Embraces are lateralized in spider monkeys (Ateles fusciceps rufiventris)
Abstract
Side biases observed in behavior are thought to reflect underlying asymmetric brain function or hemispheric specialization. Previous work in multiple species identified left side biases (associated with the right hemisphere) for processing social behavior. In highly social species such as primates, many behaviors may be categorized as social, yet differences between such behaviors have not been examined as a test of asymmetric brain function. Using Colombian spider monkeys (Ateles fusciceps rufiventris), we observed lateral positioning during two types of behaviors widely categorized as social affiliative: embracing and grooming, and identified a left bias for embracing, but not grooming. Our findings partially support prior research in hemispheric specialization, but suggest that there may be differences between social behaviors that drive specialization. We discuss these results in light of current theory on hemispheric specialization and highlight differences between embracing and grooming.
1 INTRODUCTION
Laterality can be broadly defined as a preference to utilize one side of the body over the other. Laterality has intrigued researchers from a multitude of disciplines because side biases observed in behavior are thought to reflect underlying asymmetric brain function, or hemispheric specialization (for review see MacNeilage, Rogers, & Vallortigara, 2009). One advantage of having specialized hemispheres may be the avoidance of duplication of function (Rogers, Vallortigara, & Andrew, 2013; Vallortigara & Rogers, 2005). In this way, lateralization may serve to streamline neural processing and subsequently increase behavioral efficiency (Rogers & Vallortigara, 2015). Historically, the division of labor between brain hemispheres was thought to be uniquely human. However, reports of laterality are now widespread in vertebrate and invertebrate animals, suggesting that laterality may be a general principle of brain organization (Frasnelli, 2013; Frasnelli, Vallortigara, & Rogers, 2012; Rogers et al., 2013; Vallortigara & Rogers, 2005).
The laterality literature is dominated by the visual processing domain, which has been studied across a number of species and recently summarized (MacNeilage et al., 2009; Rogers & Vallortigara, 2015). Focused attention and routine learned behavior in the pursuit of prey in avian species (Rogers, 2012) and toads (Robins & Rogers, 2004) are examples of right side behavioral biases associated with the left hemisphere. Responding to unexpected stimuli in escape from predators in fish, amphibians, avian species, and mammals (see MacNeilage et al., 2009); face recognition in sheep (Broad, Mimmack, & Kendrick, 2000); and aspects of social behavior in avian species (Daisley, Vallortigara, & Regolin, 2010) are examples of left side behavioral biases associated with the right hemisphere. Of these behaviors, focused attention and facial recognition describe specific cognitive processes that can be isolated with regional specificity within the brain using current neuroimaging techniques in humans (Jäncke, Specht, Shah, & Hugdahl, 2003; Uddin, Kaplan, Molnar-Szakacs, Zaidel, & Iacoboni, 2005). By contrast, social behavior describes a broad construct that can be assessed and characterized by several different behavioral categories that range from aggression or affiliation with associated social interactions, such as contact aggression (e.g., biting) or grooming to higher order social domains such as social reasoning via transitive inference. Social behavior, and how it is defined, thus varies greatly across species. Highly social species, such as primates, have complex behavioral repertories that may not be sub-served by a single hemisphere. Thus, a major limitation of the prevailing laterality theoretical framework is the tendency to group all social behavior together without parsing what could be critical differences between types of social behaviors and associated interactions (cf., Rogers & Vallortigara, 2015).
Social laterality has not been critically examined in non-human primates. Previous studies investigating laterality in a social setting are limited to studies that fail to probe differences in laterality across interaction types, instead defining social as presence of others or examining lateral responses within a single behavior. These include the investigation of spontaneous social behavior in orangutans (Rogers & Kaplan, 1996), approach behaviors in different groups of mangabeys (Baraud, Buytet, Bec, & Blois-Heulin, 2009), and visual orientation toward conspecifics during agonistic encounters in baboons (Casperd & Dunbar 1996). Most recently, Quaresmini et al. (2014) demonstrated that gorillas and chimpanzees maintain conspecifics on the left side during object manipulation in a social setting. In this study, social was defined by presence of conspecifics, and the distance from one individual to another instead of a specific social behavior itself. Overall, these studies provide the firsts steps toward measuring social laterality in non-human primates, particularly from Old World monkeys and apes, yet missing from the literature are studies examining social laterality across multiple behaviors and associated social interactions, particularly in New World species, and in relation to group social dynamics.
