Differences between ecological niches in northern and southern populations of Angolan black and white colobus monkeys (Colobus angolensis palliatus and Colobus angolensis sharpei) throughout Kenya and Tanzania
Abstract
Ecological niche models can be useful for clarifying relationships between environmental factors and a species’ geographic distribution. In this study, we use presence-only data and environmental layers to create an ecological niche model to better understand the distribution of the East African Angolan black and white colobus monkey, Colobus angolensis palliatus, and to assess whether the model supports considering the population as two separate subspecies, Colobus angolensis sharpei and C. a. palliatus. We found the range of the predicted distribution for suitable habitat of C. a. palliatus as currently classified to be only 12.4% of that shown in the International Union for Conservation of Nature Red List range map and to be fragmented. As C. angolensis is considered a “Least Concern” species, this difference suggests that generalized maps may lead to understating the species’ extinction risk. When presence points were divided into two previously proposed subspecies —C. a. palliatus (Kenya and Northern Tanzania) and C. a. sharpei (Southern Tanzania)—we found significant environmental differences between the distributions. The most important ecological variable for C. a. palliatus was predominantly precipitation of the driest month (69.1%) whereas for C. a. sharpei annual precipitation (44.8%) and land cover (normalized difference vegetation index, 16.4%) were the most important. When comparing suitable ranges for the separate distributions, we found only a 1.2% geographical overlap. These differences are consistent with previous subspecies delineations of C. a. palliatus and C. a. sharpei based upon morphology, pelage, and genetics. Our study suggests that extirpation of C. a. palliatus in suitable habitat areas and occurrence of this subspecies in anthropogenic environments, warrant further consideration for conservation actions.
Research Highlights
-
Ecological niche model reveals suitable habitat for C. a. palliatus in Kenya and Tanzania is 12.4% of the International Union for Conservation of Nature range map
-
Significant ecological differences exist between C. a. palliatus and C. a. sharpei populations, supporting sub-speciation
1 INTRODUCTION
Human activity and habitat decline are known to severely impact primate populations (Cowlishaw, 1999; Hall, Burgess, Lovett, Mbilinyi, & Gereau, 2009; Rovero, Mtui, Kitegile, & Nielsen, 2012). Conservation efforts can reduce or counteract some of these negative impacts, but understanding an animal's behavior, ecology, evolutionary uniqueness, and current distribution is crucial before conservation management plans can be appropriately implemented. For primate taxa affected by increasing habitat fragmentation, it is necessary to identify their ecological niche, or the range of ecological conditions necessary for them to survive and reproduce (Hutchinson, 1957).
An ecological niche describes how an organism or population responds to a distribution of resources and environmental factors. A species has a fundamental niche, representing the maximum range of ecological conditions that allow for its long-term survival, and a realized niche, which is the actual range of ecological conditions that it currently occupies (Hutchinson, 1957). Climate often determines a species’ fundamental niche, while humans (as well as geographic barriers, presence of nonsympatric species, and predation) can have a strong influence on a species’ realized niche (Marshall et al., 2009). In most cases, a species’ realized niche is more restricted than its fundamental niche, as these factors restrict a species movement in areas which would otherwise be suitable (Hutchinson, 1957; Pulliam, 2000). Using environmental variables associated with known species occurrence can predict distribution in other areas, increase our understanding of a species’ overall fundamental niche, lead to predictions of a species’ range within understudied areas, improve forecasting of future extinction risk, and address questions of taxonomy and patterns of speciation (Blair, Sterling, Dusch, Raxworthy, & Pearson, 2013; Junker et al., 2012; Kamilar & Tecot, 2016; Kamilar, Blanco, & Muldoon, 2016; McCormack, Zellmer, & Knowles, 2010). In this paper, we use Maxent (Elith et al., 2011; Phillips & Dudík, 2008) to model the ecological niche and species distribution of the Angolan black and white colobus monkey (Colobus angolensis palliatus) in Kenya and Tanzania based on presence-only data.
The genus Colobus consists of five species that can be found in West Africa, throughout Central Africa, and in East Africa (Oates, Davies, & Delson, 1994; Oates & Trocco, 1983). African colobines are arboreal folivores with group sizes typically ranging from 2 to 20 individuals (Fashing, 2006; Fashing et al., 2007; Oates et al., 1994). Rest generally makes up more than 50% of their activity budget, and while forest is essential for their survival, the vegetation structure and composition can vary widely across species (Fashing et al., 2007). Angolan black and white colobus (Colobus angolensis) are found in African forests in northeast Angola, the Democratic Republic of Congo, Rwanda, Tanzania and Kenya (Anderson, Cowlishaw, & Rowcliffe, 2007; Kingdon, 1997) and encompass seven subspecies. Group sizes and activity budgets across this species are known to vary considerably, with C. a palliatus groups in the Diani Forest, Kenya, averaging six individuals (Anderson, 2004) and super-troops of over 300 C. a. ruwenzorii individuals in the Nyungwe Forest, Rwanda (Fashing et al., 2007; Vedder & Fashing, 2002). The colobus in the Nyungwe Forest are uniquely more active than other colobus subspecies and species, have much larger group and home range sizes, and have been observed to migrate large distances (13 km) to enter new ranging areas (Fashing et al., 2007).
While C. angolensis as a species is not currently listed as threatened, understanding the ecological niches of each subspecies, and the degree to which each exhibits habitat flexibility, will provide useful insights into their conservation status and requirements. In addition, the subspecies, C. a. palliatus, has been highlighted by conservationists for being confined to the islands of fragmented forests where it resides (Kingdon, 1997; Rodgers, 1981) and it is currently considered nationally threatened in Kenya.
