006), and sun-tailed monkeys (Motsch, Gonzalez, and Verrier 2012).
The significant differences in some hematological parameters observed between the pre- and post-2012 periods may be attributed to changes in research protocols and management practice at CIRMF. The increase in lymphocyte and hematocrit levels after 2012 probably reflects a reduction in stress and inflammatory responses due to the cessation of invasive procedures that were common before 2012. Higher hematocrit levels after 2012 could also indicate improved general health and better management practices, including better nutrition and reduced exposure to experimental stressors. Hematocrit levels are often used as indicators of red blood cell concentration and oxygen-carrying capacity, both of which can be affected by stress and general health (Videan, Fritz, and Murphy 2008).
Conversely, the elevated counts of white blood cells, neutrophils, and eosinophils observed before 2012 probably indicate an increased inflammatory and immune response, likely due to frequent exposure to experimental drugs or substances. Studies have shown that invasive procedures and frequent handling can cause chronic stress and activation of the immune system in nonhuman primates, leading to altered hematological parameters (Capitanio, Del Rosso, and Spinner 2023; Hasegawa, Huffman, and Chapman 2009). For example, research on rhesus macaques has shown that chronic stress leads to an increase in neutrophils and eosinophils, while improved living conditions and reduced stress lead to normalized hematological values (Capitanio, Del Rosso, and Spinner 2023; Kessler and Rawlins 1983).
Consistent with previous studies, males had higher hematocrit and red blood cell counts than females (Hainsey et al. 1993; Howell et al. 2003). Similar sex differences have been reported in other primates, including tufted capuchin monkeys Cebus apella (Samonds, Ausman, and Hegsted 1974), rhesus macaques, Macaca mulata (Kessler and Rawlins 1983; Ramachandra et al. 1998; Tattersall et al. 1981), Tonkean macaques M. tonkeana (Thierry, André, and Imbs 2000), long-tailed macaques M. fascicularis (Schuurman and Smith 2005), African green monkeys Chlorocebus aethiops (Kagira et al. 2007), bonnet macaques M. radiata (Pierre et al. 2011), night monkeys (Takeshita et al. 2011), mandrills (Setchell et al. 2006), sun-tailed monkeys (Motsch, Gonzalez, and Verrier 2012). These differences are often attributed to hormonal status and variations in muscle mass between sexes (Bain 1985; Howell et al. 2003).
Age had a significant impact on hematological parameters, with adolescents and adults having lower lymphocyte counts and higher neutrophil and monocyte levels than infants. Adults also had significantly lower platelet counts than other age groups. These results are consistent with previous reports in chimpanzees (Hodson et al. 1967; Howell et al. 2003; Ihrig et al. 2001), hamadryas baboons Papio hamadryas (Harewood et al. 1999), rhesus macaques M. mulatta (Kessler and Rawlins 1983), mandrills (Setchell et al. 2006) and sun-tailed monkeys (Motsch, Gonzalez, and Verrier 2012). The decrease in lymphocytes with age may reflect a decline in the adaptative immune system (Jégo et al. 2014; Videan, Fritz, and Murphy 2008) or stress (Davis, Maney, and Maerz 2008). Furthermore, the higher lymphocyte count in infants is consistent with the findings of (McClure, Keeling, and Guilloud 1972). Increased neutrophil and monocyte levels could indicate an ongoing immune response to infection or inflammation, potentially related to stress in adolescents and ageing chimpanzees (Hasegawa, Huffman, and Chapman 2009). In rhesus monkeys, neutrophil variation has been associated with cortisol concentrations, suggesting a relationship between stress and the immune response (Capitanio, Del Rosso, and Spinner 2023). The decrease in platelet counts with age is consistent with findings linking lower platelet count to higher body mass (Semple, Cowlishaw, and Bennett 2002).
Seasonal variations were also evident, with higher lymphocyte counts in the rainy season and higher neutrophil counts in the dry season. These results are consistent with observations made on semi-free-ranging mandrills in the same primate center (Setchell et al. 2006) and may be related to the increased likelihood of infection and parasitemia during the rainy season (Altizer et al. 2006; Boundenga et al. 2021; Huffman et al. 1997; Setchell et al. 2007).
Although this study provides valuable information on hematological variation in captive chimpanzees, several limitations should be recognized. The methods used to collect blood samples, including the anesthetic agents and handling techniques during annual health checks, as well as invasive experimental protocols used before 2012, may have an impact on the hematological values obtained. In addition, the influence of the reproductive status of the females and of past and recent infections on hematological parameters could not be taken into account.
5 Conclusion
Our results reveal significant differences in hematological profiles between age groups and between males and females, reinforcing the need to take these factors when assessing the health status of chimpanzees in captivity. While the influence of age and sex on hematological parameters in primates is well documented, two potentially novel aspects of this report are the effect of season and the significant differences in certain hematological parameters between the period before the widespread use of chimpanzees as experimental models and after 2012, when the CIRMF banned invasive procedures. Seasonal variations observed in certain hematological parameters likely reflect the influence of environmental factors on physiological responses in captivity. Monitoring environmental conditions such as temperature, humidity and precipitation will help researchers and veterinarians in accurately interpreting these physiological responses. Overall, the findings of this study should improve our understanding and management of chimpanzee health in captivity.
Author Contributions
Barthélémy Ngoubangoye: conceptualization (equal), supervision (equal), visualization (equal), writing–review and editing (equal). Serge-Ely Dibakou: conceptualization (equal), formal analysis (lead), visualization (equal), writing–original draft (lead), writing–review and editing (equal). Désiré Otsaghé: data curation (supporting), methodology (supporting). Thierry Tsoumbou: data curation (supporting), methodology (supporting). Cyr Moussadji: data curation (supporting), methodology (supporting). Freddy Yanagha: data curation (supporting). Yasmine Okomo Nguema: data curation (equal). Larson Boudenga: supervision (supporting); visualization (supporting), writing–review and editing (supporting). Dominique Pontier: supervision (supporting), visualization (supporting), writing–review & editing (supporting).
Acknowledgments
We acknowledge the past and present staff of the CIRMF Primate Center who organized the chimpanzee sample acquisition and the Laboratoire d'Analyses Médicales of the CIRMF for their help in laboratory analyses. We are also grateful to the veterinarians for routine blood collections and to the primate centre staff for their care of the animals. We thank Joanna Setchell for English editing and comments. This study was supported by Centre of the Interdisciplinary Medical Research Centre of Franceville (CIRMF, Gabon) and the LABEX ECOFECT (ANR-11-LABX-0048) of Université de Lyon, within the program “Investissements d'Avenir” (ANR-11-IDEX-0007) operated by the French National Research Agency (ANR) and by the RESPOND program of the Université de Lyon (UDL).
Conflicts of Interest
The authors declare no conflicts of interest.
Open Research
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.




