Advances in primate stable isotope ecology—Achievements and future prospects
Abstract
Stable isotope biogeochemistry has been used to investigate foraging ecology in non-human primates for nearly 30 years. Whereas early studies focused on diet, more recently, isotopic analysis has been used to address a diversity of ecological questions ranging from niche partitioning to nutritional status to variability in life history traits. With this increasing array of applications, stable isotope analysis stands to make major contributions to our understanding of primate behavior and biology. Most notably, isotopic data provide novel insights into primate feeding behaviors that may not otherwise be detectable. This special issue brings together some of the recent advances in this relatively new field. In this introduction to the special issue, we review the state of isotopic applications in primatology and its origins and describe some developing methodological issues, including techniques for analyzing different tissue types, statistical approaches, and isotopic baselines. We then discuss the future directions we envision for the field of primate isotope ecology. Am. J. Primatol. 78:995–1003, 2016. © 2015 Wiley Periodicals, Inc.
INTRODUCTION
Stable isotopic approaches to primate diets are based upon the principle that “you are what you eat.” When you nibble a fig, or devour a slab of beef, carbon and nitrogen isotopes (among others) in these foods find their way into your tissues, such as hair or developing tooth enamel. These isotope values are archives of past meals that can then be “read” to reveal much about your diet, physiology, and habitat. For decades, this approach has been used to answer questions about the diets of humans and other mammals, with a special emphasis on those long extinct [e.g., Cerling et al., 1999; Kingston, 2011; Koch, 1998; Lee-Thorp et al., 1989; Quade et al., 1992; Vogel and van der Merwe, 1977].
By the 1980s, stable isotope analysis was used in archaeology and anthropology departments to answer questions about the adoption of C4 agriculture [Vogel and van der Merwe, 1977], consumption of animal foods [Krueger and Sullivan, 1984] and marine resources [Schoeninger et al., 1983], and to reconstruct paleoclimate [Sealy et al., 1986]. Applications were also expanded to answer more sophisticated questions about seasonal mobility [Sealy et al., 1986] and whether or not individuals grew up in the areas where they were buried [Ericson, 1985; Sealy et al., 1991].
Despite this florescence of studies on humans, there was scant attention paid to non-human primates. Sparse carbon, and sometimes nitrogen, isotopic data appeared for wild non-human primates adventitiously by the mid 1980s and early 1990s [e.g., Ambrose, 1986; Ambrose and DeNiro, 1986; Sealy et al., 1986; van der Merwe and Medina, 1991], but these were only parts of larger studies with no particular focus on the primates themselves. Probably the first study to explicitly address questions about the dietary ecology of non-hominin primates, albeit extinct ones, focused on Papio robinsoni and Theropithecus oswaldi from the Pleistocene fossil site Swartkrans in South Africa [Lee-Thorp et al., 1989]. These authors exploited the well-known difference in δ13C values of C3 plants (most trees, bushes) and C4 plants (largely tropical grasses and sedges), and found that fossil T. oswaldi, like its closest living relative Theropithecus gelada, ate a good deal of grass. Papio robinsoni, in contrast, consumed much more C3 vegetation (as does modern Papio) [Ambrose, 1986; Ambrose and DeNiro, 1986; Lee-Thorp et al., 1989; Sealy et al., 1986]. This study was soon followed by the first stable isotope studies of early hominins [Bocherens et al., 1991; Lee-Thorp et al., 1994].
By the mid 1990s, dozens of studies had been published using stable isotopes to address questions of interest to archaeologists and paleontologists, yet there had never been a study using stable isotopes to explore the ecology of extant non-human primates in natural settings. This may have been, in part, because such studies seemed to offer relatively little. Why use stable isotopes to study modern primates if you can just go out and observe them? Also, the kinds of ecological data provided by stable isotopes are generally much coarser than those provided through observation, as stable isotopes are best at distinguishing between broad classes of foods (e.g., C3 vs. C4 plants; plants vs. animals; terrestrial vs. marine foods) and habitats (e.g., xeric vs. mesic). Francis Thackeray and colleagues [1996] showed that δ13C and δ15N values for collagen from South African baboons (Papio) was affected by both diet and climate. Shortly thereafter, Margaret Schoeninger and colleagues produced a series of seminal papers [Schoeninger et al., 1997, 1998, 1999] that argued convincingly that carbon isotopes could be used to track canopy cover, rainfall, and feeding height, and nitrogen isotopes could be used to discriminate between herbivores and those with more omnivorous diets. They also maintained that among herbivorous primates, nitrogen isotopes could be used to track the importance of legume consumption. These arguments were not entirely novel. However, never before had this complete constellation of ideas been applied specifically to non-human primates.