C. a. palliatus occurs in coastal forests south of Mombasa, Kenya, to the riparian forest along the Rufiji River, Tanzania (Groves, 2001; Kingdon & Howell, 1993; Figure 1). This subspecies also ranges in forested regions in the Eastern Arc Mountains of Tanzania and in the Southern Highlands, a forested mountain region adjacent to the southern end of the Eastern Arc Mountains (Groves, 2001). These colobus monkeys are arboreal folivores, and canopy cover and vegetation height are considered significant predictors of their presence (Anderson, 2004; Anderson, Rowcliffe, & Cowlishaw, 2007aa, 2007bb; Cavada, Ciolli, Barelli, & Rovero, 2017; Davies & Oates, 1994; Marshall et al., 2009; Moreno-Black, 1977; Moreno-Black & Bent, 1982).
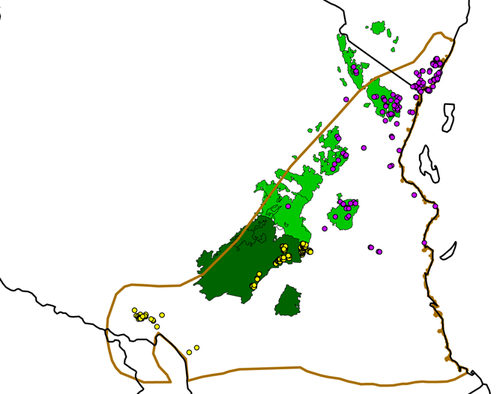
Map of study area with Eastern Arc Mountains. Brown polygon indicates the 2008 IUCN Range Map (Kingdon et al., 2008aa, 2008bb). Light green polygons represent the Eastern Arc Mountains with C. a. palliatus presence points; Dark green polygons represent the Eastern Arcs with C. a. sharpei presence points. Purple and yellow presence points, which occur inside and outside of the Eastern Arcs in the coastal regions of Kenya and the Southern Highlands were combined for Scenario 1; purple dots are points considered C. a. palliatus in Scenario 2a; yellow dots are presence points considered C. a. sharpei in Scenario 2b (as defined by Rahm, 1970)
Many areas of their habitat are fragmented. Though individuals are observed in wooded shrub land, shrub grassland, wooded grassland, mangroves, and in perennial plantations, encounter rates are low compared with closed canopy forest (Anderson, 2004). In the Udzungwa Mountains, the best predictors of primate density and encounter rate for C. a. palliatus are forest patch size (Cavada, Barelli, Ciolli, & Rovero, 2016; Marshall et al., 2009), percentage of climber trees (Barelli et al., 2015; Cavada, Barelli et al., 2016; Rovero & Struhsaker, 2007), isolation (Marshall et al., 2009), habitat protection (Araldi, Barelli, Hodges, & Rovero, 2014; Cavada, Barelli et al., 2016; Marshall et al., 2009), hunting pressure (Marshall et al., 2009; Rovero et al., 2012), and elevation (Barelli et al., 2015; Cavada, Barelli et al., 2016; Marshall et al., 2009). In the Kwale district of Kenya, fragment size and canopy cover are positively associated with colobus occurrence, and forest area, area with major food trees, and the proportion of forest change over the past 12 years are associated with colobus density (Anderson, Cowlishaw et al., 2007). One notable exception is the holiday resort town of Diani, on the southern Kenyan coast. This 7 km2 suburban center holds Kenya's second largest population of C. a. palliatus (Anderson, 2004). Groups live in remnant coastal forest patches characterized by scattered trees, exotic trees, and bushes interspersed within houses, hotels, restaurants, and shopping centers (Anderson, 2004).
The fragmentation of C. a. palliatus habitat has been proposed to be a result of both environmental changes in the Pleistocene that led to the creation of the Eastern Arc “mountain islands” (Lovett & Wasser, 1993; Wasser, 1993) and more recent anthropogenic disturbances (Preston, 2011). Hunting and deforestation are known to cause negative impacts on the density of this subspecies in unprotected (Hall et al., 2009; Rovero et al., 2012) and protected areas (e.g., Arabuko Sokoke Forest Reserve, Kenya), and extirpations are recorded at a number of sites, including Arabuko Sokoke, Kenya (Anderson, 2004; Anderson et al., 2007aa, 2007bb); Pande Game Reserve (Burgess & Clarke, 2000; Doggart, 2003); Pugu Forest Reserve (Burgess & Clarke, 2000); and likely in North Pare Mountains, Tanzania (Doggart, Leonard, Perkin, Menegon, & Rovero, 2008).
Dandelot (1971), and Rahm (1970) distinguished populations of C. a. palliatus, residing in the coastal forests of Tanzania and the Usambara and Uluguru Mountains, from C. a. sharpei, which reside in the southern mountains of Tanzania (Figure 1). This classification is based upon pelage differences and population isolation. In addition, Hull (1979) noted morphological differences in craniometrics between the two subspecies. A genetic study by McDonald and Hamilton (2010) also supported this division, as the greatest genetic distance among populations was observed between Kenyan and southern Tanzanian populations, though areas of northern and central Tanzania were not sampled. The division of C. a. palliatus and C. a. sharpei is perhaps not unlikely, considering that this geographic delineation for subspecies differentiation is found in other taxa (Fjeldså & Bowie, 2008).
The two subspecies, palliatus and sharpei, are currently classified under the original designation of C. a. palliatus with C. a. sharpei considered as synonymic (Groves, 2001). C. a. palliatus (including C. a. sharpei) was considered data deficient by the International Union for Conservation of Nature (IUCN) Red List until 2008, when it was reassessed to “Least Concern” status based on the assertion that “they remain widespread and relatively common, and do not seem to be declining fast enough to warrant listing in a higher category of threat” (IUCN Red List Data v. 3.1: C. a. palliatus). The IUCN range map of C. a. palliatus indicates the presence of the subspecies over an area of approximately 295,000 km2 (Kingdon et al., 2008aa, 2008bb; Figure 1).