Species living in complex social dynamics may experience heightened pressure during social interaction. Fission–fusion is characterized by a unique pattern of separation and reunion whereupon individuals merge into subgroups with variable composition throughout the day, placing a heightened level of complexity on dyadic interactions (Aureli et al., 2008). The fission–fusion social dynamic is hypothesized to be particularly complex given the extent of spatial variation and individual membership in a group over time (Aureli et al., 2008). The fission–fusion dynamic posits differing degrees of fission–fusion dependent on the temporal variation in three elements: spatial cohesion among group members, party size, and party composition. Low degree fission–fusion describes species that exhibit low variation in these three elements, which typically characterizes solitary or territorial species (e.g., orangutans). In contrast, high degree fission–fusion describes species (e.g., chimpanzees, spider monkeys) living in highly fluid multi male, multi-female communities that exhibit high variation in all three elements (Aureli et al., 2008).
Species living in high degree fission–fusion, such as spider monkeys, may be ideal for examining laterality across social interactions because species living in social systems characterized by a high degree of fission–fusion dynamics are more likely to exchange specific social behaviors during reunions (e.g., affiliative interactions) (Aureli et al., 2008). Affiliative interactions (e.g., embracing and grooming) often occur between two individuals (i.e., dyad), are considered pro-social, and commonly involve vocalization and tactile behavior. These behaviors are thought to reaffirm relationships and serve as a method of conflict resolution (de Waal, 2000; Schaffner & Aureli, 2005), but have also been described as related, but separate component parts of social relationships invoking different facets of social processing (Aureli & Schaffner, 2007; Schaffner & Aureli, 2005). Yet, the brain–behavior relationship of affiliative interactions is not understood, and could provide a more comprehensive understanding of the functions of these behaviors.
Ateles phylogeny is characterized by a divergence from the human line 36 million years ago, making them more distant relatives than chimpanzees, which diverged only 8 million years ago (Eizirik, Murphy, Springer, & O'Brien, 2004). Spider monkeys provide an opportunity to examine the role of convergent evolutionary processes since they share a social system with many parallels to chimpanzees and humans. Thus, the spider monkey is an ideal species for further characterizing hemispheric specialization in species living within a high degree fission–fusion dynamic. The objective of this work was to measure potential side biases in the spider monkey's affiliative social repertoire as a test of asymmetric brain function. We examined social laterality, the preference to maintain others on the left or right side during social interaction, by evaluating side biases during three social behaviors: two variations of embracing (i.e., embracing and face-embracing), and grooming. Given previous findings in other vertebrate species, we predicted that spider monkeys would exhibit left side biases (implicating right hemispheric control) during social interaction (e.g., embracing and grooming).
2 METHOD
2.1 Subjects
To evaluate side biases during social interactions, observations were collected from 15 Colombian spider monkeys (Ateles fusciceps rufiventris) housed in an outdoor enclosure viewable by the public at Monkey Jungle in Miami, Florida from May 2015 to August 2015. Observations took place when monkeys were in the main outdoor enclosure (8.84 × 3.96 × 4.47 m3). The sample consisted of six males and nine females, which ranged in age from <1 year to 48 years old.
2.2 Procedure
One hundred and eighty-six hours were collected from the visitor pathway in three intervals throughout the day: 9:30 AM–11:00 AM, 12:30 PM–2:00 PM, and 4:00 PM–5:30 PM to avoid disruptions in data collection due to husbandry procedures. We utilized a behavioral sampling method in which two variations of embracing (embrace and face-embrace) and grooming (Eisenberg, 1976; Schaffner & Aureli, 2005) were recorded upon occurrence. Figure 1 illustrates the difference between the two types of embraces. An embrace was recorded when two individuals approached one another and initiated the contact gesture of wrapping arms around the body and placing the head at the shoulder or along the abdomen. Face-embrace was recorded when two individuals articulated their heads in a way that the cheeks were in contact. Grooming was recorded when an individual presented one side of the body to an individual, and the fur was manipulated with the hands, feet, or mouth. No additional contextual information was recorded for each dyadic event. Each observation included the actor, the social behavior, the recipient, and the side bias. Data were recorded using Apple iPod 5th generation with the application Animal Behavior Pro by four trained observers. Observers achieved an inter-observer reliability of at least 85% during training prior to collecting data. The Institutional Animal Care and Use Committee of the DuMond Conservancy approved the research, and the study was conducted in accordance with the law of the United States. The research adhered to the American Society of Primatologists (ASP) Principles for the Ethical Treatment of Non Human Primates.
