Quantitative Analysis of the Carpal Tunnel and Its Inner Structures in Primates
ABSTRACT
To explore the anatomical factors potentially involved in the high incidence of carpal tunnel syndrome in humans, we have quantified the anatomical variations of the carpal tunnel and its inner structures in humans, non-hominoid primates (monkeys), and hominoid primates (apes). In specimens of six humans, eight monkeys, and three apes, we assessed the size of the carpal tunnel, the tendons of the digit flexor muscles, and the median nerve. We compared the size of the carpal tunnel normalized by the wrist size, and the size of the median nerve and the tendons of the digit flexors normalized by the size of the carpal tunnel. Differences between humans and monkeys were calculated using the T test or Mann–Whitney U test, as appropriate. Data on the apes were not included in the statistical analyses due to the small sample size. The normalized size of the carpal tunnel was similar in all specimens. The normalized size of the tendons of the digit flexors was smaller in humans, while that of the median nerve was significantly larger. The median nerve was also larger in apes than in monkeys. The relatively larger median nerve observed in humans could suggest a greater vulnerability of the nerve to compression, which could predispose humans to carpal tunnel syndrome. However, the tendons of the digit flexor muscles were smaller in humans, and moreover, the proportional size of the median nerve was similar in apes, leading us to suggest that the factors predisposing humans to carpal tunnel syndrome must be sought beyond anatomical features and may be more closely related to functional or personal parameters.
Summary
-
The relative size of the carpal tunnel is similar in humans and other hominoid and non-hominoid primates.
-
The relative size of the tendons passing through the carpal tunnel is higher in nonhuman primates than in humans.
-
The relative size of the median nerve to the size of the carpal tunnel is greater in humans and nonhuman hominoid primates than in non-hominoid primates.
Abbreviations
-
- CSA
-
- cross-sectional area
-
- CT
-
- carpal tunnel
-
- CTS
-
- carpal tunnel syndrome
-
- FCU
-
- flexor carpi ulnaris
-
- FDP
-
- flexor digitorum profundus
-
- FDS
-
- flexor digitorum superficialis
-
- FPL
-
- flexor pollicis longus
-
- HMM
-
- hand muscle mass
-
- MN
-
- median nerve
-
- T
-
- diameter of the tendons
-
- TMM1
-
- total muscle mass of the forearm and hand
-
- TMM2
-
- total muscle mass of the muscles that pass through the carpal tunnel
-
- WS
-
- wrist size
1 Introduction
Carpal tunnel syndrome (CTS), which is caused by compression of the median nerve (MN) within the carpal tunnel (CT), accounts for 90% of all entrapment neuropathies (Aroori and Spence 1999) and is the most common MN neuropathy (Atroshi 1999; Keith et al. 2009). In general, CTS is characterized by pain or dysesthesia in the first, second, and third fingers and by weakness in thumb abduction (Franzblau 1999; Iyer and Shetty 2012), although in some patients it can affect the entire hand. Symptoms tend to worsen during the night and diminish by the morning. If CTS persists, it can result in atrophy of the thenar muscles (Rea 2015). CTS has a multifactorial origin and is influenced by personal characteristics and physical workload (Palmer et al. 2007; Dale et al. 2013). Risk factors for CTS encompass obesity (Shiri et al. 2015), diabetes (Pourmemari and Shiri 2016), hypothyroidism (Shiri 2014), osteoarthritis, and rheumatoid arthritis (Shiri 2016). The prevalence of CTS is approximately 3%–6% but fluctuates, with higher rates among women and individuals aged 45–60 (Kuschner et al. 1992; Stevens et al. 1998; Atroshi 1999; Aroori and Spence 1999; Franzblau 1999; D'Arcy and McGee 2000; Atroshi 2011; Iyer and Shetty 2012). CTS occurs up to three times more frequently in women than in men (Solomon et al. 1999; McDiarmid et al. 2000; Papanicolaou, McCabe, and Firrell 2001; Becker et al. 2002; Bongers et al. 2007; Harris-Adamson et al. 2013; Jackson et al. 2018; Cazares-Manríquez et al. 2020).