In this study, we use presence-only data (Elith et al., 2006; Li, Guo, & Elkan, 2011) from multiple sources, as summarized in Table 1, combined with environmental layers to better understand the current geographical distribution and ecological niche of C. a. palliatus based upon environmental variables across the subspecies range. We develop a predictive model for the distribution of C. a. palliatus that indicates the probability of their occurrence and compare this distribution to the current IUCN range map. We also investigate the ecological differences in C. a. palliatus populations according to the previous subspecies’ delineation of C. a. palliatus and C. a. sharpei and assess the amount of niche overlap they share, as well as test whether the ecological niches are significantly different from one another.
| Country | Location | # of points | References |
|---|---|---|---|
| Kenya (C. a. palliatus) | Coastal Forest: Kenya | 67 | Anderson unpub. data (2001); Colobus Conservation, unpub. data; McDonald & Hamilton (2010) |
| Tanzania (C. a. palliatus ) | Coastal Forest: Tanzania | 10 | Clarke and Stubblefield (1995); Davenport, Nowak, and Perkin (2014); D. Klaassen (personal communication, 2017); Sheil and Burgess (1990) |
| Tanzania (C. a. palliatus ) | Gendagenda Forest | 3 | Clarke and Stubblefield (1995); Davenport et al. (2014) |
| Tanzania (C. a. palliatus) | Mikumi National Park | 1 | Cunneyworth unpub. data (2017) |
| Tanzania (C. a. palliatus) | Nguru | 6 | Bracebridge (2006); Cunneyworth unpub. data (2017); Davenport et al. (2014); Kiure and Doggart (2007) |
| Tanzania (C. a. palliatus) | Nguu | 2 | Davenport et al. (2014); Kiure (2005) |
| Tanzania (C. a. palliatus) | Pare: South | 4 | Baker and Baker (2002); Cordeiro et al. (2005); Cunneyworth unpub data (2017) |
| Tanzania (C. a. palliatus) | Rubeho | 1 | Davenport et al. (2014) |
| Tanzania (C. a. palliatus) | Rufiji riverine forest | 6 | Cunneyworth unpub. data (2017) |
| Tanzania (C. a. palliatus) | Tongwe Forest | 2 | Burgess and Clarke (2000); Clarke and Stubblefield (1995) |
| Tanzania (C. a. palliatus) | Uluguru | 15 | Cunneyworth unpub. data (2017); Davenport et al. (2014); Doggart, Lovett, Mhoro, Kiure, & Burgess (2004a, 2004b) |
| Tanzania (C. a. palliatus) | Uncategorized | 2 | Burgess & Clarke, (2000); Clarke & Stubblefield (1995); Cockle (1992); Frontier Tanzania (2002) |
| Tanzania (C. a. palliatus) | Usambara: East | 20 | Beharrell, Fanning, and Howell (2002); Cordeiro et al. (2005); Cunneyworth unpub. data (2017); Davenport et al. (2014); Doggart, Dilger, Cunneyworth, and Fanning (1999); Doggart, Dilger, Kilenga, and Fanning (1999); Doggart et al. (2008); Doggart, Joseph, Bayliss, and Fanning (1999); Doody, Beharrell, Howell, and Fanning (2001); Frontier Tanzania (1996a, 1996bb, 1996cc, 2001aa, 2001bb) |
| Tanzania (C. a. palliatus) | Usambara: West | 12 | Cunneyworth unpub. data (2017); Frontier Tanzania (2002); Mrema & Nummelin (1998); Preston (2011) |
| Tanzania (C. a. sharpei) | Udzungwa | 165 | (Cavada, Barelli et al., 2016; Cavada, Ciolli et al., 2016; Cavada et al., 2017; Davenport et al. (2014); McDonald & Hamilton, (2010); F. Rovero (personal communication July 05, 2018) |
| Tanzania (C. a. sharpei) | Southern Highlands | 65 | Davenport et al. (2014); Davenport (personal communication, September 4, 2018); McDonald & Hamilton (2010) |
| Grand Total | 381 |
2 METHODS
2.1 Colobus point presence data
We obtained locality data records (Table 1) for C. a. palliatus and C. a. sharpei from previous publications, totaling 381 presence points (151 from Kenya and northern Tanzania; 230 from southern Tanzania), to use for modeling. This study complies with the American Society of Primatologists’ principles for the ethical treatment of nonhuman primates and no permissions from an Institutional Animal Care and Use Committee or from Kenya or Tanzania were needed.
2.2 Habitat modeling
We evaluated C. a. palliatus distributions using Maxent v3.4.1. Each of the 100 replications we performed used 500 maximum iterations, a 0.0001 convergence threshold, and 10,000 maximum background points. To reduce model overfitting, we used the ENMeval R package (Muscarella et al., 2014) to select a regularization multiplier (RM) and feature classes (FC) (Table 2). We withheld 25% of the presence points to test the model's performance (Holzmann et al., 2014; Phillips, Anderson, & Schapire, 2006). To remediate oversampling not reflective of primate density (mainly of the northern Tanzania and Kenya population), we used spThin to down sample all presence points, using 100 iterations (Aiello-Lammens, Boria, Radosavljevic, Vilela, & Anderson, 2015; Holzmann et al., 2014). We constructed a model using the optimized RM and FCs for five different thinning distances (i.e., 0.9, 1,0, 1.5, 2.0, and 5.0 km; Tables 2-4). We used the lowest average area under the curve (AUC) difference between training and testing samples across all cases in selecting a thinning distance of 0.9 km.
