Landscape and conservation genetics of western black crested gibbons (Nomascus concolor) in China
Abstract
Despite decades of field study, very little is known about the molecular ecology of gibbons, particularly as it relates to their ability to disperse across degraded and fragmentary landscapes. The critically endangered western black crested gibbon (Nomascus concolor) has been reduced to a small, fragmented population with about 1300 individuals. In the largest population genetic study of free-ranging gibbons to date, we sampled 47 of these gibbons from 13 sites in China and generated 15 polymorphic autosomal microsatellite markers. We identify three population clusters of N. concolor in Yunnan centered in 1) the Wuliang and Ailao Mountains, 2) the Yongde Daxueshan Mountains, and 3) an isolated remnant near the border with Vietnam. Within the Wuliang Mountains, we identified four subclusters, three of which are bounded by high-altitude rhododendron forest, and one that is isolated from the main population by ~2 km of degraded forest and pasture. Least-cost path analysis and isolation by resistance modeling demonstrates that the population genetic distances among gibbons in Wuliangshan National Nature Reserve are significantly correlated with geographic paths that avoid use of high-altitude rhododendron forest in favor of evergreen broadleaf forest. Although these gibbons have likely undergone reductions in heterozygosity from recent consanguineous mating, we suggest that their active avoidance of inbreeding on the population level maintains higher than expected levels of genetic diversity. This research provides new insights into how gibbons interact with heterogeneous environments and expands our understanding of their molecular ecology and conservation genetics.
Research Highlights
-
We examined the how forest structure boundaries affect the population structure and genetic connectivity of western black crested gibbons.
-
Both high-altitude rhododendron thickets and lowland anthropogenic deforestation are restricting gene flow in these critically endangered apes.
Abbreviations
-
- AIC
-
- Akaike's information criterion
-
- AMOVA
-
- Analysis of molecular variance
-
- Ar
-
- Allelic richness
-
- DAPC
-
- Discriminant analysis of principle components
-
- DEM
-
- Digital elevation model
-
- EEMS
-
- Estimation of effective migration surfaces
-
- FDR
-
- False discovery rate
-
- Fis
-
- Individual inbreeding coefficient relative to the subpopulation
-
- Fit
-
- Individual inbreeding coefficient relative to the total population
-
- Fst
-
- The inbreeding coefficient of subpopulations relative to the total population
-
- Gis
-
- Nei's inbreeding coefficient
-
- Gis p
-
- p-value of Nei's inbreeding coefficient
-
- G-statistics
-
- Nei's genetic diversity statistics
-
- Ho
-
- Observed heterozygosity
-
- Hs
-
- Expected heterozygosity within a subpopulation
-
- Ht
-
- Expected total heterozygosity
-
- H't
-
- Corrected total heterozygosity
-
- IAM
-
- Infinite alleles model
-
- K
-
- Number of population clusters
-
- LCP
-
- Least cost path
-
- LDNe
-
- Linkage disequilibrium effective population size
-
- Lhome/Lmax
-
- The ratio of the highest likelihood value among all available population samples including the population where the individual was sampled (Piry et al., 2004)
-
- MLPE
-
- Maximum likelihood population effects model
-
- M ratio
-
- Number of alleles: range of alleles
-
- nDemes
-
- number of demes
-
- NDVI
-
- Normalized differential vegetation index
-
- Ne
-
- Effective population size
-
- r2
-
- Coefficient of determination
-
- Rousset's â
-
- Rousset's pairwise genetic distance
-
- SPOT
-
- Satellite pour l'observation de la terre
-
- SRTM
-
- Shuttle radar topography mission
-
- TPM
-
- Two phased model
-
- uHe
-
- Unbiased expected heterozygosity
-
- USGS
-
- United States Geological Survey
-
- WNNR
-
- Wuliangshan National Nature Reserve
-
- θ
-
- Watterson estimator
-
- μ
-
- Mutation rate
-
- ∆AIC
-
- Delta Akaike's information criterion
-
- Δg
-
- The average size of single step mutations
1 INTRODUCTION
How geographic boundaries intersect with locomotion, social structure, and life history to produce the patterns of population subdivision that lead to speciation, fragmentation, and extinction remains a fundamental question for molecular ecology and conservation genetics. However, within the primate radiation, relatively little research has focused on how small-scale geographic heterogeneity modifies the population genetic structure of primates in changing environments. As anthropogenic landscape modification continues to restrict and fragment dwindling populations of primates, it has become critical to understand how they can disperse through heterogeneous landscapes. Among the species studied, gene flow tends to be higher among those inhabiting intact habitats and can be restricted by both anthropogenic effects (e.g. deforestation and urbanization) and geographic boundaries (e.g. rivers and mountain ranges) (Blair & Melnick, 2012; Hermosilla-Albala et al., 2023; Westphal et al., 2021). However, it is important to consider that the scale of landscape barriers can be taxon specific (Westphal et al., 2021); for example, a dispersal barrier to a mouse lemur might not restrict a chimpanzee. These concerns are particularly relevant to gibbons, who not only are obligate canopy dwellers, reliant upon tall, large diameter trees and continuous forest canopies (Cheyne et al., 2013), but are also among the world's most threatened primate families, for whom 19 of 20 species are classified as endangered or critically endangered by the IUCN (Cheyne et al., 2023).
Despite over a century of research on gibbons, little is known about their molecular ecology. While our understanding of gibbon phylogenetics and phylogeography has improved substantially in the past two decades (Carbone et al., 2014, 2023; Chatterjee, 2006; Kheng et al., 2018; Kuderna et al., 2023; Roos & Geissmann, 2001; Whittaker et al., 2007), population-level molecular studies of gibbons remain exceedingly rare. The few papers that have examined the intraspecific population genetics of gibbons have focused on groups from single sampling sites, populations of exceptionally low size, and/or comprised sample data of few individuals (Barelli et al., 2013; Bryant et al., 2016; Hu et al., 2018; Kenyon et al., 2011; Kheng et al., 2018; Lappan, 2007; Oka & Takenaka, 2001). While this research offers important insights about the molecular ecology of gibbons, fundamental aspects of their landscape and conservation genetics remain poorly known. Nonetheless, the fact that gibbons form small, often pair-bonded family groups from which individuals have been observed dispersing into neighboring groups (Bartlett, 2011), coupled with their arboreal dependance, suggests that they should be highly susceptible to the population genetic consequences of habitat fragmentation and environmental heterogeneity.
The critically endangered western black crested gibbon (Nomascus concolor) occupies a highly fragmented environment spanning the borders of China, Vietnam, and Laos, with ~1300 individuals remaining globally. The vast majority of these apes ( ~ 1200) are found in two isolated mountaintop forests in China's Yunnan Province: Mt. Wuliang ( ~ 500) and Mt. Ailao ( ~ 600) (Fan, 2017; Pengfei et al., 2020). Habitat loss and hunting have contributed to an estimated 80% decline in the population size in the last 45 years (3 generations) (Pengfei et al., 2020). As such, after centuries of ubiquitous lowland and mountainside terraced farming in Yunnan, fragments of sky-island forest provide the only remaining suitable habitat for this species (Yang et al., 2021). Extant populations of N. concolor are nearly completely isolated in bands of evergreen broadleaf forest between 1800 and 2700 m, above which the forest grades into thickets of rhododendrons, bamboo, and meadows—potential barriers to gibbon dispersal, restricting gene flow within populations (Jiang et al., 2006; Liu et al., 2009; Yang et al., 2021).