Several factors have been associated with these higher rates of CTS in women, such as hormonal fluctuation or smaller wrist, hand, and CT size (McDiarmid et al. 2000; Cazares-Manríquez et al. 2020). Several studies have used animal models to explore the importance of repetitive movements and external physical load in CTS. In 1973, Anderson studied 235 rabbit CTs by inducing 10 h of electrically forced flexion-extension contractions at the radiocarpal joint and observed an increased water content of the CT tissues immediately afterwards, which was suggestive of edema and compression of the inner CT structures (Andersson 1973). Since then, several studies have associated different work-related factors to CTS, such as compression (Mackinnon et al. 1984, 1985; OʼBrien et al. 1987), vibration forces (Ho and Yu 1989), and electrically stimulated contractions (Backman et al. 1990). In the early 2000s, Barbe and Barr studied the relation between CTS and repetitive reaching and grasping tasks in trained rats and concluded that the tasks induced changes associated with inflammation in muscles, tendons, loose areolar connective tissues, and synovial connective tissues throughout the reach limb, as well as signs of tendon fray in the limb flexors (Barr et al. 2000; Barr and Barbe 2002; Barbe et al. 2003). In 2003 and 2004, the same group characterized changes in the MN associated with the same repetitive tasks and found that the MN in trained rats showed numerous changes over 3–12 weeks of task performance, including increased inflammatory markers, signs of fibrosis, and a slight but significant slowing of nerve conduction velocity in the reach limb. Moreover, they detected behavioral and physiological changes in the rats that were comparable to those observed in humans with CTS, including motor weakness, hypoalgesia, and slowed MN conduction (Clark et al. 2003, 2004). In 2007, Sommerich approached the pathophysiology of human CTS by using nonhuman primate models (Macaca fascicularis) to correlate the performance of a moderately forceful, repetitive manual task with the development of median mononeuropathy at the wrist. They observed a 25% decline in nerve conduction velocity in the working hands and enlargement of the affected nerves near the proximal end of the CT. This study provided evidence of a relationship between manual work, median mononeuropathy, and CTS, which the authors suggested could be extrapolated to humans (Sommerich et al. 2007). However, to the best of our knowledge, no anatomical dissection studies have compared the size of the CT and its inner structures between nonhuman primates and humans.
As a group, primates are characterized by having a broad diversity of locomotive and postural behaviors as well as by the capacity to use multiple manual positions to adapt to their surroundings. The anatomy of primate wrist joints has had to adapt to balance the need for both mobility in manipulation and stability in locomotion (Patel and Wunderlich 2010). Each species of primates has experienced particular modifications in the morphology of the carpus, which has allowed them to have different ranges of manipulative movements, reaching and grasping movements, and locomotion. These adaptations include changes in the surfaces, sizes and shapes of the wrist bones and the specific characteristics of tendons and ligaments (Daver, Berillon, and Grimaud-Hervé 2012). These changes have also affected the CT of primates. Although the entire structure of the carpus modifies the configuration of the CT, the bones in which the transverse ligament is inserted and the characteristics of the ligament itself have the greatest impact on the shape and depth of the CT, especially the tubercle of the scaphoid bone and the hook of hamate, also known as the hamulus (Hamrick 1996, 1997). Importantly, in both African apes and in humans, the scaphoid is fused to the os centrale—in apes as a functional adaptation to the increased shear stress during knuckle-walking and in humans due to phylogenetic “lag” or exaptation to shear stress during power-grip postures (Kivell and Begun 2007). A relatively large scaphoid tubercle in these primates has been associated with an expanded CT, acting as a “windlass mechanism” for the pollical branch of the flexor digitorum profundus (FDP) tendon. This mechanism supports the strong thumb adduction required for grasping onto supports with relatively small diameters (Hamrick 1997).