| C. a. palliatus | Thinning distance (km) | ||||
|---|---|---|---|---|---|
| combined results | 0.9 | 1.0 | 1.5 | 2.0 | 5.0 |
| AUC | 0.952 | 0.955 | 0.950 | 0.947 | 0.944 |
| ΔAUC (train-test) | 4.1e−3 | 4.6e−3 | 6.4e−3 | 7.3e−3 | 1.5e−2 |
| RM | 2 | 2 | 2 | 2 | 2 |
| FC | HQPT | HQPT | HP | HQPT | HP |
| Number of presence points | 299 | 283 | 229 | 192 | 118 |
| Number of occupied raster cells | 290 | 279 | 229 | 192 | 118 |
- * Feature classes were restricted based upon the model with the minimum Akaike information criterion with a correction for small sample sizes (AICc), from a parametric study of five regularization multipliers (RMs), that is, 1, 2, 5, 10, and 20, and 10 feature class (FC) combinations of linear (L), hinge (H), quadratic (Q), product (P), and threshold (T) factors (i.e., LQ, LQP, LP, QP, H, HQ, HP, HQP, LQPT, and HQPT), using the ENMeval R package (Muscarella et al., 2014) to reduce model overfitting.
| Thinning distance (km) | |||||
|---|---|---|---|---|---|
| C. a. palliatus results | 0.9 | 1.0 | 1.5 | 2.0 | 5.0 |
| AUC | 0.964 | 0.967 | 0.963 | 0.957 | 0.953 |
| ΔAUC (train-test) | 6.8e−3 | 8.0e−3 | 7.4e−3 | 1.1e−2 | 2.3e−2 |
| RM | 2 | 2 | 2 | 2 | 2 |
| FC | HQ | HP | HP | HQ | HP |
| Number of presence points | 133 | 130 | 120 | 111 | 79 |
| Number of occupied raster cells | 132 | 130 | 120 | 111 | 79 |
| Thinning distance (km) | |||||
|---|---|---|---|---|---|
| C. a. sharpei results | 0.9 | 1.0 | 1.5 | 2.0 | 5.0 |
| AUC | 0.991 | 0.990 | 0.987 | 0.983 | 0.979 |
| ΔAUC (train-test) | 2.7e−3 | 2.1e−3 | 2.2e−3 | 2.7e−3 | 6.4e−3 |
| RM | 2 | 2 | 2 | 1 | 1 |
| FC | HQPT | HQPT | HQPT | QP | LQPT |
| Number of presence points | 166 | 153 | 109 | 81 | 39 |
| Number of occupied raster cells | 158 | 149 | 109 | 81 | 39 |
Some locality information obtained from biodiversity surveys only listed a range of coordinates corresponding to an entire forest patch, plot or transect. In these cases, we used coordinates that corresponded to the center of this range, likely where the forest is densest. Because our raster resolution is 1 km, we reduced the sampling of points derived from Araldi et al. (2014) to the termini of the 1 km transects. While reasonable, these assumptions may have resulted in an underestimation of overall presence in these areas. After reducing point concentrations using spThin, the data continued to exhibit spatial correlation bias, with sampling concentrated in a variety of smaller study regions. While our model selection procedure suggests that we selected an optimum, this analysis could be updated if more widespread sampling is conducted throughout the study area.
It is important to consider the spatial extent from which pseudo-absence data are taken. Pseudo-absences taken from too small of an area can result in spurious results and those taken from too large of an area can lead to artificially inflated predictions and test statistics and/or potentially less informative response variables (VanDerWal, Shoo, Graham, & Williams, 2009). In this study, we chose to restrict the background area from which pseudo-absence points were drawn to the boundaries of the C. a. palliatus IUCN range map extended by a 100 km buffer to encompass the area occupied by all presence points.
Our model evaluates two scenarios, the first considering all points as representing a single subspecies (to compare range estimates to IUCN range maps) and the second representing the data as two populations based upon the subspecies-level taxonomic categorization of C. a. palliatus and C. a. sharpei as indicated previously by Rahm (1970) and supported by Hull (1979) and McDonald and Hamilton (2010) to evaluate the validity of a separate subspecies designation based on niche similarity. The combined scenario (Scenario 1) used 213 presence points for training and 71 for testing (299 total, 15 removed due to missing predictor values), and assumes all presence data is for a single subspecies (C. a. palliatus) to predict a distribution for comparison with the existing IUCN distribution map. Scenario 2 assigns presence points to either C. a. palliatus (for Kenyan and northern Tanzanian populations) or C. a. sharpei (for central and southern Tanzanian populations) to assess ecological differences between populations at the extremes of their north–south range. For C. a. palliatus (Scenario 2a), 95 presence points were used for training and 31 for testing (133 total, 7 removed due to missing predictor values), and for C. a. sharpei (Scenario 2b), 119 presence points were used for training and 39 for testing (166 total, 8 removed).
2.2.1 Scenarios
Previous research has found that spatial effects (Bannar-Martin, 2014) and forest area (Marshall et al., 2009) may be more important than climatic factors for most primate communities, particularly those that are arboreal. However, temperature and precipitation are important for plant production (Marshall et al., 2009) and in assuring water sources are available for animal consumption. As Angolan black and white colobus monkeys are arboreal folivores, spending most of their time in the upper canopy of trees (Bocian & Anderson, 2013), we predicted that areas having at least partial forest would be necessary for their long-term survival (Pulliam, 2000) and that bioclimatic variables should be evaluated for this species. For the model, we obtained values for 19 WorldClim bioclimatic variables, USGS land cover data, NASA GIMMS imagery, and altitude (elevation) at a 1 km2 resolution.
We assessed two different metrics for vegetation: A normalized index from spectral data and USGS classifications of land use. We obtained the normalized difference vegetation index (NDVI) from NASA GIMMS satellite imagery and resampled onto the same raster as the WorldClim data for the spectral index. We reclassified the USGS land cover data according to the implied presence/absence of forest cover to form the land use raster layer. For both NDVI and USGS raster layers, we randomly sampled points to manually quality check against satellite imagery and ground-based classifications of ground cover when available. We determined that the USGS classifications contained high error rates, biased towards underestimation of forest cover at presence points; however, the NDVI data agreed well with vegetation presence in these areas. We, therefore, excluded USGS land cover data from subsequent analyses and used NDVI data to characterize land cover.