One of the chief virtues of isotopic analysis is that it allows one to study the foraging ecology of primate species that are difficult (or impossible) to observe because they are small, nocturnal, forage high in the canopy, or are extinct. There also the potential also exists to isotopically address questions about reproductive status and weaning [Fuller et al., 2006; Reitsema et al., 2016], resource allocation [Dalerum et al., 2007; O'Brien et al., 2000], and life history [Macho and Lee-Thorp, 2014]. Additionally, the technique offers the possibility of broadening the temporal scales at which we regularly operate. One might, for instance, ask how human encroachment has influenced the diets and habitats of primates over time so long as archives of feces, hair, or other tissues are available [e.g., Gibson, 2011]. One could just as readily trace ecological change within a lineage over millions of years [e.g., Cerling et al., 2013]. Stable isotope analysis also allows one to ask questions at different spatial scales than traditional observational studies. For instance, Codron and colleagues [2008] analyzed baboon feces from eight localities in Waterberg and Kruger National Park, South Africa (some sampling locations were more than 300 km apart) nearly simultaneously. Fecal δ13C values revealed differences in the consumption of grasses and CAM succulents in the two regions. Work at such a broad spatial scale would be impractical, if not impossible, using observational methods.
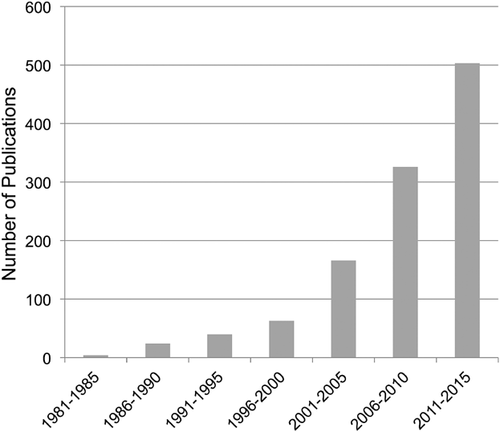
In the past few years, we have witnessed a relative explosion of primate isotope studies. A Google Scholar search indicates an exponential increase in the number of papers on the stable isotope ecology of non-human primates over the past 30 years (Fig. 1). Similarly, a search in the Web of Science using “primate,” “diet,” “carbon,” and “isotope,” produces eight publications for the year 2000, but 56 for the year 2014. This is mirrored in terms of citations, which grew from 287 to 1928 during the same period. These increases reflect the growing popularity of the technique as well as the increasing diversity of isotopic applications in primatology. The time has come to step back and take stock of what we know, question our assumptions, retool our methods, and evaluate new areas to explore. This special issue, which stems from a session at the 2014 meeting for the American Association of Physical Anthropologists, represents a small step in this direction. There are three primary themes in this special issue devoted to primate stable isotope ecology: (i) methodological issues; (ii) environment and habitat reconstructions; and (iii) diet and niche partitioning.
Methodological Issues
Choosing appropriate materials for stable isotopic analysis
Given the omnipresence of carbon and nitrogen in organic materials, these commonly used isotope systems can be analyzed in a variety of Tissues and products from an animal's body as well as from dietary items in the animal's habitat. Articles in this special issue report isotopic data for blood serum [Reitsema et al., 2016], hair [Loudon et al., 2016; Mundry and Oelze, 2016; Oelze, 2016; Schoeninger et al., 2016], bone collagen [Godfrey et al., 2016], bone carbonate [Carter and Bradbury, 2016], and plants [Blumenthal et al., 2016; Carlson and Crowley, 2016]. Skeletal tissues, such as bone collagen, bone apatite, dentine, or enamel can provide valuable information about long-term dietary or environmental trends. Skeletal materials have been the most widely utilized in primate isotope studies, in part because they are relevant to extinct primate taxa, including fossil hominins [e.g., Cerling et al., 2011, 2013, 2004; Codron et al., 2005; Krigbaum et al., 2013; Lee-Thorp et al., 1989; Smith et al., 2010; Sponheimer et al., 2005, 2006; Thackeray et al., 1996].