In digitigrade and palmigrade non-hominoid primates (monkeys), the scaphoid tubercle is also enlarged, restricting extension and entailing a concurrent increase in the depth of the CT. The hamulus, which also influences the depth of the CT, is more developed in nonhuman hominoids (apes) than in monkeys (Hamrick 1997). For example, Papio has a short hamulus and a relatively shallow CT in comparison with apes. In contrast, a well-developed hamulus in apes is most clearly associated with a deep CT and strong digit flexor muscles, which are needed for vertical climbing and suspensory behavior (Hamrick 1997; Ward et al. 1999; Ward 2002). Alongside the hamulus, the pisiform delineates the medial boundary of the CT, providing attachment points for the flexor carpi ulnaris (FCU) and the abductor digiti minimi (Diogo and Wood 2011). Nevertheless, the function of a lengthened pisiform seems to be more closely related to enhancing the moment arm of the FCU than to deepening the CT (Sarmiento 1988; Lewis 1989; Hamrick 1997).
Despite numerous studies that have independently compared the individual bones comprising the CT across various primate groups, there is a lack of comprehensive comparative analyses regarding the structures passing through the CT. Such analyses could expose potential variations in the architecture of the CT and its inner structures and provide insights into the etiology of CTS, a condition highly prevalent in humans but not assessed in other primates. In the present study, we have compared the relationship between the size of the CT and that of its inner structures (tendons of the digit flexor muscles and MN) in humans, apes, and monkeys (Figure 1). To the best of our knowledge, this study is the first to perform a detailed anatomical comparison of the CT and its structures in human and nonhuman primates. The central question of this study was to determine if, compared to nonhuman primates, humans have a relatively smaller CT in relation to its inner structures and whether such a smaller CT could explain the high incidence of CTS in humans. Our working hypothesis postulates that the human CT is relatively small in relation to its structures, so the risk of compression of the MN may increase as a result of repetitive flexion-extension movements of the wrist. If our hypothesis were confirmed, it would suggest that there is an anatomical substrate for the high prevalence of CTS in humans. Therefore, we believe that our study, using a comparative approach, will help investigate potential anatomical factors in the etiology of CTS.
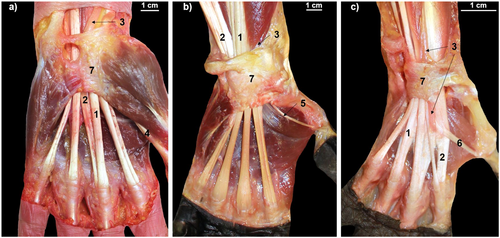
2 Methods
2.1 Ethics Statement
The research complied with protocols approved by the Institutional Animal Care and Use Committee of the University of Barcelona (IRB00003099) and adhered to the legal requirements of Spain and to the principles of the American Society of Primatologists for the ethical treatment of nonhuman primates.
2.2 CT Samples
We dissected a total of 11 nonhuman primates provided by the Anatomical Museum of the University of Valladolid (Spain), and six humans provided by the Body Donation Service of the University of Barcelona (Spain). The individuals were stored post-mortem at −18°C, and then unfrozen at 4°C between 24 and 48 h before the study. We performed macroscopic dissections of the forearms and hands of human specimens in the Unit of Human Anatomy and Embryology of the University of Barcelona and of the nonhuman primate specimens in the Anatomical Museum of the University of Valladolid. Three of the humans were men, with a mean age of 80.3 years (range, 72–86 years), and three were women, with a mean age of 80.0 years (range, 76–84 years). Eight nonhuman primates were monkeys (one male Chlorocebus aethiops, one male and one female Macaca silenus, one male Cercopithecus neglectus, one male Macaca tonkeana, one male and one female Colobus guereza, and one male Cercopithecus ascanius), all of which had palmigrade locomotor behavior. The three remaining nonhuman primates were apes: a 2-year-old female Nomascus gabriellae (gibbon), with a brachiation type of locomotion, and two knuckle-walkers, one female Pan troglodytes (chimpanzee) and one male Gorilla gorilla (gorilla). All the dissected primates were adult specimens, except for the young female N. gabriellae, and had died from causes unrelated to our study.