For the remaining bioclimatic variables, we generated a collinearity matrix (using the Pearson correlation matrix from ENMTools raster.cor.matrix function) and removed variables with a correlation value (r) of 0.80 or higher, to reduce multicollinearity (Bannar-Martin, 2014; Holzmann et al., 2014; Kramer-Schadt et al., 2013). The reduced variable set included: altitude, mean annual temperature, mean diurnal temperature range, seasonality, annual precipitation, precipitation in the driest month, and NDVI.
We used AUC values generated in Maxent to evaluate model performance for each scenario. An AUC of 1 signifies that the model can discriminate between areas with presence and absence well, whereas an AUC of 0.5 or less indicates model prediction equal to or less than a random outcome. We generated raw and logistic outputs. Though the cloglog of the raw data may be more theoretically appropriate, the cloglog output is unlikely to have a measurable effect on model performance (Phillips, Anderson, Dudík, Schapire, & Blair, 2017), so we used traditional logistic outputs, which are presented on a 0–1 scale, where higher values indicate more favorable conditions for the species. Rather than a subjective fixed threshold approach, the objective minimum training presence (MTP) threshold was used to establish binary suitability. This threshold approach was chosen because it is a conservative and robust objective approach that integrates the prevalence of model-building data as the threshold (Liu, Berry, Dawson, & Pearson, 2005) and also exhibits the lowest realized omission error rates for the Maxent models among those generated. We suggest that values above the MTP threshold for each scenario (i.e., MTP fractional predicted area for each) represent areas of suitability (C. a. sharpei = 0.130; C. a. palliatus = 0.621; and combined = 0.544). Figure 2 shows habitat suitability for each scenario, where values below the MTP for each are not shown (i.e., unsuitable) and the remaining suitability results are normalized into quartiles between 0 and 1.

Predicted Kenyan and Tanzanian distributions of (a) C. a. palliatus combined (Scenario 1), (b) C. a. palliatus, and (c) C. a. sharpei (Scenario 2) according to probability of suitability. White indicates unsuitable habitat (<MTP) and all other areas of suitability are normalized on a 0–1 scale where darker colors are closer to 1.0. (d) Areas of overlap for Scenario 2. Brown polygon represents the 2008 IUCN distribution map (Kingdon et al., 2008aa, 2008bb). MTP: minimum training presence
We calculated the overall areas (in km2) of habitat suitability for each scenario and calculated the areas of overlapping suitable habitat for C. a. palliatus and C. a. sharpei (Scenario 2a vs. Scenario 2b). We also generated niche overlap statistics and a niche identity test to determine whether we can reject the null hypothesis that the two subspecies models were drawn from the same underlying distribution of environmental variables (Kamilar & Tecot, 2016; McCormack et al., 2010; Warren, Glor, & Turelli, 2008, 2010).
3 RESULTS
3.1 Scenario 1: Overall distribution of C. a. palliatus in Kenya and Tanzania
The model assessing the overall distribution of C. a. palliatus as one species (Groves, 2001) throughout their range in Kenya and Tanzania, results in a replicate mean AUC of 0.935 (standard deviation = 0.048; Figure 2a). In this model, the variables with the highest average model contribution (Figure 3) are annual precipitation (29.1%), precipitation of the driest month (25.6%), and land cover (17.8%). Those with the highest average permutation importance are precipitation in the driest month (26.5%) and mean diurnal range (21.8%). The MTP for a representative replicate model near the mean is 0.544, so results above this were considered suitable habitat. The total predicted area of suitable habitat when considering all points belonging to the same subspecies was 36,655 km2 from a study area of the IUCN map buffered by 100 km (i.e., in order to include all presence data) and subtracting water areas (total study area of 503,240 km2).
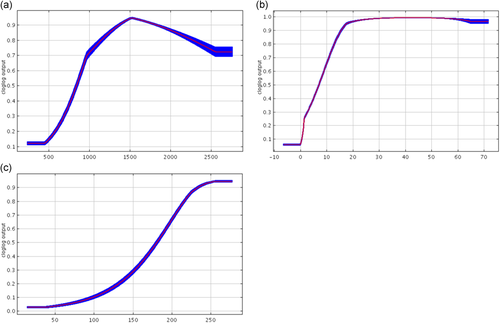
Response curves for Scenario 1 C. a. palliatus combined from Maxent replicates for (a) annual precipitation (29.1%), (b) precipitation in the driest month (25.6%), and (c) land cover (17.8%), where parenthetical percentages indicate contribution of the variable to the model
3.2 Scenario 2: Potential distribution of C. a. palliatus and C. a. sharpei
For Scenario 2, we divided the subspecies into two according to Rahm (1970). We included points north of the Udzungwa Mountains (and the Rufiji River) in the scenario to represent C. a. palliatus and the remaining as C. a. sharpei.
The replicate model for C. a. palliatus in this scenario results in an average AUC score of 0.949 (standard deviation = 0.092; Figure 2b). The variable with the highest average model contribution (Figure 4) is precipitation of the driest month (69.1%), and the contribution of each remaining variable ranged from 1.5% to 13.2%. In this case, the variable with the highest average permutation importance was also precipitation of the driest month (77.6%). Again, the contribution of each remaining variable was low, ranging from 0.2% to 10.2%. The MTP for a representative replicate model near the mean is 0.621, so results above this were considered suitable habitat and resulted in a total predicted area of suitable habitat for C. a. palliatus of 20,383 km2. This model predicted areas of suitability where the species does not occur. Some of these were areas where Colobus guereza currently resides (e.g., C. g. caudatus near Kilimanjaro in Tanzania), suggesting that these C. angolensis and C. guereza subspecies may have similar ecological niche requirements but that the absence of C. angolensis in these locations is a result of historical migration patterns over differing timescales. Other areas were places where they were known to exist until hunting resulted in their recent extirpation (e.g., Arabuko Sokoke Forest north of Mombasa in Kenya; Anderson, 2004).