3 RESULTS
In order to examine side bias, a Laterality Index (LI) was calculated for each monkey for each social behavior with the formula LI = (R − L)/(R + L), where R is the number of right side responses, and L is the number of left side responses. Individuals were only included in analyses if they met a minimum cutoff of 15 responses per behavioral category. A one-sample Wilcoxon signed-rank test was performed on LI scores with a test value of 0 to test for population-level biases. Sex and age effects were examined using Mann–Whitney U-tests. Individual preferences were calculated with two-tailed binomial probabilities. Alpha was 0.05 for all tests.
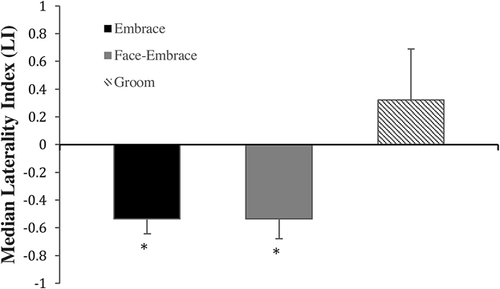
A total of 2,363 occurrences were recorded across all behaviors (1,458 embraces, 368 face-embraces, and 537 grooming interactions). Individual data are provided in Table 1. A significant group-level left side bias was found for embrace (N = 13, Z = −3.180, P = 0.001) and face-embrace (N = 8, Z = −2.521, P = 0.012; see Figure 2). No bias was found for grooming (N = 7, Z = 1.183, P = 0.237). Mann–Whitney U-tests found no sex differences for any of the behaviors. For embrace, 12 monkeys were left-preferent and 1 monkey had no preference. For face-embrace, seven monkeys were left-preferent and one monkey had no preference. For groom, four monkeys were right-preferent, one monkey was left-preferent, and one monkey had no preference.
| Embrace | Face-embrace | Groom | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Subject | Gender | Age | Left | Right | LI | Pref | Left | Right | LI | Pref | Left | Right | LI | Pref |
| Bon Jovi | M | A | 74 | 18 | −0.61 | L | 15 | 1 | −0.88 | L | 3 | 5 | – | – |
| Butch | M | A | 83 | 24 | −0.55 | L | 29 | 10 | −0.49 | L | 10 | 27 | 0.46 | R |
| Carmelita | F | A | 30 | 6 | −0.67 | L | 12 | 5 | −0.41 | NP | 2 | 5 | – | – |
| Cary | F | A | 26 | 11 | −0.41 | L | 0 | 0 | – | – | 12 | 36 | 0.50 | R |
| CJ | F | A | 60 | 18 | −0.54 | L | 12 | 2 | – | – | 2 | 374 | – | – |
| Cleo | F | A | 95 | 31 | −0.51 | L | 48 | 15 | −0.52 | L | 11 | 24 | 0.37 | R |
| Dusky | F | A | 71 | 21 | −0.54 | L | 21 | 6 | −0.56 | L | 14 | 23 | 0.24 | NP |
| Jasper | M | J | 17 | 10 | −0.26 | NP | 0 | 0 | – | – | 2 | 1 | – | – |
| Jenny | F | J | 7 | 1 | – | – | 0 | 0 | – | – | 2 | 1 | – | – |
| Mason | M | A | 139 | 42 | −0.54 | L | 49 | 12 | −0.61 | L | 0 | 5 | – | – |
| Marley | M | J | 7 | 2 | – | – | 1 | 0 | – | – | 2 | 1 | – | – |
| Mints | F | A | 33 | 14 | −0.40 | L | 2 | 0 | – | – | 3 | 8 | – | – |
| Molly | F | A | 64 | 17 | −0.58 | L | 9 | 2 | – | – | 39 | 76 | 0.32 | R |
| Sunday | M | A | 109 | 42 | −0.44 | L | 17 | 5 | −0.55 | L | 14 | 25 | – | – |
| Uva | M | A | 140 | 58 | −0.41 | L | 57 | 23 | −0.43 | L | 15 | 4 | −0.58 | L |
- M, male; F, female; A, adult; J, juvenile; L, left preference; R, right preference; NP, no preference.
- Dash (−) indicates subject did not meet the minimum of 15 observed responses.