None of the individuals included in the study presented muscular atrophy or nerve impingement as macroscopic signs of CTS.
2.3 Anatomical Dissections and Measurements
All dissections were performed by the same researchers (P.R. and J.M.P.). Before the dissections, we measured the anteroposterior and transverse diameters of the wrists with a digital caliper to calculate the wrist size (WS) by multiplying the two measurements. This parameter was later used to normalize the size of the CT and the MN and the mass of the hand muscles in the different species. Next, we carefully removed the epidermis, adipose tissue, and connective tissue from the samples to access the muscle layers. The muscles of the forearm and hand were dissected individually and weighed with a precision scale (Sartorius PT610, resolution of 0.1 g). The portion of the MN and the tendons passing through the CT (the flexor digitorum superficialis [FDS], the FDP and, only in humans, the flexor pollicis longus [FPL]), were collected and preserved in a 5% formaldehyde solution. Finally, the carpal bones were separated from the radius, the ulna and the metacarpal bones, and the CT inlet and outlet were photographed with a Canon EOS-50 digital camera to obtain its cross-sectional area (CSA) (Figure 2). The samples of the MN and the tendons passing through the CT were preserved in formaldehyde for 7 days. Subsequently, 2-mm-thick sections were obtained from their proximal and distal ends and photographed with the same camera to calculate their CSAs.
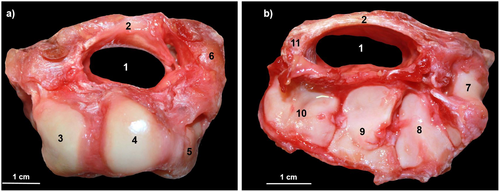
2.4 Quantitative Analyses
From these dissections, we obtained the following parameters: the total mass of all the muscles of the forearm and hand (TMM1); total mass of muscles passing through the CT (TMM2); total mass of all the muscles of the hand (HMM); the percentage of the TMM2 relative to the TMM1 (%TMM2); the CSA of the CT (CT-CSA); the CSA of the tendons passing through the CT (T-CSA); and the CSA of the MN (MN-CSA). The CSAs of the CT, of the tendons that pass through it, and of the MN were determined from the images taken of the CT inlet and outlet and from the images taken of the proximal and distal ends of the tendons and the MN by using ImageJ, an open-source software for image processing (Abramoff, Magalhaes, and Ram 2004). The proximal and distal CSAs of the CT, and the proximal and distal CSAs of the tendons and the MN were added and divided by 2, providing the parameters CT-CSA, T-CSA, and MN-CSA. Considering the differences between species in terms of absolute size, we then normalized the absolute values to compare the different species: the CT-CSA was normalized relative to the WS (CT-CSA/WS); the T-CSA was normalized relative to the CT-CSA (T-CSA/CT-CSA); the MN-CSA was normalized to the CT-CSA (MN-CSA/CT-CSA); the HMM was normalized relative to the WS (HMM/WS); and the MN-CSA was normalized relative to the WS (MN-CSA/WS). We then compared these normalized values in humans, apes, and monkeys.
2.5 Statistical Analyses
We tested the sample normality using the Shapiro–Wilk test. Significant differences between humans and monkeys were assessed with the parametric T test for variables with a normal distribution and with the nonparametric Mann–Whitney U test for variables without a normal distribution. Since there were only three apes, they were not included in the statistical analyses. All analyses were performed using SPSS (IBM SPSS Statics for Windows, version 24.0) and significance was set at p ≤ 0.05.