This pattern of large-scale fragmentation between populations and fine-grained landscape barriers within them provides an optimal setting to examine the landscape genetics and population structure of gibbons. However, the ecology and behavior of gibbons present challenges to a traditional landscape genetic analysis. Gibbons form small groups of related individuals, have been observed dispersing into neighboring social groups, and are extremely difficult to sample genetically (Bartlett, 2011; Orkin et al., 2016). As a result, an adequate collection of specimens for population genetic analysis will likely rely on an overabundance of related individuals that are unevenly distributed. Furthermore, the potentially high degree of inbreeding at some sites (Hu et al., 2018) could confound pairwise estimates of relatedness, resulting in the unwarranted removal of individuals from analysis. To circumvent this problem, we test whether observable patterns of genetic connectivity in N. concolor are consistent with explicit a priori hypotheses rooted in landscape ecology (see Methods). While spurious population genetic subdivision can result from sampling bias, it is unlikely that such a nonrandom distribution of gametes would emerge and be consistent with multiple a priori hypotheses about how those genetic discontinuities should be distributed. As such, by assembling the largest geo-referenced collection of genetic samples from free-ranging gibbons to date, we: 1) conduct the first multi-site landscape genetic study of a gibbon species; 2) provide evidence that gene flow in western black crested gibbons is strongly affected by environmental heterogeneity; and 3) demonstrate that their system of mating could be facilitating higher than expected levels of genetic diversity. Our results provide a crucial understanding of how gibbons are dispersing through complex, heterogeneous environments with direct relevance to small ape conservation.
2 METHODS
All methods were carried out in accordance with the Principles for Ethical Treatment of Nonhuman Primates set forth by The American Society of Primatologists and the guidelines of the Institutional Animal Care and Use Committee (IACUC) of Washington University in St. Louis. All experimental protocols were approved by the Washington University in St. Louis Animal Studies Committee (A-3381-01 20090204). Permits to collect fecal samples were issued by the Yunnan Forestry Department (云林 保护许准55[2009]号).
2.1 Species distribution and study area
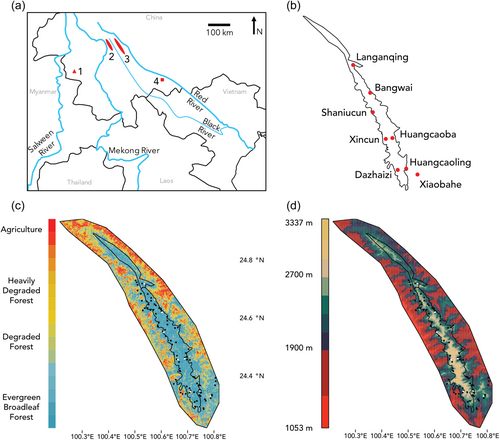
Previous research has proposed four putative subspecies of N. concolor corresponding to riverine boundaries (Figure 1a): N. concolor furvogaster, between the Salween and Mekong Rivers in China; N. concolor jingdongensis between the Mekong and Black Rivers in China; N. concolor concolor between the Black and Red Rivers in China and Vietnam; and N. concolor lu, southeast of the Mekong River in northwestern Laos (Fan, 2017). We collected samples from all four remaining populations of N. concolor in Yunnan, China, accounting for three of the four putative subspecies (Table 1). Samples of N. concolor furvogaster were collected from Mt. Yongde Daxueshan (hereafter Mt. Yongde); N. concolor jingdongensis from Mt. Wuliang, and N. concolor concolor from Mt. Ailao and a forest fragment near Bajiaohe village.
| Putative Subspecies | Region | Site | Barcoded N. concolor | Unique Individuals |
|---|---|---|---|---|
| Nomascus concolor jingdongensis | Mt. Wuliang | Bangwai | 12 | 4 |
| Dazhaizi | 11 | 10 | ||
| Huangcaoba | 4 | 2 | ||
| Huangcaoling | 14 | 7 | ||
| Langanqing | 1 | 1 | ||
| Shaniucun | 16 | 5 | ||
| Xiaobahe | 18 | 6 | ||
| Xincun | 17 | 3 | ||
| Nomascus concolor concolor | Mt. Ailao | Pinghe | 2 | 2 |
| Xujiaba | 2 | 2 | ||
| Bajiaohe | Bajiaohe | 3 | 3 | |
| Nomascus concolor furvogaster | Mt. Yongde | Mt. Yongde | 3 | 2 |
| Nomascus concolor (TOTAL) | 103 | 47 | ||
Each of the three mountain sites is an isolated sky-island forest fragment, wherein gibbon habitat is composed primarily of evergreen broadleaf forest (Fan, 2017). Mt. Wuliang is located immediately between the Mekong and Black rivers in central Yunnan, and the Wuliangshan National Nature Reserve (WNNR) is established at elevations roughly > ~ 2200 m. WNNR is about 85 km in length with a variable width (generally 5–15 km), totaling 30,938 ha. The vast majority of the ~ 500 gibbons inhabiting Mt. Wuliang reside within WNNR. Mt. Ailao is located immediately between the Red and Black rivers in central Yunnan. ~ 600 gibbons are believed to reside there, predominantly in the Ailaoshan National Nature Reserve, although they have not been as well studied as those from Mt. Wuliang. Mt. Yongde is in southwestern Yunnan, wherein the only confirmed population of N. concolor furgovaster remains (14 individuals). This is the only Nomascus population known west of the Mekong River. Bajiaohe is an isolated, unprotected forest fragment of about 30 ha located in southeastern Yunnan (Jinping County) about 30 km south of the Red River. Only four individuals remained (two groups) during our sampling, which according to interviews with local elders had been isolated from other gibbons for at least 80 years (Ni et al., 2014).
Additionally, we targeted a broad cross-section of sites within Mt. Wuliang for landscape genetic analysis (Figure 1b). To maximize effect of the high-altitude central mountain ridge, we collected samples from three sites on the western slope (Dazhaizi, Xincun, Shaniucun), and four on the eastern slope (Huangcaoling, Huangcaoba, Bangwai, and Langanqing). Sites were evenly dispersed from north to south. Additionally, we sampled an area of minimally disturbed forest (Xiaobahe) outside the reserve in the southeast of Mt. Wuliang that it is isolated by a 2 km wide gap from an area of degraded forest and pasture.
2.2 Landscape genetic hypotheses
- 1.
Within WNNR, areas of genetic discontinuity will correspond to high-altitude forest structure barriers
- a.
Pairwise genetic distances among gibbons will correspond to the distribution of low-altitude evergreen broadleaf forest, not isolation by distance.
- b.
Population clustering will be more evident in the south of WNNR where high-altitude forest structure barriers are more abundant than in the north.
- a.
- 2.
Within Mt. Wuliang, areas of genetic discontinuity will also correspond to regions with high levels of anthropogenically degraded forest.
- a.
Xiaobahe will form a cluster separate from the main population in WNNR.
- a.
- 3.
Subdivision across the four N. concolor populations will correspond to river boundaries and match the proposed subspecies taxonomy
- a.
We expect to identify three population clusters: 1) Mt. Wuliang, 2) Mt. Yongde, and 3) Mt. Ailao and Bajiaohe.
- a.