4 DISCUSSION
The primary goal of this study was to quantify social laterality in spider monkeys by examining behavioral side biases during dyadic social interactions. We hypothesized that spider monkeys would maintain conspecifics in the left hemifield for social behaviors, implicating the right hemisphere. We found a significant population-level left side bias for embrace and face-embrace, but not for grooming. Notably, the difference in patterning between embracing and grooming suggests that not all social behaviors are associated with the right hemisphere. That these social behaviors did not show similar hemispheric specialization suggests that their functions may vary in spider monkeys. In relating these social behaviors to brain organization, we build upon previous work that has suggested a functional difference between embraces and grooming, whereupon embraces but not grooming regulate social relationships in spider monkeys (Aureli & Schaffner, 2007; Schaffner & Aureli, 2005).
There are several factors that may functionally distinguish embracing and grooming. Both embracing and grooming are broadly classified as social affiliative behaviors in spider monkeys (Fedigan & Baxter, 1984; Pastor-Nieto, 2001); however, there are components of embracing that may necessitate higher levels of arousal than grooming. The most conspicuous element of embracing is the vocalization (e.g., whinny) in combination with the tactile gesture, or interpersonal touch. Developmentally, touch is the first sense to fully emerge and it has repeatedly been implicated as being the most arousing of any sense in humans (Gallace & Spence, 2010). However, both embracing and grooming involve touch. It may be that the specific sequence of the gesture in combination with vocalization as a communicative greeting after separation makes it more arousing. This idea builds on Rebecchini et al. (2011) in which embracing and grooming were identified as related but separate components of social behavior. Principle component analysis (PCA) was utilized to parse types of social behavior in spider monkeys (Ateles geoffroyi). Embracing (along with aggression) loaded positively onto a component the authors termed “risk,” while grooming (along with proximity) loaded positively onto a component the authors were unable to narrow to a specific term, but suggested it was related to compatibility and relationship value. Embracing was distinguished as a behavior involving risk. Although Rebecchini et al. (2011) did not distinguish the name of the component grooming behavior loaded onto, we suggest an appropriate term for that component may be “routine.” The difference in components is supported by our data in which there is a clear split in hemispheric specialization between a behavior involving risk during an event in which there is close exposure of the body and or face (embrace), and a behavior that occurs during a routine state of social touch (grooming). This risk may be related to the high degree fission–fusion dynamic in which social interactions across party composition, party size, and group cohesion over time are highly variable (Aureli et al., 2008). Furthermore, previous research has shown that aggression occurs significantly more often following fusion, yet it is reduced almost completely with the exchange of embraces (Aureli & Schaffner, 2007), suggesting the embrace may be a behavior requiring a higher level of arousal that is associated with monitoring the environment for aggression. Moreover, the gesture occurring very close to the face during embracing may create risk in which a higher level of arousal is warranted. It has been suggested that different forms of touch on different parts of the body might communicate different kinds of information in monkeys (Boccia, Reite, & Laudenslager, 1989). Interpersonal touch in embracing may engender a different emotional response than grooming, which may promote differential functional activation of brain regions. Neuroimaging data from humans have shown stronger brain activation during pleasant and painful touch rather than neutral touch (Rolls & Grabenhorst, 2008; Rolls et al., 2003). Thus, more affective touch may implicate different levels of activation in the brain, which may drive hemispheric specialization. Further research is needed in nonhuman primates to characterize the affective nature of embracing and grooming, perhaps between the sexes. Although we found no sex differences within our data, we are limited in interpreting this finding by the low number of subjects in each group (6 males, 9 females). Future work should include more subjects to adequately address the question of whether sex influences social laterality in spider monkeys.
In considering the left side bias for embracing, our data build upon previous findings supporting right hemisphere processing of social stimuli in multiple species (Rogers & Vallortigara, 2015). Moreover, our data support results in ecologically similar primate models (i.e., chimpanzees and gorillas) in which a trend toward a left-side bias during behaviors involving object manipulation in a social setting was demonstrated (Quaresmini, Forrester, Spiezio, & Vallortigara, 2014). However, a key difference in the data presented here is that we targeted specific social behaviors occurring as interactions. Our approach differs from the study on apes where social behavior was defined by presence of conspecifics, and the distance from one individual to another instead of social interaction (Quaresmini et al., 2014), and other studies targeting a single behavioral mode (Casperd & Dunbar, 1996). Both studies highlight the potential influence of the social environment to elicit lateralized responses, but our data allow us to begin to tease apart lateral differences among multiple behaviors within the social category in understanding asymmetric brain function.