3 Results
The main results of the quantitative analyses are summarized in Table 1. The size of the CT (CT-CSA) was highest in the gorilla (352.0 mm²) and the chimpanzee (201.4 mm²), while the smallest CT-CSA value was in the female M. silenus (27.3 mm²). The CT-CSA in humans was 152.9 ± 25.8 mm², compared to 49.2 ± 15.5 mm² in monkeys. These differences in the CT-CSA absolute values disappeared when the CT-CSA value was normalized relative to the WS. The CT-CSA/WS was 0.12 ± 0.02 in humans, 0.11 ± 0.01 in apes, and 0.12 ± 0.03 in monkeys, with no significant differences between humans and monkeys (p = 1.00).
| Sample | Sex | Age (years) | CT-CSA/WS | %TMM2 | T-CSA/CT-CSA | MN-CSA/CT-CSA | HMM/WS | MN-CSA/WS | |
|---|---|---|---|---|---|---|---|---|---|
| Humans | HS1 | F | 80 | 0.10 | 21.8 | 0.67 | 0.08 | 0.018 | 0.008 |
| HS2 | F | 84 | 0.14 | 31.4 | 0.58 | 0.09 | 0.051 | 0.012 | |
| HS3 | F | 76 | 0.09 | 31.2 | 0.61 | 0.08 | 0.022 | 0.008 | |
| HS4 | M | 72 | 0.11 | 31.5 | 0.42 | 0.08 | 0.042 | 0.009 | |
| HS5 | M | 83 | 0.13 | 33.2 | 0.60 | 0.08 | 0.046 | 0.011 | |
| HS6 | M | 86 | 0.12 | 29.3 | 0.70 | 0.09 | 0.042 | 0.011 | |
| Mean | 0.12 | 29.7 | 0.60 | 0.08 | 0.037 | 0.010 | |||
| SD | 0.02 | 4.1 | 0.10 | 0.00 | 0.013 | 0.002 | |||
| Monkeys | MS1 | M | A | 0.14 | 38.0 | 0.75 | 0.05 | 0.019 | 0.007 |
| MS2 | F | A | 0.09 | 38.8 | 0.74 | 0.05 | 0.014 | 0.004 | |
| MT1 | M | A | 0.12 | 33.4 | 0.69 | 0.07 | 0.023 | 0.008 | |
| CN1 | M | A | 0.19 | 34.3 | 0.35 | 0.05 | 0.008 | 0.010 | |
| CAs1 | M | A | 0.11 | 37.4 | 0.59 | 0.05 | 0.011 | 0.005 | |
| CAe1 | M | A | 0.10 | 33.3 | 0.81 | 0.04 | 0.009 | 0.004 | |
| CG1 | F | A | 0.10 | 33.5 | 0.79 | 0.04 | 0.015 | 0.004 | |
| CG2 | M | A | 0.11 | 35.8 | 0.77 | 0.04 | 0.021 | 0.004 | |
| Mean | 0.12 | 35.6 | 0.69 | 0.05 | 0.015 | 0.006 | |||
| SD | 0.03 | 4.5 | 0.15 | 0.01 | 0.006 | 0.002 | |||
| p = 1.00 | p = 0.005a | p = 0.09 | p = 0.002a | p = 0.01a | p = 0.004a | ||||
| Apes | PT1 | F | A | 0.12 | 33.1 | 0.77 | 0.08 | 0.050 | 0.009 |
| GG1 | M | A | 0.11 | 30.7 | 1.15 | 0.09 | 0.048 | 0.007 | |
| NG1 | F | Y | 0.10 | 47.4 | 0.91 | 0.06 | 0.015 | 0.006 | |
| Mean | 0.11 | 37.1 | 0.94 | 0.08 | 0.038 | 0.008 | |||
| SD | 0.01 | 9.1 | 0.19 | 0.02 | 0.020 | 0.002 |
- Abbreviations: A = adult; CAe = Chlorocebus aethiops; CAs = Cercopithecus ascanius; CG = Colobus guereza; CN = Cercopithecus neglectus; CSA = cross-sectional area; CT = carpal tunnel; F = female; GG = Gorilla gorilla; HMM = hand muscle mass; HS = Homo sapiens; M = male; MN = median nerve; MS = Macaca silenus; MT = Macaca tonkeana; NG = Nomascus gabriellae; PT = Pan troglodytes; SD = standard deviation; T = tendons; TMM = total muscle mass; WS = wrist size; Y = young.