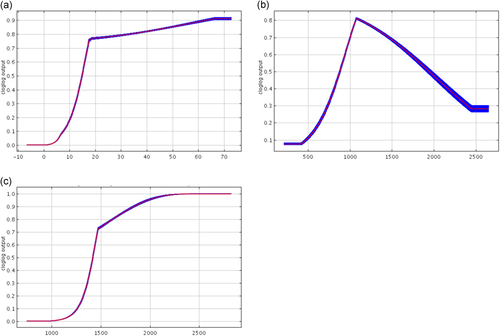
Response curves for Scenario 2 C. a. palliatus from Maxent replicates for (a) precipitation in the driest month (69.1%), (b) annual precipitation (13.2%), and (c) seasonality (8.4%), where parenthetical percentages indicate contribution of the variable to the model
The replicate model for C. a. sharpei in this scenario results in an average AUC score of 0.979 (standard deviation = 0.023; Figure 2c). In this model, the variables with the highest average contribution (Figure 5) are annual precipitation (44.8%) and land cover (16.4%); the contribution of each remaining variable ranged from 2.3% to 12.8%. The variables with the highest average permutation importance were mean diurnal range (43.7%), altitude (20.7%), and annual precipitation (20.5%), and the contribution of each remaining variable was comparatively low, ranging from 0.6% to 5.1%. The MTP for a representative replicate model near the mean is 0.130, so results above this were considered suitable habitat. The total predicted area of suitable habitat for C. a. sharpei was 45,191 km2.
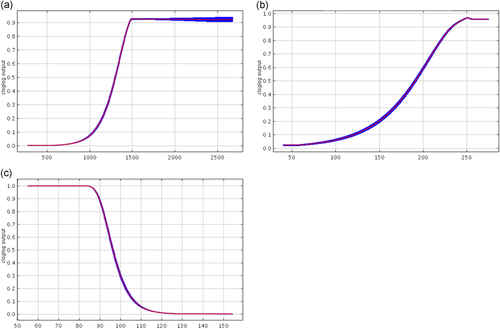
Response curves for Scenario 2 C. a. sharpei from Maxent replicates for (a) annual precipitation (44.8%), (b) land cover (16.4%), and (c) mean diurnal range (12.4%), where parenthetical percentages indicate contribution of the variable to the model
3.3 Ecological differences between C. a. palliatus and C. a. sharpei
The area of predicted overlap for these two species was comparatively small (754 km2), or approximately 1.2% of the total habitable area of the two subspecies’ ranges. We additionally generated niche overlap statistics, that is, Schoener's D (Schoener, 1968), I (Warren et al., 2008) and (for Geographic and Environment cases) the Pearson correlation (cor), to evaluate whether C. a. palliatus and C. a. sharpei occupy similar niches (Table 5). All approaches indicate relatively low overlap in suitability.
| Overlap Type | D | I | cor |
|---|---|---|---|
| Environment | 0.13 | 0.30 | −0.06 |
| Geographic | 0.17 | 0.40 | 0.41 |
| Geographic/Raw | 0.06 | 0.21 | – |
- These were determined by using a raster overlap of the Maxent suitability distributions in geographic space (Geographic) via ENMTools (raster.overlap), a Latin hypercube sampling of the n-Dimensional space of environmental variables (Environment) via ENMTools (env.overlap, tolerance 1e−3) and overlap in geographic space (Geographic/Raw) via ENMeval (calc.niche.overlap).
We also performed a niche identity test, which compares observed measures of niche similarity between the distribution in areas occupied by a population and a generated null distribution that assumes no niche differentiation, then tests whether the observed results are significantly different from the null distribution (Warren et al., 2010). Our values for I, D, and correlation in both geographic and environmental space are all significantly lower than the values expected from the generated null datasets (Figure 6), suggesting that C. a. palliatus and C. a. sharpei reside in significantly different niches.
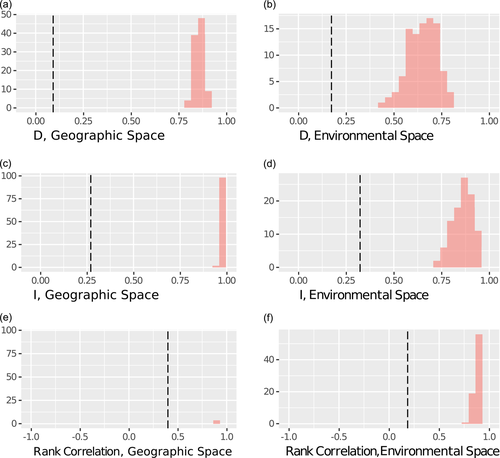
Results of identity test, where the dashed line indicates the observed measure and the pink histograms indicate the generated null distribution that assumes no differentiation for (a) D in geographic space, (b) D in environmental space, (c) I in geographic space, (d) I in environmental space, (e) Pearson's correlation in geographic space, and (f) Pearson's correlation in environmental space. Note: In all cases, the dashed line lies far from the null distribution, indicating that we can reject the hypothesis that there is no differentiation between the niches occupied by the C. a. palliatus and C. a. sharpei populations
4 DISCUSSION
Previous studies have assessed factors related to the abundance of Angolan colobus monkeys in specific localities throughout Kenya and Tanzania. However, this study is the first to take a broader look at the ecology of these populations across their entire range in Kenya and Tanzania, knowing that factors affecting their abundance in some geographic areas may not be generalizable into other areas. This is also the first study to compare ecological differences between C. a. palliatus in Kenya and northern Tanzania to populations (previously considered C. a. sharpei) in Central and Southern Tanzania.