2.3 Sample collection and molecular lab work
We collected 137 DNA-confirmed primate fecal samples with the aid of our scat detection dog team, 71 of which were from N. concolor (Orkin et al., 2016). Our scat detection dog, Pinkerton, was trained by the Kunming Police Dog Training Base of the Chinese Ministry of Public Security and handled by JDO, as described elsewhere (Orkin et al., 2016). Combined with samples collected from habituated individuals, we obtained a total of 47 unique individuals from known territories in four regions across Yunnan (Figure 1, Table 1). Details of fecal sample identification, molecular lab work, and the selection of 15 polymorphic microsatellite markers are provided in the Supporting Information S1, Supporting Information S2: Tables S1–3, and Supporting Information S3: Figures S1.
2.4 Population structure
Population subdivision was identified using STRUCTURE (Pritchard et al., 2000) and DAPC (Jombart et al., 2010). 10 iterations of STRUCTURE were run for each value of K [1,8], each with 2 million iterations and discarding 10% of those as burn-in, using admixture and LOCPRIOR models with correlated allele frequencies. The effects of uneven sampling were accounted for in two ways. K was determined using the cross-validation error method of Puechmaille (2016) with StructureSelector (Li & Liu, 2018) (Supporting Information S3: Figures S2,3). Because STRUCTURE can be sensitive to the effects of related individuals and uneven sampling, we also ran a discriminant analysis of principle components with DAPC, using ADEGENET in R (Jombart et al., 2010). We used sampling site membership as a known group to inform cluster identity. Both within the Mt. Wuliang population and across all populations in Yunnan, the number of retained principal components was determined with the a-score approach (six in Mt. Wuliang and eight across Yunnan; Supporting Information S3: Figure S4).
Weir and Cockerham's (1984) Fst (the inbreeding coefficient of subpopulations relative to the total population) was calculated among the subpopulations identified by STRUCTURE both within Mt. Wuliang and across Yunnan using an analysis of molecular variance (AMOVA) in Genodive (Meirmans & Van Tienderen, 2004) with 10,000 permutations to test for significance. Pairwise Fst values were generated between all subpopulations and all sampled sites, independent of the assignment algorithm, with Genalex 6.5 (Peakall & Smouse, 2006, 2012) and FDR corrected.
2.5 Detection of migration
GeneClass2 was used to detect first generation migrants by identifying if the multilocus genotypes of individuals are misaligned with the allele frequencies of given populations (Paetkau et al., 2004; Piry et al., 2004). First, GeneClass2 was used to assign individuals to a single population by probabilistically excluding them from membership in other populations through random permutations of the data set (Paetkau et al., 2004). Second, we compared an individual's likelihood of inclusion in its home population against the ratio of the likelihood of its inclusion in the home population to the likelihood of its inclusion in the second most likely population (Lhome and Lhome/Lmax) (Paetkau & Strobeck, 1995; Paetkau et al., 2004; Piry et al., 2004). Each inference was permuted 10,000 times.
2.6 Population history and genetic diversity
Within-population and global heterozygosities (observed and expected) were calculated for each locus with Genodive (Meirmans & Van Tienderen, 2004) using Nei's (1987) heterozygosities and G-statistics. We used a rarefaction procedure (implemented in HP-RARE (Kalinowski, 2005)) to generate a randomized measure of allelic variation among our sampling sites that was not influenced by variable sampling depth. In this case, we selected four random alleles from each site, which corresponded to the 2n number of the smallest number of individuals per site in our data set. While this remains a low number of alleles, we include the result to demonstrate that patterns of inter-site genetic diversity remain consistent in a rarefied data set. Both within Mt. Wuliang and across Yunnan, the system of mating inbreeding coefficient (the correlation of gametes within a population due to inbreeding, which is the within population deviation from Hardy-Weinberg equilibrium) was measured at each locus with Fisher's exact tests of Weir and Cockerham's (1984) Fis using Genepop 4.2 (Rousset, 2008).
Single generation inbreeding effective population sizes (Ne) from the Mt. Wuliang population and the broader distribution of N. concolor across Yunnan were inferred through linkage disequilibrium (Waples & Do, 2008) in NeEstimator v2 (Do et al., 2014). Minimum and maximum values were recorded to establish a range of potential effective population sizes. Because the mating system of these gibbons is closer to monogamy than random mating, we only used the former model of the linkage disequilibrium estimation in NeEstimator.
BOTTLENECK (Piry et al., 1999) was used to detect the presence of excess heterozygosity relative to allelic number in the data sets with one-tailed Wilcoxon signed rank tests and variable rates of non-stepwise mutation. To account for the fact that the microsatellite mutation mode and rate in gibbons is unknown, BOTTLENECK was parameterized with the following options: Infinite alleles model (IAM) (0% stepwise), relaxed TPM (70% stepwise), and strict TPM (95% stepwise). The TPM variance was set to 12% (Peery et al., 2012; Piry et al., 1999). Because statistical tests from BOTTLENECK often suffer from low power (Peery et al., 2012), a second bottleneck test using the program M_P_val (Garza & Williamson, 2001), that examines M ratios (number of alleles: range of alleles) was employed. During a reduction in population size, the M ratio should decline, because genetic drift will reduce the total number of alleles more quickly than it will the range of alleles (Garza & Williamson, 2001). We set the average size of non-one-step mutations (Δg) to 2.8, the microsatellite mutation rate (μ) to 5 × 10−4 (Garza & Williamson, 2001), and used a variable proportion of stepwise mutations as above. For each M_P_val model, the minimum and maximum effective population sizes generated in NeEstimator plus Ne of 500 (θ = 1), 1,000 (θ = 2), and 10,000 (θ = 20) were run to account for a broad range of possible pre-decline Ne.
2.7 Landscape genetics of Mt. Wuliang gibbons
To identify areas of genetic discontinuity in the Mt. Wuliang gibbon population that could be associated with landscape features, both within the nature reserve and across the entire population, we generated an estimated effective migration surface using EEMS (Petkova et al., 2016). EEMS is adept at identifying genetic discontinuities in populations where gene flow otherwise fits an isolation by distance model (Petkova et al., 2016), and as such is well suited to the long and narrow geographic profile of the gibbon population in Mt. Wuliang. We parameterized EEMS with default settings and a moderate grid density (nDemes = 400), using the geographic coordinates of each sampling site, a polygon enclosing the external geographic limits of Mt. Wuliang, and a matrix of average pairwise genetic distances.