Body tissues and products with relatively rapid turnover times (on the order of hours to months) such as blood, feces, urine, and hair increasingly are being used to investigate the ecology of primates [e.g., Blumenthal et al., 2012; Codron et al., 2006, 2008; Crowley et al., 2011; Loudon et al., 2007; Reitsema, 2012; Reitsema et al., 2016; Schillaci et al., 2014; Schurr et al., 2012; Vogel et al., 2012]. These materials provide shorter-term information about an individual's diet, habitat, or life history in comparison to skeletal materials. For example, blood plasma has one of the quickest turnover rates of any body tissue; the half-life for carbon is on the order of 24 hours [e.g., Podlesak et al., 2005]. Given its rapid turnover, blood plasma may prove particularly valuable for future isotope validation studies concerning diet and physiology in captive settings. In the wild, blood can occasionally be obtained during primate monitoring efforts that require capture-recapture and often include veterinarian examination, although one should take caution to ensure samples are preserved in a manner that will minimally impact isotope values [Hobson et al., 1997; Sweeting et al., 2004]. Oxygen and carbon isotope values in exhaled CO2 also have very rapid turnover rates, and closely track those in blood plasma [e.g., Pantaleev et al., 1999; Podlesak et al., 2005]. Sampling breath is less invasive than sampling blood, although it does require briefly restraining individuals [Ayliffe et al., 2004; Voigt, 2010].
Hair keratin records a longer period than blood, feces, or urine. Two papers in this special issue present novel hair isotopic data from free-ranging chimpanzee (Pan troglodytes) populations from different regions in Africa [Loudon et al., 2016; Schoeninger et al., 2016]. Isotopic differences among populations, particularly for carbon, underscore the effects of climate and microhabitat (including mean annual precipitation, forest cover, and anthropogenic influences) on stable isotope variation in hair keratin. It is also possible to analyze sections from a single strand of long hair to monitor ecological changes over time for a single individual [Oelze, 2016]. Hair can be readily trimmed or shaved from wild animals during capture-recapture studies. It may also be possible to collect hair from primate nests. In this special issue Oelze [2016] presents a methodological framework for isotope studies using hair to reconstruct temporal variation in feeding behavior. This paper also highlights the potential drawbacks of keratin, and suggests standardized sample preparation guidelines for future work, particularly for studies seeking to use sequential sampling along the hair strand to gain an isotopic chronology of single individuals.
Considering isotope baselines
It has long been recognized that climatic and structural differences within and among habitats affect the isotope values of plants [e.g., Amundson, 2003; Codron et al., 2005; Martinelli et al., 1999; Medina and Minchin, 1980]. Several articles in this special issue specifically address the influences of baseline variation on interpretations of primate behavior [Blumenthal et al., 2016; Carlson and Crowley, 2016; Carter and Bradbury, 2016; Godfrey et al., 2016; Oelze, 2016; Reitsema et al., 2016]. As these manuscripts illustrate, the most appropriate type of baseline data will depend on the research question. Whereas studies explicitly focused on quantifying primate diet (e.g., estimating differences in consumed foods among individuals or assessing the relative importance of a particular dietary resource) may require samples of consumed resources [Blumenthal et al., 2016; Carlson and Crowley, 2016], studies interested in general ecological interpretations may not need this level of detail. Studies on historic or fossil primates may use plant data from comparable modern habitats. For example, Godfrey and colleagues [2016] use δ13C and δ15N values for plants from a diversity of localities in Madagascar to explain spatial variability in the isotope values for the extinct lemur Hadropithecus stenognathus. Alternatively, researchers could rely on data from sympatric taxa with relatively straightforward foraging ecologies, such as ungulate browsers or grazers [Carter and Bradbury, 2016]. While sampling a completely representative baseline is unrealistic in most isotopic studies, for most questions we ask of the fossil record, approximated baselines should be adequate. Researchers investigating suckling infants may choose to use data from lactating females as an isotope baseline [Oelze, 2016; Reitsema et al., 2016]. Reitsema and colleagues point out that attention should be paid to selecting the actual mothers as baselines, as isotope values may vary among females with different reproductive statuses.