- a Statistical significance (comparison realized only between humans and monkeys).
The total muscle mass (TMM1 and TMM2) values were highest in the knuckle-walking apes (2247.6 and 689.6 g in the gorilla; 793.3 and 262.5 g in the chimpanzee), followed by those in humans (383.7 ± 136.3 and 117.3 ± 49.7 g), and finally by those in monkeys (117.0 ± 73.0 and 41.1 ± 24.5 g). However, when these values were normalized, the %TMM2 values were lower in humans (29.7 ± 4.1) than in monkeys (35.6 ± 4.5) and in apes (37.1 ± 9.1). The difference between humans and monkeys was significant (p = 0.005).
The size of the tendons (T-CSA) was largest in the gorilla (403.31 mm²) and the chimpanzee (154.65 mm²), followed by humans (90.1 ± 16.0 mm²), monkeys (33.3 ± 12.2 mm²), and N. gabriellae (26.4 mm²). A similar distribution was observed in the size of the MN (MN-CSA), with higher values in the gorilla (33.2 mm²) and the chimpanzee (15.9 mm²), followed by humans (12.9 ± 2.1 mm²), monkeys (2.5 ± 1.3 mm²), and N. gabriellae (1.8 mm²). However, when the T-CSA parameter was normalized by the CT size (CT-CSA), the highest T-CSA/CT-CSA values were found in apes (0.94 ± 0.19), followed by monkeys (0.69 ± 0.15), and humans (0.60 ± 0.10). The difference between humans and monkeys was not significant (p = 0.09). When the MN-CSA values were normalized relative to the CT-CSA, humans and apes had similar MN-CSA/CT-CSA values (0.08 ± 0.00 in humans and 0.08 ± 0.02 in apes), while monkeys had lower values (0.05 ± 0.01). A significant difference was observed between humans and monkeys (p = 0.002).
Finally, when the HMM was normalized relative to WS, humans and apes had similar HMM/WS values (0.037 ± 0.013 in humans and 0.038 ± 0.020 in apes). However, knuckle-walking apes had clearly higher HMM/WS values than humans (0.048 in the gorilla and 0.050 in the chimpanzee), while N. gabriellae had clearly lower values (0.015). Monkeys as a group had clearly lower HMM/WS values (0.015 ± 0.006) than humans (p = 0.01). When the MN-CSA was normalized relative to the WS (MN-CSA/WS), humans had the highest values (0.010 ± 0.002), followed by apes (0.008 ± 0.002), and monkeys (0.006 ± 0.002). The difference between humans and monkeys was significant (p = 0.004).
4 Discussion
Studies of the hominid hand have focused on hand morphology, function and evolutionary adaptations in extant and fossil primate species (Kivell et al. 2016). Although few studies have explored the structure and configuration of the CT and its structures, a comparative study of the anatomy of the CT could provide valuable insights into evolutionary adaptations related to locomotion and manual dexterity and shed light on anatomical differences that could predispose an individual to CTS.