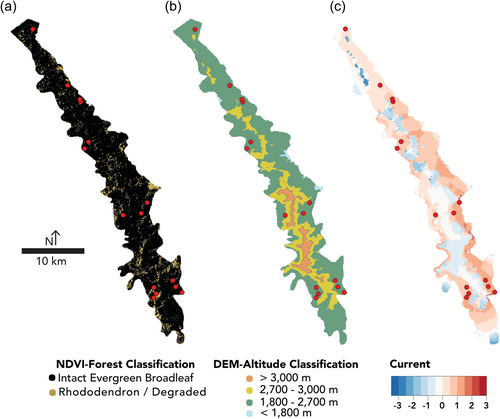
To test if gene flow among gibbon groups is restricted by high altitude rhododendron forests, we ran spatially explicit models of landscape connectivity using least cost path and isolation by resistance modelling. First, we generated raster maps of forest structure and altitude (as a proxy of forest structure) in the WNNR. Forest structure within WNNR was classified using a normalized differential vegetation index (NDVI) and altitude. First, LANDSAT 8 multispectral imagery (bands four and three) of Yunnan from 2013 (corresponding to the gibbon fecal sampling date) was downloaded from the United States Geological Survey (USGS) and imported into ArcMap 10.2 (ESRI). A supervised classification was generated in ArcMap by importing georeferenced coordinates collected from observed vegetation categories using high resolution SPOT imagery available from Google Earth. 100 sample coordinates of evergreen broadleaf forest, degraded forest, barren land, farmland, and urban zones were collected. Based upon these sample points of known vegetation, we used a maximum likelihood model to predict the regions of each landscape category throughout Yunnan province. The resulting classified imagery yielded a map of available preferred habitat (evergreen broadleaf forest), potential barriers (degraded and rhododendron forests), and presumed barriers (farms and urban areas) for Yunnan's primates. Secondly, a digital elevation model (DEM) was generated from SRTM imagery, from which the altitude of each georeferenced map pixel was obtained. Forest structure was classified by altitude based upon existing botanical knowledge (Fan et al., 2009; Jiang et al., 2006; Shi & Zhu, 2009), with altitude being a proxy for the same forest boundaries identified in the NDVI. The boundary between rhododendron forest (high altitude) and evergreen broadleaf forest (moderate altitude) was set at 2700 m within the WNNR. The lower limit of the evergreen broadleaf forest was set to 1800 m, which was almost entirely excluded from the nature reserve (Figure 2). Habitat within WNNR was merged into binary preferred and non-preferred habitat types based on landscape classifications (<2700 m vs >2700 m; intact forest vs degraded forest) to account for fine-grained local variation between pixels resulting from differences in slope, aspect, altitude, etc. in an effort to increase discriminatory power. In both cases, friction maps were generated by reclassifying pixels from raster maps in R. Following Blair and Melnick (2012), six arbitrary resistance categories were chosen for non-preferred habitat to account for sensitivity: 10, 50, 100, 1000, 5000, and 10,000. Preferred habitat was classified with a resistance of one.
Geographic distance matrices were constructed using pairwise distances (Euclidian, least-cost, and resistance) between individuals (i.e. the GPS coordinates of gibbon fecal collection sites). To account for slight irregularities between “crow's flight” distances and the external shape of WNNR, constrained Euclidian distances were recalculated as least-cost path distances within a raster map of WNNR with no resistances to exclude the effects of anthropogenic disruptions shaping the edge of the nature reserve. Least-cost path distances between all pairs of sampled individuals within each friction map were generated using the costDistance function in the gdistance package (van Etten & Hijmans, 2010) in R. To account for the effect of multiple gene flow pathways (i.e. multiple dispersal pathways), isolation by resistance modeling was performed in Circuitscape 4.0 (McRae et al., 2008). Resistances were iterated across all pairwise focal nodes and raster cells were connected with eight neighbors. All geographic distance matrices were log-transformed to control for a two-dimensional habitat (McRae & McRae, 2006).
A matrix of Rousset's â pairwise genetic distances between all pairs of individuals was constructed in SPAGeDI v1.3 (Hardy & Vekemans, 2002). Significant associations between genetic distance and landscape effects were assessed with Clarke's maximum likelihood population effects model (MLPE) (Clarke et al., 2002), following (Jha, 2015). MLPE was run with the corMLPE package in R (https://github.com/nspope/corMLPE). ∆AIC scores were ranked separately for each of four categories of cost models: least-cost altitude, least-cost NDVI, resistance altitude, and resistance NDVI.
3 RESULTS
3.1 Population structure
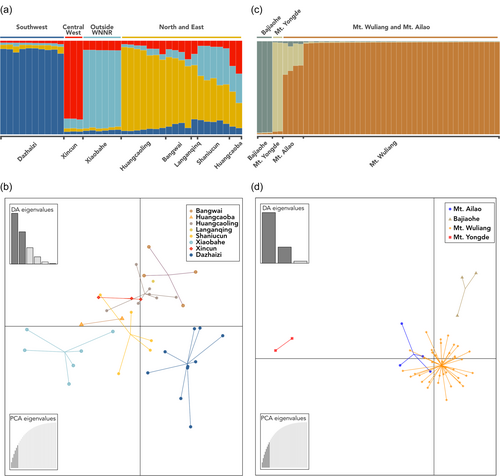
STRUCTURE inferred four population clusters in Mt. Wuliang corresponding to the following geographic areas: 1) the southwest (Dazhaizi), 2) the central west (Xincun), 3) a long area of connectivity including the north and east (Huangcaoling, Huangcaoba, Bangwai, Shaniucun, and Langanqing); and 4) the area outside the nature reserve in the southeast (Xiaobahe) (Figure 3a). Within the third population cluster, the sampling sites of Shaniucun and Huangcaoba appear to show more admixed ancestry than do the others. DAPC largely confirmed these results and correctly reproduced the geographic position of these four clusters along the long axis of Mt. Wuliang, although it overlapped Xincun with the larger northern/eastern cluster. Significant population structure as identified by Fst with AMOVA was found within Mt. Wuliang (Fst = 0.085) (Table 2). Additionally, we identified significant pairwise Fst values between all STRUCTURE identified subpopulations within Mt. Wuliang (Table 3). Fst was highest between the central western population (Xincun) and all three other subpopulations (0.124−0.177). Fst values among the other subpopulations ranged from 0.052 to 0.090, with stronger subdivision between the subpopulations inside WNNR and the external subpopulation (Xiaobahe). The inbreeding coefficient (Fis) was negative and significant within Mt. Wuliang (Fis = −0.121).
| % Variance | Std. Dev | Value | 95% CI | P-value | ||
|---|---|---|---|---|---|---|
| Yunnan | ||||||
| Within individuals | 0.948 | 0.035 | Fit | 0.052 | −0.012 – 0.122 | -- |
| Among individuals | −0.051 | 0.021 | Fis | −0.057 | −0.095 – −0.015 | 0.998* |
| Among populations | 0.102 | 0.027 | Fst | 0.102 | 0.057 – 0.158 | 0.000 |
| Mt. Wuliang | ||||||
| Within individuals | 1.026 | 0.021 | Fit | −0.026 | −0.067 – −0.013 | -- |
| Among individuals | −0.111 | 0.018 | Fis | −0.121 | −0.158 – −0.087 | 1.000* |
| Among populations | 0.085 | 0.012 | Fst | 0.085 | 0.062 – 0.107 | 0.000 |
- Note. Significant values (p < 0.05) for each data set are in bold. * Test of Fis value is one-sided, thus the corrected p-value for a negative Fis value would be (1 - p).
- Abbreviations: Fis, individual inbreeding coefficient relative to the subpopulations; Fit, individual inbreeding coefficient relative to the total population; Fis, individual inbreeding coefficient relative to the subpopulation; Fst, inbreeding coefficient of subpopulations relative to the total population.
| Within Wuliang | ||||
|---|---|---|---|---|
| Southwest | Central West | North & East | Outside Reserve | |
| Southwest | -- | 0.129 | 0.052 | 0.090 |
| Central West | 0.0000 | -- | 0.124 | 0.177 |
| North & East | 0.0000 | 0.0000 | -- | 0.078 |
| Outside Reserve | 0.0000 | 0.0000 | 0.0000 | -- |
| Across Yunnan | ||||
|---|---|---|---|---|
| Mt. Wuliang | Mt. Ailao | Mt. Yongde | Bajiaohe | |
| Mt. Wuliang | -- | 0.011 | 0.169 | 0.164 |
| Mt. Ailao | 0.2167 | -- | 0.176 | 0.137 |
| Mt. Yongde | 0.0003 | 0.0110 | -- | 0.274 |
| Bajiaohe | 0.0003 | 0.0110 | 0.0110 | -- |
- Note. Fst values are above the diagonal and false discovery rate-corrected p-values are below it. Significant values (p < 0.05) for each data set are in bold.
