Environmental variables across Pan troglodytes study sites correspond with the carbon, but not the nitrogen, stable isotope ratios of chimpanzee hair
Abstract
Diet influences the stable isotope ratios of carbon and nitrogen (δ13C and δ15N values) in animal tissue; but here we explore the influences of particular aspects of the local environment on those values in chimpanzees (Pan troglodytes). In this article we present new δ13C and δ15N values in Gombe chimpanzees using hairs collected from night nests in 1989. Then, we explore the influence of environmental factors by comparing our Gombe data to those from eight additional Pan study sites with previously published stable isotope data. We compare chimpanzee δ13Chair and δ15Nhar values to specific characteristics of local site ecology (biome and ecoregion) and to local Mean Annual Precipitation (MAP) to test hypotheses based on known effects of these variables on the δ13C and δ15N values in plant tissues. The comparison shows that hair from chimpanzees living in savanna sites with lower MAP have higher δ13Chair values than do chimpanzees living in woodland and forested sites with higher MAP. These results demonstrate the potential of using δ13C values in primate tissue to indicate aspects of their local ecology in cases where the ecology is uncertain, such as samples collected early in the last century and in fossil hominins. In contrast to expectations, however, chimpanzee δ15Nhair values from some savanna sites with lower MAP are lower, not higher, than those living in more forested areas with higher MAP. It is likely that diet selectivity by chimpanzees affects δ15Nhair values to a greater extent than does the influence of precipitation on plants. Am. J. Primatol. 78:1055–1069, 2016. © 2015 Wiley Periodicals, Inc
INTRODUCTION
Chimpanzees (Pan troglodytes) often serve as referential models in human evolution studies or as parts of conceptual models describing species close to the last common ancestor of the human and ape lineages [see Moore, 1996]. Today, chimpanzees are found in a range of ecosystems, from closed canopy rainforests [e.g., Boesch & Boesch-Achermann, 2000] to closed woodlands [Goodall, 1986; Nishida & Uehara, 1983a] to more open “savanna” sites consisting of grasslands and variable amounts of closed and/or open woodland [McGrew et al., 1981; Moore, 1992; Ogawa et al., 2007]. Multiple lines of evidence suggest that the habitats of our early relatives, that is the australopithecines and early Homo sp. varied as well. For example, fossil fauna and the stable carbon isotope ratios (δ13C values in per mil, ‰, notation) of fossil soil organics and pedogenic carbonates from several sites suggest that these early hominins inhabited woodland, bush savanna, and more open savanna sites, or mosaic ecological regions where plant communities varied over space within a single site [Cerling et al., 2011; Kingston, 2007; Reed, 1997; Wynn, 2000].
Observations of chimpanzee feeding behavior and the composition of their fecal material indicate that diets consist largely of fruit and leaves in all habitats [Basabose, 2002; Newton-Fisher, 1999; Nishida, et al., 1983; Nishida & Uehara, 1983b; Potts et al., 2011; Watts et al., 2012; Wrangham, 1977], and although ingested plant foods vary across seasons and habitats, the focus remains on fruit and leaves with little ingestion of high fiber foods [Macho & Lee-Thorp, 2014; Oelze et al., 2014; Tutin & Fernandez, 1993; Wrangham et al., 1998]. The sites with published feeding information on chimpanzee diets are few [Wrangham, 1977], and those with long-term records [Boesch & Boesch-Achermann, 2000; Goodall, 1986] are biased towards woodland and forest regions [Collins & McGrew, 1988; McGrew et al., 1988]. However, published δ13C values in chimpanzee tissues (hair, bone) from more open sites also show no indication of high fiber C4/CAM foods such as grasses, sedges, and succulents [Schoeninger et al., 1999, 2006a]. In contrast, fossil hominin tooth enamel δ13C data appear to indicate that the hominins ate some amounts of C4/CAM foods [Cerling et al., 2013; Sponheimer & Lee-Thorp, 1999; Sponheimer et al., 2013, 2005; Wynn et al., 2013] with intra-annual dietary variation in some species [Sponheimer et al., 2006]. Indeed, some researchers suggest that the ingestion of C4 foods was fundamental to the separation of the ape and human lineages [Codron et al., 2008].