In summary, the selection of accurate baseline datasets is highly dependent on the research question and the feeding regime of the group of interest (taxon, age group, etc.). For those researchers interested in diet, the main aim should be to cover the most important items consumed by the primate(s) of interest, including animal foods as well as plants. Both the dietary diversity of a given primate species and the diversity of isotope ratios among food resources should be taken into consideration (including habitat, plant part, canopy position, taxon, and temporal variability), although this can be difficult, and in some cases, impossible. This is where understanding the study system in the context of the research question is crucial. If one wants, for instance, to quantify consumption of foods that differ only moderately in their relevant isotopic compositions (e.g., tree fruits and leaves), one must have much better baseline control than if one only wants to distinguish between consumption of broader food classes (e.g., C3 vs. C4 plants, plant vs. animal foods).
Statistics
Technological advancements combined with the increasing accessibility of mass spectrometers have resulted in larger and more complex isotopic datasets. Interpreting ecological patterns in these large datasets can require advanced statistical approaches. For example, stable isotope mixing models have proven to be a valuable tool for evaluating the contribution of different food items to an individual's or population's diet [e.g., Blumenthal et al., 2012]. Isotope mixing models incorporate categories of food items with distinct isotope signatures such as C3- and C4- plants, canopy and understory fruits or leaves, legumes with nitrogen fixing symbionts, vertebrate flesh, and arthropods [e.g., Phillips and Gregg 2001]. Mixing models can also account for differences in the elemental concentration of sources [Isoconc; Phillips and Koch, 2002]. Traditional mixing models are somewhat limited in the number of discrete dietary resources they can distinguish [reviewed in Phillips and Gregg, 2001, 2003; Phillips et al., 2005]. As a result, probabilistic models are increasingly being used by researchers because they provide distributions of feasible resources and can also calculate uncertainties in model estimates [e.g., Blumenthal et al., 2016; Crowley et al., 2015a]. Several freely available software packages offer Bayesian stable isotope mixing models including “SIAR” [Parnell et al., 2010] and “MixSIR” [Moore and Semmens, 2008]. It is also possible to use probabilistic models to estimate niche overlap (or separation) between two or more sympatric species [Jackson et al., 2011; Crowley et al. 2015a]. Nevertheless, it is critical to keep in mind that these models are not a panacea. They can only produce meaningful results in so much as the user provides meaningful inputs of relevance to the system and question.
Linear mixed models, and their extensions, the generalized linear models (GLM) and generalized linear mixed models (GLMMs), provide particularly informative tools for hypothesis testing in large, complex, natural datasets [e.g., Oelze et al., 2014; Vogel et al., 2012]. These statistical models test multiple predictor variables that potentially affect isotope ratios in primates using likelihood ratio tests. They additionally control for other factors (so called random effects) that potentially explain some of the isotopic variation observed. In this issue, Carlson and Crowley use stepwise linear mixed models to assess the influence of plant type, altitude, and canopy level on plant carbon isotope values. They find that plant part and canopy height have the strongest influence on the δ13C values of plants. Linear mixed models also enable one to control for potential biases resulting from multiple measurements of the same sample (e.g., the same hair strand) or individual. Mundry and Oelze [2016] discuss the effect of pseudoreplication in stable isotope analysis of primate hair and illustrate the potential drawbacks for statistical analysis (type I and type II errors) if pseudoreplication is not controlled for during data analyses.