4.1 Comparison to IUCN range map
When considering all populations in Kenya and Tanzania as C. a. palliatus (Groves, 2001), our predicted distribution of suitability in eastern Kenya and Tanzania is considerably smaller than that of the 2008 IUCN range map (Kingdon et al., 2008aa, 2008bb) (36,655 km2 vs. 286,562 km2 of land area, or 12.8%). In contrast, the combined suitable range under (the two subspecies) Scenario 2 is larger (64,819 km2 or 22.6% of the estimated IUCN land area). The current IUCN range map does not consider the quality and/or type of environment (e.g., land cover, temperature, and/or precipitation constraints), and in cases where sampling is sparse and/or spatially correlated, as is the case with C. a. palliatus, the estimate is based on a subjective assessment. The distribution maps from this study have greater support than the IUCN range map and should serve as a more robust base against which conservation considerations should be weighed.
4.2 Ecological similarities and differences among scenarios
When considering populations in Kenya and northern Tanzania as C. a. palliatus, and those in central and southern Tanzania as C. a. sharpei (Hull, 1979; McDonald & Hamilton, 2010; Rahm, 1970), it is clear that both precipitation and land cover are important in their overall distributions. Precipitation is strongly coupled with plant productivity (Marshall et al., 2009), and land cover is a reasonable proxy for the presence of trees on which they rely for diet and locomotion, as is the case for other arboreal primates. In this study, we used NDVI as our measure of land cover. While NDVI (i.e., the proxy for land cover used here) could possibly be a liberal measure of this requirement (compared with detailed measures of vegetation height, canopy cover, type, etc.), NDVI can be used to identify forest habitat using a particular spectral index (National Center for Atmospheric Research, 2018), without making assumptions regarding a species’ specific forest preferences. Our approach is appropriate for a large-scale study of this kind, as detailed studies in some areas have suggested that Angolan colobus monkeys show considerable resilience to moderate forest disturbance (Barelli et al., 2015; Cavada, Barelli et al., 2016; Cavada et al., 2017). Vegetation height and percentage of climber species, which can exist in both primary and secondary forests (Barelli et al., 2015; Cavada et al., 2017), are considered more important for this species than canopy cover, at least in some areas.
While precipitation and land cover were consistently important in our model, once the populations were divided into C. a. palliatus and C. a. sharpei, the influence of the temporal distribution of precipitation and the types of land cover that characterized each subspecies’ habitat differed substantially. For C. a. palliatus, the most influential variable was precipitation of the driest month. When compared with the predicted distribution of C. a. sharpei in Kenya and central and northern Tanzania, our model suggests C. a. palliatus’ niche is characterized by less vegetation, larger temperature ranges, and more precipitation in the “dry” months. These results are consistent with Marshall et al. (2009), who found that high maximum temperature, high maximum precipitation in the driest month, and high precipitation range can result in the high richness of primate species. For C. a. sharpei, the most influential variables were annual precipitation and land cover, as their distribution in Tanzania was characterized by more vegetation, lower average temperatures and temperature range, and more precipitation. These populations also seem to be more reliant on large forest patches (Cavada, Barelli et al., 2016; Marshall et al., 2009). These environmental differences can be further illustrated by the small area of overlap in their suitability distributions (1.2%) and by the differences in key factors characterizing the environments of these two populations.
4.3 Considerations regarding the Southern Kenyan population
The differences in the environments that characterize these two populations can be partially explained by differences in the level of human disturbance. Diani is a suburban center on the south coast of Kenya and represents a relatively unusual case of high primate density for arboreal primates. Diani has remnant forest patches and isolated trees from the original forest as well as substantial amounts of anthropogenic disturbance, including high human population density and high levels of tourism (Anderson, 2004; McDonald, 2009). On the basis of the results of other studies (Arroyo-Rodríguez, González-Perez, Garmendia, Solà, & Estrada, 2013; Bannar-Martin, 2014; Marshall et al., 2009), it would be expected that these areas of small forest patch size might be undesirable. However, the suburban center, approximately 7 km2 , holds the second largest population of colobus in Kenya with the 2018 census counting 220 individuals in 36 groups (Anderson, 2004). This population has been stable for 20 years (Cunneyworth, unpub. data, 2017). This high density is likely a result of historically large populations that have been subject to drastic forest reduction in the past few decades resulting in limited dispersal opportunities (Bannar-Martin, 2014). A similar explanation has been given for high Angolan colobus densities in the Magombera forest near the Udzungwa mountains in Tanzania. Araldi et al. (2014) attributed these high population densities to the rapid shrinking of forest patch over the last three decades and the colobus’ inability to migrate to other locations due to isolation. While diminished, options for immigration and emigration in the Diani areas of Kenya do exist (and are not as drastic as the sugarcane and rice fields surrounding Magombera); they are generally limited to the south. It is likely that while dispersal is possible, it is difficult, leading to the high population densities. An alternative explanation, though not necessarily exclusive, may be that while this environment is highly fragmented, these forest fragments are relatively close to each other and have high matrix permeability across them. Adding to this, Colobus Conservation—a nonprofit organization that carries out primate and forest conservation in this area—has been actively working to protect colobus (and other primates) in this area since 1997. Therefore, it is possible that the high degree of protection within (and high spatial gradient of protection around the periphery) has made Diani more desirable than it would have been otherwise. These results are consistent with Chapman et al. (2013), who saw declines in the number C. guereza in all unprotected fragments that they studied outside of Kibale National Park except for the fragment where their study site was located. These ideas are also supported by studies that have found that that Angolan colobus monkeys are more adaptable to habitat change and have greater dietary flexibility than red colobus (Anderson, 2004; Marshall et al., 2009). While forest area is important, fragments that are less isolated from one another, those with high matrix permeability and those with good levels of protection can be just as important (Anderson, 2004; Anderson et al., 2007; Araldi et al., 2014; Arroyo-Rodríguez et al., 2013; Cavada et al., 2016; Marshall et al., 2009). It is possible that the areas between forest fragments even serve as supplementary food sources for them, which in turn contribute to their survival in this area (Arroyo-Rodríguez et al., 2013).