Across Yunnan, STRUCTURE identified the individuals from Bajiaohe and Mt. Yongde as internally distinct clusters; however, it also clustered the Mt. Ailao and Mt. Wuliang individuals together (Figure 3b). DAPC produced a similar pattern, except that it placed Mt. Yongde as substantially separate from the other three sampling sites, which is more consistent with their geographic separation. Population clustering results were confirmed by the identification of significant population structure across Yunnan with an AMOVA (Fst = 0.102) (Table 2). Significant pairwise Fst values were found in four of six cases (Table 3). The Mt. Wuliang population differed significantly from Yongde (Fst = 0.169), and Bajiaohe (Fst = 0.164), but not from Mt. Ailao (Fst = 0.011). Bajiaohe also differed significantly from Mt. Ailao and Mt. Yongde (Fst = 0.137, 0.274, respectively). Mt. Yongde also significantly differed from Mt. Ailao (Fst = 0.176). The inbreeding coefficient (Fis) was negative and significant across Yunnan (Fis = −0.057). Because permutation based significance tests run in an AMOVA use one-tailed p-values, testing for the probability that each F-statistic is greater than the random distribution of permuted values, the p-values for Fis in Mt. Wuliang (p = 1.000) and across Yunnan (p = 0.998) do not correctly test the significance of a negative value, which should be tested as the probability of the value being smaller than the permuted values. Thus, comparison to the 95% confidence intervals obtained by bootstrapping over all loci are more justifiable and indicate that Fis is significantly negative.
3.2 Genetic diversity
The number of alleles, allelic richness, and Nei's (1978) indices of genetic diversity within and across populations are presented in Table 4. Within Mt. Wuliang we observed heterozygosity (Ho) is highest in the north/east subpopulation, followed by the groups outside the nature reserve (Xiaobahe), the central west (Xincun), and the southwest (Dazhaizi). In contrast, expected heterozygosities (uHe) are highest in the north/east subpopulation, followed by the southwest (Dazhaizi), outside the nature reserve (Xiaobahe), and the central west (Xincun). Rarefied allelic richness (Ar) and both the absolute and effective number of alleles follow the same pattern as the expected heterozygosity. Nei's inbreeding coefficients (Gis) are all negative, indicating a mating system that avoids inbreeding that is most strong in the central west (−0.320) and outside the reserve (−0.197), followed by the north/east (−0.106) and southwest (−0.080).
| Indiv. | Alleles | Effective Alleles | Ar | Ho | uHe | Hs | Ht | H't | Gis | Gis (p) | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Mt. Wuliang | 38 | 5.533 | 2.415 | -- | 0.708 | 0.569 | 0.611 | 0.672 | 0.692 | −0.159 | 0.000 |
| North & East | 19 | 4.733 | 3.777 | 3.08 | 0.739 | 0.653 | 0.668 | −0.106 | |||
| Dazhaizi | 10 | 3.800 | 2.687 | 2.79 | 0.661 | 0.582 | 0.612 | −0.080 | |||
| Xiaobahe | 6 | 3.333 | 2.607 | 2.75 | 0.722 | 0.561 | 0.603 | −0.197 | |||
| Xincun | 3 | 2.467 | 2.097 | 2.47 | 0.711 | 0.478 | 0.539 | −0.320 | |||
| Yunnan | 47 | 5.933 | 1.960 | -- | 0.579 | 0.530 | 0.536 | 0.631 | 0.662 | −0.081 | 0.015 |
| Mt. Wuliang | 38 | 5.533 | 3.352 | 2.54 | 0.712 | 0.675 | 0.674 | −0.056 | |||
| Mt. Ailao | 4 | 3.000 | 2.620 | 2.41 | 0.683 | 0.637 | 0.628 | −0.088 | |||
| Bajiaohe | 3 | 2.000 | 1.795 | 1.90 | 0.589 | 0.473 | 0.433 | −0.359 | |||
| Mt. Yongde | 2 | 1.667 | 1.516 | 1.67 | 0.333 | 0.333 | 0.333 | -- |
- Note. P-values generated from 10,000 permutations. Population subdivisions within Wuliang Mountain a not accounted for in the Yunnan-wide test.
- Abbreviations: Ar, allelic richness; Gis, inbreeding coefficient; Gis p, p-value of; Ho, observed heterozygosity; Hs, expected heterozygosity within a subpopulation; Ht, expected total heterozygosity; H't, corrected total heterozygosity; uHe, unbiased expected heterozygosity.
Across Yunnan, the Mt. Ailao and Mt. Wuliang groups have roughly the same observed and expected heterozygosities, with the former slightly higher, and the observed and expected heterozygosities within Bajiaohe and Mt. Yongde substantially lower (Table 4). All three allelic diversity metrics follow the same pattern, except that Mt. Wuliang has substantially more alleles (absolute, not effective), which is likely a response to the substantially larger sample size. Again, Nei's inbreeding coefficients (Gis) are all negative, indicating avoidance of inbreeding. The Bajiaohe group is strongest (−0.359), followed by Mt. Ailao (−0.088) and Mt. Wuliang (−0.046).
3.3 Detection of first-generation migrants
GeneClass2 assigned all 38 individuals from Mt. Wuliang to their subpopulation of origin (Supporting Information S2: Table S4), indicating that no individual is likely to have migrated between the four subpopulations identified by STRUCTURE. When testing for first generation migrants moving from one sampling site to another within a subpopulation, four individuals were identified as having a significant probability of migration (Lhome/Lmax; p < 0.01). Each first-generation migrant was classified as having moved between sites in the North/East cluster (HCB03 from Huangcaoling to Huangcaoba, HCB22 from Huangcaoling to Huangcaoba, HCL12 from Bangwai to Huangcaoling, and LGQ01 from Bangwai to Langanqing).
3.4 Population history and effective population size
Effective population sizes modeled in NeEstimator were higher than sample sizes of the census populations in both the Mt. Wuliang population (LDNe monogamy: 72.0 (53.9–101.9)) and the broader trans-Yunnan sample LDNe monogamy: 88.8 (66.9–125.0)). Effective population size estimates higher than the census size of the sampled individuals are consistent with declining populations of N. concolor both in Mt. Wuliang and across Yunnan (Waples, 2005).
The two bottleneck tests (BOTTLENECK and M_P_val) produced conflicting results (Supporting Information S1: Table S5). BOTTLENECK only found significant (p < 0.05) excess of heterozygosity relative to the number of alleles when the infinite alleles model was employed. Significant relationships were not found under the more realistic TPM models or the stepwise model. A mode shift in the distribution of rare alleles was not observed in either data set. On the other hand, M_P_val identified M ratios (number of alleles:range in alleles) that were beneath critical values in both the TPM-strict (95% stepwise) and stepwise models. M values were below the critical M values (indicating significant support for a bottleneck) for all tested Ne values under 10000 across Yunnan and under 10000 (SSM) and 1000 (TPMs) for the Mt. Wuliang population (Supporting Information S2: Table S5).