Environment and Habitat Reconstructions
Several of the manuscripts in this special issue focus on the utility of stable isotope biogeochemistry for characterizing modern habitats or reconstructing past environments. Blumenthal and colleagues [2016] determine that variability in the δ13C and δ15N values of plant samples from Kibale National Park is primarily driven by leaf age (young vs. mature leaves) and plant part (leaves, fruit, or bark). Carlson and Crowley [2016] also use carbon isotope values in plants to define baseline isotopic variability within and among two moist forest sites with varying structures and elevations in western Uganda. The results from these studies demonstrate that isotopic variability associated with vertical position in the canopy, as well as food type, is inconsistent among sites, reiterating the importance of site-specific baseline isotopic data. Loudon and colleagues [2016] and Schoeninger and colleagues [2016] both examine stable isotope values in the hair of wild chimpanzees, comparing new results with previously published data for Pan [Carter and Bradbury, 2016; Fahy et al., 2013; Macho and Lee-Thorp, 2014; Oelze et al., 2011, 2014; Schoeninger et al., 1999; Smith et al., 2010; Sponheimer et al., 2006]. Their results suggest that carbon isotope values can distinguish populations in open, closed, and anthropogenically-disturbed habitats.
Diet and Niche Partitioning
Stable isotope analysis may provide novel insights into primate feeding behavior that cannot be detected using direct observation or indirect monitoring techniques such as fecal analysis or camera trapping. Because isotope ratios in tissues reflect what was consumed (and digested), and not just what might have been consumed, isotopic data may reveal “invisible” behaviors, such as cryptic feeding in living primates or the diets of extinct taxa.
Weaning is a cryptic, but important, aspect of diet among non-human primates. Otherwise hidden night-nursing and comfort nursing are rendered visible through an isotopic approach. In this special issue, Reitsema and colleagues [Reitsema et al., 2016] report how stable isotopic data for blood serum collected throughout the weaning process of captive rhesus macaques provide an objective, longitudinal record of infants’ transitions to nutritional independence. Using carbon and nitrogen isotope ratios in serum, these authors show how characteristics of infants (e.g., infant sex) and mothers (e.g., body size) relate to differences in the timing of weaning events. Isotopic data have also demonstrated that for most infants, suckling persists into subsequent pregnancies, which contributes to growing doubts about the inhibitory effect lactation is presumed to have on ovulation [e.g., Rosetta et al., 2011].
As articles in this special issue demonstrate, stable isotopic data can also help identify how multiple sympatric taxa coexist [Carter and Bradbury, 2016; Godfrey et al., 2016]. This is in part because stable carbon, nitrogen, and oxygen isotope ratios are variable across plant types, plant parts (e.g., leaves vs. fruits), and microhabitats (e.g., vertical niches in a forest) [see Blumenthal et al., 2016; Carlson and Crowley, 2016]. Teasing apart the dual roles of microhabitat and diet on stable isotope variation in animal tissue is important for interpreting isotopic variation of extinct taxa and past forest dynamics. In this issue, Carter and Bradbury [2016] use δ13C and δ18O values from bone apatite of Pan troglodytes, Papio anubis, Procolobus rufomitratus, and Cercopithecus ascanius to confirm the degree to which isotope values track differences in diet as well as foraging height among species. Stable oxygen isotope ratios differ significantly among the taxa, with baboons exhibiting low values and colobines exhibiting high values. Although canopy height and vertical stratification of plant δ18O ratios may explain some of this variation, the authors draw particular attention to the perhaps stronger influence of dietary sources of oxygen: leaves are 18O-enriched compared to other foods, and the authors document a positive relationship between folivory and δ18O values.
FUTURE DIRECTIONS
Looking forward, we discuss emerging directions for the field of primate isotope ecology. Articles in this special issue reiterate and expand on the utility of isotopic data for tracking diet and habitat. More studies on habituated primate groups with well-constrained diets will make it easier to interpret data from free-ranging, non-habituated groups [e.g., Deschner et al., 2012; Koike and Chisholm, 1988; O'Grady et al., 2012; Reitsema, 2012]. Studies increasingly demonstrate that stress and growth affect stable carbon and nitrogen isotope values in animal tissues [Deschner et al., 2012; Fuller et al., 2005; Hatch, 2012; Hatch et al., 2006; Mekota et al., 2006; Reitsema, 2013; Reitsema and Muir, 2015; Vogel et al., 2012; Waters-Rist and Katzenberg, 2010]. Some studies suggest that age, social rank, sex, and reproductive status are factors that influence not only diet, but possibly also the fractionation of isotopes within the body [Crowley et al., 2014; Fahy et al., 2013; Oelze et al., 2011]. However, the mechanisms and effects of these physiological processes remain poorly understood. Given persistent uncertainties surrounding tissue-diet isotopic spacing, additional research in both controlled and natural settings is warranted.