The question then remains as to whether these populations are sustainable. Studies of black and white colobus and red colobus in unprotected fragments outside of Kibale National Park revealed drastic decreases in the number of individuals of both species in these areas over an 8-year period as well as the decimation of several forest fragments altogether (Chapman et al., 2013; Chapman, Naughton-Treves, Lawes, Wasserman, & Gillespie, 2007). Fragments with increased numbers of individuals could be attributed to the migration of individuals from one location to another out of sheer necessity. Arroyo-Rodriguez et al. (2013) found that howler monkeys in certain areas of Mexico are relatively resilient to the initial phases of disturbance but that sharp increases in population density in highly isolated patches may suggest there is an extinction debt to be paid. Over time, higher population densities in small fragments may result in the reduction in food availability, increases in inter- and intraspecific competition for resources, and higher endoparasite loads, which could have negative consequences for the long-term survival of the species (Arroyo-Rodríguez et al., 2013).
On the basis of the results of this study, we suggest that the source/sink dynamics in this area be investigated in greater detail (Kuussaari et al., 2009; Pulliam, 1988) and that genetic studies of relatedness and dispersal would be beneficial for understanding the levels of gene flow and inbreeding. This should also help assess the population's sustainability and help identify future conservation action plans, such as increasing migration options and prioritizing reforestation efforts surrounding this suburban center.
4.4 Suitability prediction in areas of known C. a. palliatus absence
The habitat suitability maps for the Angolan colobus in Kenya suggest suitable habitat in areas of known and highly likely extirpations (Arabuko Sokoke Forest, Kenya and North Pare Mountains, Tanzania). Our results suggest that while the climatic variables in these areas are desirable for the colobus (or at least as desirable as that in the Diani areas), the differences lie in levels of protection and forms of human influence. While not officially regarded as such, the Diani area is effectively highly protected with limited hunting, while Angolan colobus in the areas north of Mombasa were not historically well protected and were extirpated due to hunting and land conversion to agriculture (Anderson et al., 2007aa, 2007bb).
Other areas of predicted C. a. palliatus habitat in known areas of extirpation (Pande Game Reserve and Pugu Forest Reserve, Tanzania) were likely not the result of hunting, but habitat degradation. In the Pande Game Reserve, most of the canopy trees and “emergents” have been removed and only secondary growth that rarely exceeds eight meters in height remains (Doggart, 2003). The Pugu Forest Reserve is under considerable threat from hunting and deforestation due to its proximity to the city of Dar es Salaam (Hall & Rodgers, 1986).
Overall, the “overpredictions” from our analyses are consistent with studies that have found climate and land cover to be important (but not exclusively important) for species presence and emphasize the need to integrate appropriate measures of anthropogenic disturbance into future models. Kamilar and Tecot (2016) found that adding anthropogenic factors to the model (distance to dense settlements, villages, and croplands) improved the climate models and was found to be the most important factor in most cases. While adding anthropogenic factors to the model may have been beneficial, these data were not part of our study design. Such data could be useful variables to incorporate into future studies, if available.
4.5 Significance
The results of this study provide detailed information on the distribution of Angolan colobus monkeys in Kenya and Tanzania. The distribution maps from this study reveal that areas of suitable habitat for these populations are considerably less than that suggested by the IUCN 2008 range map (Kingdon et al., 2008aa, 2008bb). Because the IUCN map does not consider habitat and/or climatic preference, the maps from this study may provide future researchers with more appropriate estimates of population presence. This could be useful for locating populations in previously unsampled locations and for identifying areas to prioritize for future data collection and conservation actions. Updated censuses and/or estimates of population density in these areas will help assess population decline over recent decades and will provide the data necessary to eliminate the spatially correlated sampling that currently challenges ecological niche modeling efforts. Sample collection across the range will also provide the necessary data to assess the genetic variation of this subspecies and help determine extinction risk.
These results provide ecological support that significant differences exist between C. a. palliatus (populations north of the Udzungwa Mountains) and C. a. sharpei (populations within and to the south of the Udzungwa Mountains). This information, when added to the existing morphological, pelage, and genetic data, further supports the assertion that these populations deserve separate subspecies designations, including distinction in IUCN Red List assessments. It also raises new questions about the extent of environmental difference between coastal and mountainous populations of C. a. palliatus and whether the existence of suburban coastal populations is evidence of ecological flexibility or is a precursor to extinction. More behavioral and genetic studies are needed to investigate these questions.
Given the support for subspeciation, conservation organizations can use these results to help maintain population numbers and increase gene flow among neighboring populations. Specifically, the suitability maps imply an extent of fragmentation, and along with protection level and so forth, these characteristics can aid in the prioritization of conservation efforts towards fragments that are particularly useful for corridor creation (i.e., for increased gene flow) or those that are larger or more protected, which may be more likely to maintain sustainable populations. Finally, incorporating this information into community education programs can help illustrate the importance of particular forest patches in the overall sustainability of each subspecies.
UNCITED REFERENCE
The Climate Data Guide: NDVI: Normalized Difference Vegetation Index − 3rd generation: NASA/GFSC GIMMS, 2018.
ACKNOWLEDGMENTS
We would like to thank Francesco Rovero and Nathalie Cavada for the use of locality data near the Udzungwa Mountains and Tim Davenport for the use of locality data near the Southern Highlands of Tanzania. We would also like to thank Michael Frachetti and the Spatial Analysis, Interpretation, and Exploration laboratory at Washington University in St. Louis for use of its computing facilities for the initial scoping studies and Julie Anderson for providing her original colobus census data set for Kwale District, Kenya. Finally, we would like to specially thank Karen DeMatteo, Lana Kerker Oliver, Corinne Kozlowski, and two anonymous reviewers for their advice and edits on various versions of this manuscript.