3.5 Landscape genetics of Mt. Wuliang gibbons
We identified a strong signal of landscape effects biasing geneflow in the Mt. Wuliang gibbon population. We observed significant correlations between geographic distances (resistance and least-cost path) and genetic distance (Rousset's â) within WNNR that are consistent with our hypotheses (Table 5). Overall, the DEM altitudinal classification models outperformed the NDVI-forest structure classification models, although both generated significant correlations. Among the DEM altitudinal classification models, the lowest AIC values occurred when we classified altitudes above 2700 m with a friction of 1000 for both LCP (r2 = 0.197) and resistance models (r2 = 0.199), although ∆AIC values < 2 were also identified for friction values of 500 (LCP) and 500 and 5000 (resistance). For the NDVI-forest structure classification models, ∆AIC values < 2 were only observed for resistance models with friction values of 500 (r2 = 0.116) and 1000 (r2 = 0.118) assigned to rhododendron forest. With the exception of the NDVI rhododendron model generated from Circuitscape, resistances models outperformed the null model of Euclidian distance alone.
| Model | Model structure | Geographic distance | Landscape cost | Cost value | df | r2 | AIC | ∆AIC |
|---|---|---|---|---|---|---|---|---|
| 1 | Genetic Distance ~ Least Cost Path Distance + Euclidian Distance, correlation = corMLPE(form = ~row+col) | Least Cost Path | High Altitude ( >2700 m) | 0 | 4 | 0.015 | −1016.803 | 53.756 |
| 2 | 10 | 5 | 0.075 | −1043.534 | 27.025 | |||
| 3 | 50 | 5 | 0.097 | −1051.458 | 19.101 | |||
| 4 | 100 | 5 | 0.114 | −1055.911 | 14.648 | |||
| 5 | 500 | 5 | 0.173 | −1068.615 | 1.944 | |||
| 6 | 1000 | 5 | 0.197 | −1070.559 | 0.000 | |||
| 7 | 5000 | 5 | 0.219 | −1067.354 | 3.205 | |||
| 8 | 10000 | 5 | 0.219 | −1065.134 | 5.425 | |||
| 9 | Genetic Distance ~ Resistance Distance + Euclidian Distance, correlation = corMLPE(form = ~row+col) | Resistance | High Altitude ( >2700 m) | 0 | 4 | 0.013 | −1015.116 | 75.738 |
| 10 | 10 | 5 | 0.181 | −1083.623 | 7.231 | |||
| 11 | 50 | 5 | 0.167 | −1085.034 | 5.820 | |||
| 12 | 100 | 5 | 0.166 | −1085.894 | 4.960 | |||
| 13 | 500 | 5 | 0.186 | −1089.68 | 1.174 | |||
| 14 | 1000 | 5 | 0.199 | −1090.854 | 0.000 | |||
| 15 | 5000 | 5 | 0.228 | −1089.989 | 0.865 | |||
| 16 | 10000 | 5 | 0.237 | −1088.29 | 2.564 | |||
| 17 | Genetic Distance ~ Least Cost Path Distance + Euclidian Distance, correlation = corMLPE(form = ~row+col) | Least Cost Path | Rhododendron and Degraded Forest (NDVI Classification) | 0 | 4 | 0.102 | −1515.808 | 0.000 |
| 18 | 10 | 5 | 0.100 | −1515.648 | 0.160 | |||
| 19 | 50 | 5 | 0.100 | −1515.278 | 0.530 | |||
| 20 | 100 | 5 | 0.100 | −1515.087 | 0.721 | |||
| 21 | 500 | 5 | 0.099 | −1514.786 | 1.022 | |||
| 22 | 1000 | 5 | 0.099 | −1514.739 | 1.069 | |||
| 23 | 5000 | 5 | 0.099 | −1514.817 | 0.991 | |||
| 24 | 10000 | 5 | 0.099 | −1514.878 | 0.930 | |||
| 25 | Genetic Distance ~ Resistance Distance + Euclidian Distance, correlation = corMLPE(form = ~row+col) | Resistance | Rhododendron and Degraded Forest (NDVI Classification) | 0 | 4 | 0.004 | −1432.497 | 73.204 |
| 26 | 10 | 5 | 0.069 | −1479.718 | 25.983 | |||
| 27 | 50 | 5 | 0.095 | −1499.185 | 6.516 | |||
| 28 | 100 | 5 | 0.104 | −1502.74 | 2.961 | |||
| 29 | 500 | 5 | 0.116 | −1505.701 | 0.000 | |||
| 30 | 1000 | 5 | 0.118 | −1505.382 | 0.319 | |||
| 31 | 5000 | 5 | 0.116 | −1502.543 | 3.158 | |||
| 32 | 10000 | 5 | 0.113 | −1500.944 | 4.757 |
- Note: Best-fitting alternative models (those with ΔAIC values < 2) are indicated in bold.
- Abbreviations: AIC, Akaike's information criterion; MLPE, maximum likelihood population effects.
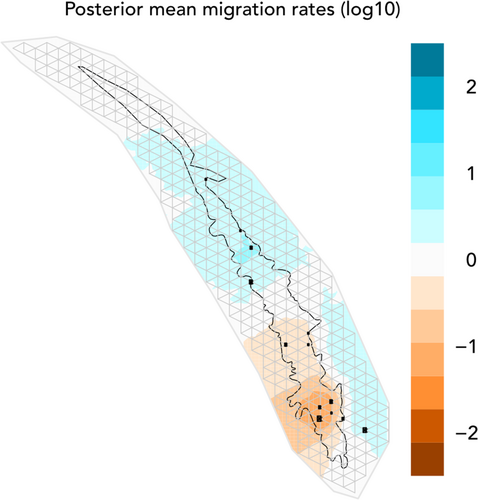
The estimated effective migration surface generated by EEMS reveals a clear area of restricted gene flow in the southern portion of Mt. Wuliang (Figure 4). The posterior mean migration rate is substantially lower in the high-altitude southern region of Mt. Wuliang (Dazhaizi, Xincun, Huangcaoling, and Huangcaoba) than in the lower altitude northern region of the mountain (Shaniucun, Bangwai, Langanqing), which is more connected by evergreen broadleaf forest. The site outside the nature reserve, Xiaobahe, is isolated from both regions by an area of low connectivity.
4 DISCUSSION
Our results demonstrate that both anthropogenic and natural landscape barriers substantially restrict dispersal and gene flow in western black crested gibbons. Landscape connectivity modelling (isolation by resistance, least-cost paths, and EEMS), Fst, and population assignment algorithms (STRUCTURE and DAPC) all indicate that not only is population subdivision present among the gibbons of Mt. Wuliang, but that it corresponds with the variable distribution of evergreen broadleaf forest. Overall molecular results are consistent with N. concolor being a locally declining population that has maintained higher than expected levels of genetic diversity through a system of mating that actively avoids inbreeding. While these results must be taken cautiously because of small sample sizes outside Mt. Wuliang, the pattern is consistent with demographic, behavioral, and a priori genetic expectations. We suggest that the innate biological characteristics of gibbons—their arboreally dependent locomotion, dispersal behavior, and social dynamics—have interacted with the variable forest structure within Mt. Wuliang and the anthropogenic deforestation surrounding the nature reserve to rapidly produce population subdivision.