Both embrace and face-embrace expose one side of the body to another individual during an interaction, but visual inspection of these behaviors establishes an appreciation for critical differences between them. The face-embrace requires initial contact of the face without use of the limbs, whereas the embrace requires initial contact of the limbs with the head placed further down along the body, such as on the shoulder or trunk. Although these initial contact gestures are often sequential to other behaviors (e.g., pectoral sniff), in the current study, we only recorded and classified behaviors based on initial contact, which was relevant to our hypothesis. Previous research in wild spider monkeys (A. geoffroyi) has followed a different method, in which each component of a sequential behavior was fractionated in order to identify social traditions (Santorelli et al., 2011). From this work, the “kiss” was identified in spider monkeys. Although similar-sounding to face-embrace, “kiss” is not identical. The kiss involves positioning the side of the face near to another individual with little or no contact and may be sequential to other behaviors (Santorelli et al., 2011). The face-embrace recorded in our group involves direct and initial contact of the cheeks. Data from humans indicates side biases for kissing behavior but these studies introduce additional variation in the term with “kiss” that is not captured in our data set (Chapelain et al., 2015; Ocklenburg & Güntürkün, 2009; Sedgewick & Elias, 2016; van der Kamp & Canal-Bruland, 2011). It is possible that certain social greeting gestures vary among groups of spider monkeys in a similar way that cheek kissing varies in humans in which some cultures kiss once on one side of the cheek, while others kiss on both sides, and others preferring to kiss directly onto the lips. Future work should aim to capture data from multiple sites and integrate wild and captive comparisons so that the development of this potential variation may be comprehensively examined.
Contrary to our prediction based on prior work on asymmetric brain function and side biases, we found that spider monkeys do not maintain conspecifics on the left side during grooming behaviors. It is possible that the lateralized response to the right side in four of the six monkeys (implicating the left hemisphere) may be related to grooming occurring as a state within established subgroups as a simple, routine affective behavior instead of an arousing event as in embracing, lending further support to the distinction between risk and routine among social behaviors in spider monkeys. Neurological research on interpersonal touch in humans is often discussed as social grooming or nurturing tactile contact (Gallace & Spence, 2010; Olausson et al., 2002; Wessberg, Olausson, Fernström, & Vallbo, 2003). Thus, given the potential difference in function of these behaviors, one hemisphere may not be functionally responsible for all social behaviors. Rather, our findings suggest a delineation between grooming and embracing in which embracing is allocated to the left side (implicating the right hemisphere) as a communicative greeting behavior while grooming may be allocated to the right side (implicating the left hemisphere) as a routine, social nurturing behavior. Neuroimaging research in primate models is needed in order to identify specific regions that may be implicated for social behaviors. Furthermore, we suggest additional data are needed to better quantify lateralization of grooming in spider monkeys. Although we showed a trend toward a right side bias, this finding did not reach statistical significance. However, previous research suggests that grooming rates may be lower in spider monkeys compared to other primates and may occur less often than embraces (Schaffner & Aureli, 2005). Future studies should aim to acquire data from larger samples, when possible, or include data from multiple study sites.
Although the general pattern of laterality summarized in the introduction by Rogers and Vallortigara (2015) is appealing, our data show that not all side biases in social behavior can be linked with the right hemisphere. In order to gain a more comprehensive understanding of the evolution of laterality, we suggest a focus on specific social interactions is needed to accurately subdivide social behavior into functional categories within species, and that behavioral observation of lateralized responses is a first step of elucidating functional brain–behavior relationships. Furthermore, we suggest that future work should compare interactive patterns in species living in degrees of fission–fusion in relation to brain organization. Fission–fusion has not previously been discussed as a catalyst for hemispheric specialization. High degree fission–fusion may be a uniquely complex social dynamic in which some social interactions pose a greater risk and in which the cognitive resources required for remembering social details across space and time necessitates efficient neural processing. In this vein, the level of social risk together with the cognitive demand of maintaining relationships in high degree fission–fusion may drive hemispheric specialization. Future work investigating sociality in Ateles should focus on elucidating the social dynamics across social interaction types by employing network-based computation (Wey, Blumstein, Shen, & Jordán, 2008) in order to further examine potential differences among interaction partners between embrace, face-embrace, and grooming, along with structural differences between cohesion patterns in wild and captive populations.
ACKNOWLEDGMENTS
The authors would like to thank Alyssa Seidler for providing the drawings in Figure 1; Giulianna Kendall, Vanessa Padilla, and Andrea Perez-Villarreal for assisting with data collection; and Monkey Jungle staff for their support. This is DuMond Conservancy publication No. 57.