The configuration of the CT in primates depends on the scaphoid tubercle and the hamulus (Hamrick 1997; Ward et al. 1999; Ward 2002), both of which are related to a strong digit flexor musculature. This musculature is highly conserved in primates, and most forearm and hand muscles identified in humans can be observed in other primate species (Patel, Larson, and Stern 2012). However, the range of locomotion patterns, grasping techniques and hand or tool manipulation have contributed to a differential development of the FDS and FDP muscles (Kikuchi 2010). In our study, we have focused not on the anatomical variations in the digit flexors but rather on the mass of all flexors that pass through the carpal tunnel (FDS and FDP in nonhuman primates, and FDS, FDP, and FPL in humans) (Figure 1). We have found that the relative muscle mass of the digit flexors (%TMM2) was significantly greater in monkeys than in humans. The higher %TMM2 in monkeys indicates the importance of the flexor muscles in their locomotion, since all the primates included in our study use arboreal grasping, in which the digit flexor muscles are very important (Granatosky and Young 2023). In fact, among apes, N. gabriellae, the most arboreal of the three species studied, had the highest %TMM2, while the chimpanzee and the gorilla, which alternate arboreal locomotion with terrestrial knuckle-walking (Tuttle 1967; Tuttle et al. 1972; Inouye 1994), had intermediate values between monkeys and humans. These results are consistent with previous anatomical studies (Tuttle 1969; Kikuchi 2010) and with the EMG data collected in chimpanzees and baboons (Susman and Stern 1979; Patel, Larson, and Stern 2012), showing that locomotor behaviors like knuckle-walking elicit less activity of the FDS and FDP compared to the hook grips used during suspensory postures, and underscoring the fundamental role played by the extrinsic digital flexors for basic grasping functions of the hand (Kivell et al. 2023).
To evaluate whether this greater muscle mass in nonhuman primates correlated with thicker tendons, which would help these primates to withstand the increased mechanical demands placed upon them during arboreal locomotion, we looked at the size of the flexor tendons relative to the size of the CT (T-CSA/CT-CSA). Monkeys had a higher value than humans, although the difference was not significant (p = 0.09), which could be due to the small sample size. The three apes also had high T-CSA/CT-CSA values, with the highest values in the gorilla, which uses both arboreal locomotion and terrestrial knuckle-walking (Remis 1995). The humans in our study had smaller flexor tendons relative to the size of the CT, possibly due to the loss of arboreal locomotion and the resulting decrease in the relative muscle mass of the digit flexors. This result does not support our working hypothesis that the human CT is relatively small in relation to its inner structures; to the contrary, it indicates that humans have a relatively large CT in relation to the size of the tendons of the digit flexor muscles.
We found no significant differences between monkeys and humans in the size of the CT relative to the WS (CT-CSA/WS); the apes also had similar values, indicating that the relative size of the CT was similar in all the primates. Since the relative size of the CT was similar but the relative size of the tendons was smaller in humans, we could conclude that there is potentially more available space within the human CT, which could possibly be a protector against CTS. In contrast, when we examine the relative value of the MN size with respect to the CT size (MN-CSA/CT-CSA), we observe that humans exhibit a significantly larger MN compared to the monkeys, and we also identify that apes have similar relative values of the MN than humans. In this case, the result does support our working hypothesis, since it indicates that humans have a relatively small CT compared to the MN. This observation could support the idea that the high prevalence of CTS in humans may be due to a reduction in the relative size of the CT with respect to the size of the MN (Nattrass et al. 1994; Bower, Stanisz, and Keir 2006; Peterson et al. 2013; Lakshminarayanan, Shah, and Li 2019). However, this view may be counterbalanced by the fact that humans also have relatively smaller flexor tendons and by our observation that the large size of the MN in humans with respect to the CT size is also shared by apes. However, this result regarding the similar value of the parameter MN-CSA/CT-CSA in humans and apes should be treated with caution due to the small sample size of dissected apes in our study.