With the continued improvement of analytical techniques, we anticipate seeing an increase in the number of studies that use multiple isotope systems, as well as “less traditional” isotope systems, such as strontium (87Sr/86Sr), calcium (δ44Ca), magnesium (δ26Mg), iron (δ56Fe), copper (δ65Cu), zinc (δ66Zn), and sulfur (δ34S). Their potential utility in the study of non-human primates is virtually untapped. Additionally, those elements that are preserved in tooth enamel (Ca, Mg, Fe, Cu, Sr, Zn) may be particularly useful for the study of fossil primate species [Jaouen et al., 2013; Martin et al., 2014, 2015; Melin et al., 2014]. Strontium isotopes have the potential to track mobility of living and extinct species [e.g., Copeland et al., 2012; Crowley et al., 2015b; Richards et al., 2008; Sillen et al., 1995, 1998] and to identify the provenance of confiscated poached material [Beard and Johnson, 2000; Vogel et al., 1990], which will be increasingly important as primate populations continue to dwindle around the world. Sulfur isotopes could be used to detect utilization of coastal habitat or marine foods by primate species (e.g., Barbary macaques, baboons, or crab-eating macaques). They may also be able to identify consumption of freshwater aquatic resources [reviewed in Nehlich, 2015]. Additionally, sulfur isotopes may be able to trace anthropogenic pollution in primate food webs [Thode, 1991; Winner et al., 1988].
A final emerging research direction in non-human primate isotope ecology is the combination of isotopic and other measures of primate diets, habitats, and physiology. Stable isotopes will never replace traditional studies, but in some instances can be an important complement [Crowley et al., 2014; Fahy et al., 2013], and provide extra value from material (e.g., hair) collected for other reasons, such as DNA analysis. In many instances, stable isotopic data have proven most powerful when they are combined with other behavioral and biological datasets. For example, a combination of stable isotope and observational data have shown that stable isotopes track disease status [e.g., Loudon et al., 2007; Reitsema and Crews, 2011], anthropogenic habitat use [Gibson, 2011; Loudon et al., 2007; Schurr et al., 2012], social dominance [Oelze et al., 2011], and hunting prowess [Fahy et al., 2013] in primates. Similarly, the combination of dental wear and isotopic data can provide a more refined picture of an individual's long-term diet, and may be particularly informative for palaeodietary reconstructions of fossil hominin species [Ungar and Sponheimer, 2011]. Lastly, the combination of endocrinological and isotopic analysis can clarify physiological responses to nutritional or social stress [Deschner et al., 2012; Surbeck et al., 2012; Vogel et al., 2012]. While most hormone metabolites are commonly measured in urine and feces, it may also be possible to measure some (e.g., cortisol) in hair keratin [Carlitz et al., 2014].
In summary, isotopic analysis shines as a useful tool for understanding non-human primates, whose sociality, life history traits, omnivorous and learned foraging behaviors, and diverse metabolic adaptations to climate and food stress create unique opportunities to explore the degree to which biology and behavior impact isotopic variability. Isotopic analysis, with its capacity to explore invisible behaviors at individual (from a life history perspective) as well as population levels, stands to play a key role in our understanding of primate diversity and evolution. We anticipate that continued application of isotopic analysis in non-human primates will foster dialogues among paleoanthropologists, primatologists, and human biologists, and as such, play a critical role in advancing anthropology's purpose to explain the human condition.
ACKNOWLEDGMENTS
The motivation for this special issue began with a symposium for the American Association of Physical Anthropologists. We thank the participants in that original symposium as well as the contributors to this special issue. We also thank Paul Garber for editing and including this special issue in the American Journal of Primatology. This manuscript adhered to the American Society of Primatologists principles for the ethical treatment of primates.