4.1 Landscape genetics of gibbons in Mt. Wuliang
Terraced farming along the slopes of Mt. Wuliang—practiced by local people for decades if not centuries—has removed nearly all suitable forest below the nature reserve. As such, anthropogenic deforestation along the edges of the nature reserve likely acts as an external barrier. Simultaneously, behavioral observations indicate that the gibbons of Mt. Wuliang avoid travelling through and feeding in the rhododendron forest that is dominant above ~2700 m (Fan & Jiang, 2010; Fan et al., 2009; Fei et al., 2012; Jiang et al., 2006). However, it has remained uncertain if gibbons are able to maneuver through these high-altitude thickets of rhododendrons over the long distances necessary for intergroup dispersal, resulting in a patchwork of internal barriers. Our results indicate that while there are areas of broad connectivity within Mt. Wuliang, in others, gibbons appear penned into pockets of suitable habitat, restricted from dispersing to other sites.
First, consistent with landscape hypotheses 1A and 1B, our results suggest that these high-altitude areas, often dominated by rhododendrons, bamboo thickets, and meadows are restricting gene flow between groups of gibbons within Mt. Wuliang. Least-cost path and resistance modeling demonstrate that pairwise genetic distances among individuals correlate with pathways around both high-altitude regions and rhododendron forest, which indicates that gibbons preferentially avoid dispersing through these areas of low-quality forest (Hypothesis 1A). Second, the arrangement of population clusters in Mt. Wuliang and the pattern of migration among them correspond with altitude and forest structure (Hypothesis 1B). In the high-altitude south, Xincun, Dazhaizi, and Huangcaoling are three of the more geographically proximate sites in Mt. Wuliang, yet each belongs to a different subpopulation. In contrast, the lower altitude northern sites of Shaniucun, Bangwai, Raomalu, and Langanqing are equivalently far away from each other yet belong to a single subpopulation. Given that the gibbon groups from Xincun and Dazhaizi are nearly surrounded by areas of high-altitude, there is a clear causal explanation for why these two populations to the exclusion of others would be isolated from the rest of the Mt. Wuliang population. Furthermore, because EEMS reconstructed an estimated effective migration surface in Mt. Wuliang without the use of forest structural or altitudinal data, it provides strong, independent support for our landscape hypotheses. This is borne out by the placement of the four sites in the high altitude south—Dazhaizi, Xincun, Huangcaoling, and Huangcaoba—in a defined region with the lowest posterior mean migration rate (Figure 4). Additionally, there is little evidence to suggest that gibbons are migrating between subpopulations, as evidenced by the lack of first-generation migrants from GENECLASS2 (all of which were identified within the north/east subpopulation), which accords with an avoidance of high-altitude and low-quality forest. Third, consistent with landscape hypothesis 2, we identified population subdivision that is occurring in response to human landscape modification across the broader Mt. Wuliang population. Xiaobahe has been isolated from WNNR by a ~ 2 km wide area of degraded forest and pasture for at least fifty years—and likely longer—given that disturbance is visible in the earliest available remote sensing data (Landsat 1 from 1974). Despite the anthropogenic timescale, these gibbons have formed a distinct population cluster and area of low diversity, suggesting that not only rhododendrons, but degraded forest more generally poses a problem for gibbon dispersal.
We suggest that because of their arboreal reliance of brachiation, gibbons are ineffective dispersers over long ranges of degraded (either naturally or anthropogenically) habitat. While gibbons have been observed to cross short gaps in the forest, they have been described as “obligate canopy dwellers” (Cheyne et al., 2013) and are not known to engage in the kind of diverse locomotor behavior that long distance dispersal through non-preferred terrain requires (Cannon & Leighton, 1994; Das et al., 2009). Although smaller-bodied and arboreal primates seem to be at greater risk of having their gene flow restricted by anthropogenic boundaries such as deforestation, agriculture, and roads than do larger primates (Westphal et al., 2021), the specific link between primate locomotion and its genetic consequences has remained stubbornly unexplored. Despite the fact that the role of forest structure heterogeneity in changing environments has long served as the foundation for models of primate and hominid locomotor evolution, our results provide the first population genetic evidence consistent with the notion that an ape morphologically adapted for arboreal dependence could be less adept at long-distance dispersal than a terrestrial ape.
We caution that that some degree of relatedness among individuals could be biasing the amount of population structure we observed. Gibbons form small family groups, and the geographic isolation of many sites in Mt. Wuliang renders it nearly impossible to collect a representative sample that excludes all related individuals. While this is not ideal, our study examines the largest known collection of free-ranging gibbon samples and offers the best current explanation of how gibbon population structure interacts with landscape. The fact that our results are confirmed by multiple methodological techniques and are consistent with a priori expectations strengthens the likelihood of their veracity. While we acknowledge that spurious population genetic subdivision could have resulted from biases in our sampling design, it remains implausible that such a nonrandom distribution of gametes would emerge and be consistent with multiple a priori hypotheses about how those genetic discontinuities should be distributed.
4.2 Genetic diversity of N. concolor
The level of observed heterozygosity in N. concolor is higher than expected, most likely as a result of their engaging in a system of mating that actively avoids inbreeding. As expected with an excess of heterozygosity, significant negative Fis values were found across Yunnan, within Mt. Wuliang, and within each subpopulation. Negative Fis values are indicative of inbreeding avoidance at the demic level (Templeton, 2006). However, because of the small population sizes and localized isolation of groups, it is likely that N. concolor are inbred in the pedigree sense (i.e. mating regularly with related individuals (Hu et al., 2018), thereby increasing the probability of two alleles being identical by descent). These results suggest that relative to the available genetic diversity, these gibbons are mating in a way that maintains genetic diversity within the population.
An inbreeding avoidance system of mating is consistent with the behavioral ecology of Nomascus. We have observed that the habituated groups of Nomascus at Dazhaizi (N. concolor) and Bangliang (N. nasutus) avoid mating with close relatives to the extent possible, despite their small, fragmentary distributions. Secondly, while N. concolor groups typically include one adult male and one or two adult females, extrapair copulations are common, increasing paternity uncertainty (Hu et al., 2018; Huang et al., 2013). As a result, variance in male reproductive success appears low, relative to other mammals and primates (Storz, 1999). Finally, it remains unclear if the dispersal pattern of N. concolor contributes to their higher-than-expected level of heterozygosity (and thus negative Fis). Both sexes of N. concolor disperse from their natal groups, which should reduce mating among kin, but recent observations from Dazhaizi indicate that fewer females than males are dispersing. However, the generalizability of this dispersal pattern remains unclear, because of a substantial male bias in the sex ratio at the site, coupled with an increase in human disturbance (Huang et al., 2013). Nonetheless, the pattern of negative Fis and positive Fst in N. concolor could have been influenced by a combination of sex-biased dispersal, polygyny, and female philopatry (Melnick & Hoelzer, 1992; Pope, 1992). Male-biased dispersal has been observed in other gibbons (Hylobates lar (Brockelman et al., 1998)), and negative Fis has also been found among the congeneric N. hainanus, which form large, polygynous social groups (Bryant et al., 2016) (and possibly also found in H. moloch (Kheng et al., 2018), but see (Nijman et al., 2019)). Because observed dispersal events are known only from a few groups of N. concolor, social behavior could have a non-trivial effect on observed heterozygote excess and population structure. To address this issue fully, it will be necessary to conduct further behavioral observations, test for differences in inbreeding levels between sexes, and examine sex-linked genetic markers using genomic approaches.