In interpreting δ13C data in extant and fossil animals, however, commonly overlooked are the influences of the local ecology or ecosystem type (subsequently referred to more specifically as biome and ecoregion following The Nature Conservancy nomenclature) and Mean Annual Precipitation (MAP). These variables can affect the rate of local photosynthetic activity and the isotopic composition of the carbon dioxide available to plants [Farquhar et al., 1989, 1982; O'Leary, 1981], which consequently could affect the δ13C values in primate plant foods. The resultant variation in plant food δ13C values could affect the δ13C values in the animals that feed on them. Most stable isotope studies on nonhuman primates and on fossil hominins focus on diet, which is estimated based on the animal's position along a straight mixing line drawn between the worldwide average δ13C values for C3 plants and C4 plants [commonly taken from O'Leary, 1981, 1988]. Yet, small early studies on New World and Old World primates, including chimpanzees, showed a correlation between aspects of local ecology and the δ13C values in primate tissues, which showed a range of almost 4‰ [Schoeninger et al., 1997, 1999]. Using the average δ13C values for C3 and C4 plants to estimate the percent C3 plants in the diets of the primates with the highest δ13C values would have suggested that they fed on some amount of C4 plants, which observational data disputes.
Studies of living and recent primates (including humans) also interpret tissue nitrogen stable isotope ratios (δ15N values, ‰ notation) in terms of diet [for example: Fahy et al., 2013; Oelze et al., 2014; Richards et al., 2001, 2008; Schoeninger et al., 1999]. Yet, we have evidence in support of an early proposal [Heaton et al., 1986, 1987] that nitrogen stable isotope ratios in plants can be influenced by aridity. Analyses of multiple precipitation gradients from across the globe find that plant δ15N values exhibit significant negative correlations with MAP [Amundson et al., 2003; Aranibar et al., 2004; Austin & Vitousek, 1998; Craine et al., 2009; Martinelli et al., 1999; Schulze et al., 1998]. Animals incorporate δ15N values of consumed plants in their bodily tissues, and several studies have demonstrated significant correlations between bone collagen δ15N values and MAP [Ambrose, 1991; Hartman, 2011; Johnson et al., 1998; Murphy & Bowman, 2009; Pate & Anson, 2008] and/or temperature [Stevens et al., 2006] in a variety of animals although not in primates. Such relationships do not manifest in all species. Studies on North American jackrabbits, for example, found no correlations between bone collagen δ15N values and precipitation or temperature [Ugan & Coltrain, 2011].
In the present study, we investigate whether there is a correlation between several environmental indicators, on the one hand, and the δ13C values and δ15N values in chimpanzee hair on the other. More specifically, we test the hypothesis that chimpanzee hair stable isotope ratios correlate with ecoregion, plant biome and precipitation. To do this, we analyze samples from one site (i.e., Gombe, Tanzania) for which there were no published stable isotope data, and compare the results with published data from other chimpanzee research sites across Africa. A negative correlation between the δ13C values in chimpanzees and their local MAP would support previous arguments against using single δ13C values as the end member of C3 plants in diet estimates for extant primates and in fossil hominins. Instead, as presented most clearly by Murphy & Bowman [2009] the full range of δ13C values of C3 plants must be considered. If there is a negative correlation between MAP and chimpanzee δ15N values, then some measure of aridity must be considered when interpreting the δ15N values in living and recent primates (including humans). Also, if supported, these broader comparisons should improve future modeling of chimpanzee habitats from animal samples that were collected without specific ecological information. For earlier material such as fossil hominins, such results of the comparison would indicate that tooth enamel δ13C values should be interpreted with attention to habitat ecology in addition to diet.
Over the past several decades, analyses of carbon and nitrogen stable isotope ratios have become routine in studies of animal diet [Koch, 2007; Sandberg et al., 2012]. The approach is based on the premise that animal stable isotope ratios vary in direct relation with the stable isotope ratios in the animal's diet [DeNiro & Epstein 1978, 1981], which in the case of primates is largely plant based. In those cases where we lack the ideal of baseline data [Crowley, 2012; Warinner et al., 2013], we base expectations on well-established principles of stable isotope biogeochemistry [Fry, 2006; Hoefs, 2009; Lajtha & Marshall, 1994; Rundel et al., 1989]. This latter approach is the one employed in the present study.