The large MN in humans and in apes may be related to the fact that once it has passed through the CT, the MN innervates many of the muscles in the hand and registers cutaneous sensitivity in the first three digits (Diogo and Wood 2011; Martinez-Pereira and Zancan 2015; Drake, Mitchell, and Vogl 2020). To explore this possibility, we calculated the size of the MN and the hand muscles relative to the WS (MN/WS and HMM/WS). The value of HMM/WS was significantly lower in monkeys, while humans and apes, specifically the chimpanzee and the gorilla, had higher values, indicative of a higher relative mass of hand muscles in these two groups. The larger size of the muscles of the hand in humans and apes could potentially explain the expanded diameter of the MN, due to an increased number of motor fibers. Other studies have also found a larger relative size of the hand muscles in apes. In the Japanese monkey, the hand muscles constituted 6.8% of the total size of the forelimb muscles (Ogihara and Oishi 2012), while in chimpanzees and humans, this percentage was 11.39% and 12.06%, respectively (Ogihara, Kunai, and Nakatsukasa 2005). This larger relative size of the hand muscles in apes is reflected in their larger physiological CSA (Ogihara, Kunai, and Nakatsukasa 2005; Ogihara and Oishi 2012), which is directly related to the capacity of a muscle to generate force (Michilsens et al. 2009). The larger relative size of the hand muscles in apes and humans compared to that in monkeys is due to the larger palmar muscles in apes—more pronounced in gorillas and chimpanzees than in gibbons—and to the larger thenar muscles, which control the thumb, in humans (Zihlman and Underwood 2019; Vanhoof et al. 2021).
In contrast, when normalized to the WS, the MN has a significantly lower value in monkeys than in humans, with that of apes in an intermediate position. We can speculate that this higher relative size of the MN in humans may reflect an increased sensitivity in the hands, particularly in the first three digits, which enables precision grasping and object manipulation (Jones 2006; Sobinov and Bensmaia 2021). Some evidence for this association lies in the fact that humans have the widest distal phalanges of all the hominoids (Aiello and Dean 1990), which has been linked to a finer precision grip and a larger tactile surface (Shrewsbury et al. 2003; Almécija, Alba, and Moyà-Solà 2009). Again, considering the small sample size of apes in our study, comparison of the MN-CSA/WS parameter between humans and apes should be considered with caution. Although it is true that considered as a group, the apes had an intermediate value between humans and monkeys, this could be affected by the small relative size of the MN in N. gabriellae. Moreover, the value obtained in our specimen of Pan troglodytes is similar to that obtained for humans (Table 1).
In conclusion, the higher MN-CSA/CT-CSA values observed in our human specimens could lead us to conclude that there is a greater vulnerability of the MN to compression within the CT, which could predispose individuals to CTS. However, we cannot draw definite conclusions from this finding, since our apes had MN-CSA/CT-CSA values similar to those observed in humans. In any case, more dissections of the CT of apes will be needed to confirm or disprove this result. Moreover, the relatively large size of the MN in humans may be counterbalanced by their relatively smaller tendons of the digit flexors. In fact, Sommerich et al. (2007) found that repetitive motions can induce CTS even in monkeys, which had MN-CSA/CT-CSA values significantly lower than the humans in our study. We therefore conclude that the factors predisposing humans to CTS must be sought beyond purely anatomical features and may be more closely related to functional parameters, such as intense workloads on the wrist (Palmer et al. 2007; Van Rijn et al. 2009; Dale et al. 2013), or to personal parameters, such as obesity, diabetes mellitus or hypothyroidism (Shiri 2014; Shiri et al. 2015; Pourmemari and Shiri 2016).
Author Contributions
Patrícia Rodríguez: conceptualization, data curation, formal analysis, investigation, methodology, validation, visualization, writing-original draft preparation. Aroa Casado: data curation, formal analysis, investigation, methodology, validation, visualization. Neus Ciurana: data curation, formal analysis, investigation, methodology, validation, visualization. Marcel García: data curation, formal analysis, investigation, methodology, validation, visualization. Francisco Pastor: data curation, formal analysis, investigation, methodology, resources, supervision, validation, visualization. Josep Maria Potau: conceptualization, data curation, formal analysis, funding acquisition, investigation, methodology, project administration, resources, supervision, validation, visualization, writing–original draft preparation.
Acknowledgments
The authors would like to thank Manuel J. Taboada (University of Valladolid) for his support and collaboration and Renee Grupp for her assistance in drafting the manuscript. This work is part of the project PID2022-138176NB-100 to J.M.P., financed by MICIU/AEI/10.13039/501100011033/FEDER/UE.
Conflicts of Interest
The authors declare no conflicts of interest.
Open Research
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.