Another possibility is that the random sampling bias of very small populations is exacerbating heterozygote excess, where the effect of inbreeding avoidance can be substantial. For example, the excess of heterozygosity in N. hainanus, comprised of 23 individuals, could stem from binomial sampling error (Bryant et al., 2016). Alternatively, among captive Speke's gazelles, negative Fis emerged rapidly and to great effect from a founding population of four individuals, when mating among related individuals was minimized (Templeton & Read, 1994). These small population effects could explain why Bajiaohe and the central western Mt. Wuliang population (Xincun) have such highly negative Gis values (−0.359 and −0.320, respectively). Given that they are two of the most geographically isolated subpopulations, it is likely that the number of available outbred mating options is limited.
The general pattern of genetic diversity in N. concolor is consistent with a declining population that is losing genetic diversity between generations. Overall, the effective population sizes (LDNe monogamy) that we estimated are substantially larger than the sample sizes from which they were generated. However, given that NeEstimator assumes discrete generations and constant population sizes, we caution that these could be artificially low estimates of effective population size. Nonetheless, this is most apparent in the two smallest populations (Mt. Yongde and Bajiaohe). The sampled individuals from Mt. Wuliang (n = 38/500 individuals or 8% of population) and Mt. Ailao (n = 4/600 individuals or 0.7% of population) have nearly identical heterozygosity and rarefied allelic richness, despite an order of magnitude difference in sample size (Table 3). In contrast, heterozygosity and allelic richness is substantially lower at Bajiaohe and Mt. Yongde. Given that only four gibbons remain at Bajiaohe and 14 at Mt. Yongde, the individuals analyzed here represent 75% and 14% of the populations, respectively. In light of these absolutely small populations, it is plausible that little additional genetic diversity exists from the unsampled individuals. Nonetheless, a larger sample, particularly from Mt. Ailao, is warranted for further investigation.
We did not find consistent evidence for genetic bottlenecking in N. concolor. During the past millennium, it is unlikely that N. concolor has recovered at any point during its population decline, so this is not surprising. Modifications of mutational parameters for BOTTLENECK and M Ratio resulted in significant results at opposite ends of the mutation step frequency continuum. In the absence of samples from previous generations, it is not readily apparent from genetic data if changes in effective population sizes, heterozygosity, and allelic richness have occurred recently or have remained stable over multiple generations. Genetic data from museum specimens could clarify this issue. Nonetheless, the most likely explanation is that N. concolor has been undergoing a long-term population decline and has not entered a demographic recovery phase.
4.3 Population subdivision and conservation implications in Yunnan
We identified population subdivision throughout the Yunnan population of N. concolor, but our results are inconsistent with pre-existing classifications. Scant morphological evidence has been used to suggest that three subspecies exist within Chinese N. concolor (N. c. concolor between the Red and Black rivers, N. c. jingdongensis between the Black and Mekong rivers, and N. c. furvogaster west of the Mekong River), but mitochondrial phylogenies have been ambiguous or non-monophyletic (Monda et al., 2007; Thinh et al., 2010). Our microsatellite results reflect more recent fragmentation, indicating that while the Mekong River is isolating the Mt. Yongde individuals, the Black River is not a strong geographic barrier between Mt. Wuliang and the eastern groups (Mt. Ailao and Bajiaohe). Before anthropogenic deforestation, it is likely that the Mt. Ailao gibbon population was connected to Mt. Wuliang north of the Black River's headwaters, and more broadly to the gibbons in the southeast (e.g. Bajiaohe). This arrangement suggests the centrality of the Mt. Ailao and Mt. Wuliang populations and warrants further genetic sampling of the gibbons residing in Mt. Ailao.
Given our small sample sizes outside Mt. Wuliang, we urge cautious interpretation of our results until sampling is improved. Nonetheless, because of the critical threat to gibbons in Mt. Yongde and Bajiaohe, we contend that they should be treated as separate conservation management units, apart from Mt. Wuliang and Mt. Ailao. Unfortunately, in the years since our initial sampling occurred, the Bajiaohe population has been reduced to two aged individuals who appear unable to reproduce, which likely means that this unique genetic unit has been lost. Further sampling of N. concolor in northern Vietnam (and in the remaining forests of southeastern Yunnan if any N. concolor remain there) could reveal that Bajiaohe belongs to a larger, regional population cluster, but it is possible that there isn't a literal connection to Vietnam. Improved sampling more generally could demonstrate broader genetic diversity, but in light of the critical threat of population extirpation, diversity is likely declining. It is important to note that while a broader sampling scheme across Yunnan could clarify some of these issues (Fünfstück et al., 2014), the clumped distribution of N. concolor poses a challenge. Overall, the population structure fits a pattern of genetic drift in a declining population on a recent time scale rather than a pattern of incipient speciation. Finally, the Wuliangshan National Nature Reserve should be expanded to include the unprotected forest around Xiaobahe. Given that groups sampled outside the nature reserve harbor distinct genetic variation, increasing their protected status will improve the long-term viability of western black crested gibbons. Recognition of these populations as genetically distinct management units is not only reasonable given the data presented here, but also justifies devoting conservation resources to save these populations of gibbons.
AUTHOR CONTRIBUTIONS
Joseph D. Orkin and Xuelong Jiang designed the research, Joseph D. Orkin, Nai-qing Hu, Kai He, Zhen-hua Guan, Bei Huang, Chunyan Yang, and Peng-fei Fan performed the research, and Joseph D. Orkin analyzed the data and wrote the paper. All authors reviewed the paper.
ACKNOWLEDGEMENTS
We would like to thank the Wuliangshan National Nature Reserve Management Bureaus of Jingdong and Nanjian Counties, the Ailaoshan Station for Subtropical Forest Ecosystem Studies, the Wuliang-Ailao Mountains Wildlife Observation and Research Station of Yunnan Province, and the Yongde Daxueshan National Nature Reserve Management Bureau for making this work possible. We appreciate the graciousness and hard work of our many field assistants and their families in the Wuliang and Yongde Daxue Mountains. We thank Douglas W. Yu, Shiyuan Zhao, Yuming Yang, Yinqiu Ji, Guozheng Sun, Tongmei Shu, Ni Qingyong, Yaping Zhang, Rui Min, Yun Gao, for critical assistance, support, and advice. We would also like to thank Richard Smith, Amanda Melin, Alan Templeton, Jane Phillips-Conroy, Tony Di Fiore, and Crickette Sanz for comments on an earlier draft of this paper. Joseph D. Orkin was supported by the National Science Foundation (BCS-1155904), the Leakey Foundation, The Natural Sciences and Engineering Research Council of Canada (RGPIN-2023-04399, DGECR-2023-00272), the American Philosophical Society Louis and Clark Fund for Exploration and Field Research, Lambda Alpha National Anthropology Honor Society, and Washington University in St. Louis. Xuelong Jiang was supported by the National Natural Science Foundation of China (31570386). Xuelong Jiang was also supported by the State Key Laboratory of Genetic Resources and Evolution at the Kunming Institute of Zoology.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflict of interest.