The primary source of variation in δ13Cplant values is the plant's method of photosynthesis [Smith & Epstein, 1971], but we now know that several variables other than the photosynthetic pathway affect the final δ13Cplant value [Marshall et al., 2007]. The following discussion focuses largely on C3 plants because these variables affect C3 plants to a far greater extent than they do C4 plants [Marino & McElroy, 1991] and, as discussed below, living primates largely feed on C3 plants. The most commonly cited average for C3 plants worldwide is −27‰; but the values show a range from −37‰ to −20‰ [O'Leary, 1988]. Specific values result from a balance between the photosynthetic rate and the δ13C value of the carbon dioxide (CO2) available to the plant [Farquhar et al., 1989] both of which are affected by available water, altitude, temperature, plant phylogeny, canopy cover, and leaf type [Farquhar et al., 1982; Marshall et al., 2007]. Global distributions of δ13C plant values, however, demonstrate that of all these variables MAP has the strongest influence on C3 plants [Diefendorf et al., 2010; Kohn, 2010]. A compilation of over 1,300 C3 plant samples from 570 individual sites that span a large range of MAP, Mean Annual Temperature (MAT), altitude, and latitude show a monotonic decrease between δ13Cplant values and increasing MAP with far lower effects of MAT, altitude, latitude, and other variables than expected [Kohn, 2010]. The highest δ13Cplant values come from scrub bushlands like those in Israel [−22‰ see Hartman & Danin, 2010] and extremely arid environments (e.g., the Atacama Desert) [up to −20‰ see Kohn, 2010] while those below −31.5‰ come from tropical closed canopy forests that result from the uptake of recycled 13C-depleted CO2 released from soil respiration as well as lower light levels [Bonafini et al., 2013; van der Merwe & Medina, 1991; Vogel, 1978; Yakir & Israeli, 1995]. In addition to the distribution of δ13Cplant values, overall plant biomass varies linearly with precipitation globally [Kohn, 2010]. Usually there is a greater amount of canopy cover with lower δ13Cplant values in regions with higher MAP although there are regions where local temperature and specific soil types can affect these relationships [Michaletz et al., 2014] .
In contrast to C3 plants the range in C4 plants (arid adapted grasses and a few species within the sedge family) is much smaller, approximately 4‰, with a range from −15‰ to −11‰ and a mean δ13Cplant value of −13‰ (O'Leary, 1988). Crassulacean Acid Metabolism plants (CAM), which are also arid adapted plants [Ehleringer, 1978; Stowe & Teeri, 1978; Tieszen et al., 1979] include cacti and succulents with δ13C values that can overlap with C4 plants [Smith & Epstein, 1971].
The habitats of most primates are characterized as having predominantly C3 plant species [Cerling et al., 2004] and their diets reflect this bias. Thus far, there is little evidence that either C4 or CAM plants are significant foods for living nonhuman primates [Crowley et al., 2010; Sandberg et al., 2012; Schoeninger, 2014]. While macaques observed raiding maize fields on a seasonal basis had somewhat elevated δ13Chair values, their bone collagen indicated a pure C3 diet [O'Regan et al., 2008]. Although baboon fecal samples recovered beneath a succulent plant (i.e., CAM) in a marginal environment had δ13C values in the C4 range, baboon tooth samples from the same area indicated a complete C3 diet [Codron et al., 2006]. Even geladas, which feed extensively on grass live in regions of high altitude that are relatively cool with relatively high rainfall where most plant species, including grasses, are C3 [Fashing et al., 2014].
Because primates feed so extensively on C3 plants, it was unsurprising that primate δ13C values varied with canopy cover (which, in turn, varies with MAP) rather than dietary specifics [Schoeninger et al., 1999]. Still, recent work demonstrates that some C3 plant foods can have strikingly different δ13C values than other foods within the same ecoregion [representative examples included in: Cerling et al., 2004; Codron et al., 2005; Oelze et al., 2014]. For example, some fruits within the Ituri Forest had carbon stable isotope ratios that were much higher (e.g., −30‰ to −31‰) than those in leaves taken from the canopy floor (−37‰) [Cerling et al., 2003]. Yet, within the canopy (i.e., the main area of feeding for primates) both leaves and fruits had values that were 5‰ higher than leaves and fruits from the subcanopy. Significantly, there was no association between primate δ13C values and the differential mix of fruit and leaves among sympatric folivorous, frugivorous, and omnivorous primate species; all species had δ13C values within 1‰ of each other [Cerling et al., 2004].
Some, although not all, fruits eaten by both chimpanzees and gorillas at Loango, Gabon had δ13C values that are about 1‰ higher, on average, than leaves, and the chimpanzees there had higher δ13Chair values than did the gorillas [Oelze et al., 2014]. Because approximately 60–75% of the carbon used in synthesizing tissue proteins comes from dietary protein [Fernandez in press; Froehle et al., 2010] Oelze et al. [2014] concluded that the higher δ13C values in chimpanzees reflected their higher fruit intake relative to gorilla, and that fruit provided significant amounts of protein to the chimpanzees. However, fruits normally consist of carbohydrates with little to no protein [Fleagle, 2013; Murray et al., 2001] and the C:N ratios in fruits at Loango [Oelze et al., 2014] are extremely high, which is consistent with general expectations for fruit. To be certain of the situation with regards to protein at Loango, nutritional analyses must be done on the various plant foods before it can be concluded whether or not the fruits provide chimpanzee dietary protein. On the other hand, 25–40‰ of the carbon in collagen (and hair) comes from nutritional sources other than protein, and fruit carbohydrate (simple sugar and nonstructural carbohydrate) is the most likely source. Therefore, the higher δ13Chair values in chimpanzees versus gorillas could still be a result of fruit intake even though it is not the protein fraction of the diet that is the source. Alternatively, perhaps the situation at Loango is similar to that at Taï where chimpanzees have a similar focus on fruit. Researchers there concluded that the fruit diet at Taï provided overall protein intake lower than recommended by the National Research Council resulting in diets that could be protein deficient [N'guessan et al., 2009].
The variation in nitrogen stable isotope ratios (15N/14N represented by δ15N values) within the biosphere differs from that of carbon stable isotope values. The variation in plant δ15N values is determined by the δ15N value of the nitrogen used by plants, which can be atmospheric (N2) via bacterial nodules on plant roots or, more commonly, soil nitrogen [Shearer & Kohl, 1994]. Animal δ15N values are determined by δ15N values in their foods [DeNiro & Epstein, 1981], which as mentioned previously are mostly plant based. In general, herbivores overall have δ15N values in their tissues that are approximately 3‰ higher than the plants on which they feed, and carnivores are approximately 3‰ higher than the animals on which they feed [Minagawa & Wada, 1984; Schoeninger & DeNiro, 1984; Schoeninger, 1985] although there can be quite a bit of variation around these values [Koch, 2007]. Individual chimpanzees observed eating animal material had higher δ15Nhair values than did their more herbivorous companions [Fahy et al., 2013], the results of a similar study on bonobos were less clear [Oelze et al., 2011] and in both cases, the overall effect was quite small.
Global soil δ15N values vary between −2.0‰ and 10.3‰ [Amundson et al., 2003] depending on the amount of biomass degradation, the parent material, precipitation, salinity, and various processes such as leaching, ammonia volatilization, and denitrification [Heaton, 1987; Shearer & Kohl, 1994]. But, based on the available data thus far African soil δ15N values vary by less than 3‰, on average, and within the region where the chimpanzee sites are located, the soil δ15N values vary by less than 2‰ (9–10.3‰) [Amundson et al., 2003]. In sum, the range of baseline δ15N values across the chimpanzee sites is, on average, much lower than global distributions and nitrogen cycling processes would suggest.
Plant δ15N values are normally lower than soil and across Africa they can be 2–5‰ lower [Amundson et al., 2003], which increases the expected range of δ15N values within plants. Several studies, based on work begun in the early part of the last century, demonstrate that the greatest effects on plant δ15N values are MAP and MAT [Amundson et al., 2003; Austin & Vitousek, 1998; Handley et al., 1999; Martinelli et al., 1999]. In general, systems that are wet and cool have lower δ15N values [Evans, 2001] compared to those systems that are open, warm and dry [Amundson et al., 2003; Austin & Vitousek, 1998; Shearer & Kohl, 1986]. Some studies suggest that in temperate latitudes with marked seasonal variation in MAT, the animals most affected are ones that must drink water [Cormie & Schwarcz, 1996, 1994]. Water independent species, such as jackrabbits in North America and northern Mexico, can show no correlation between the δ15N values of bone collagen and MAP [Ugan & Coltrain, 2011], or show significant positive correlation with MAT rather than with MAP (Somerville, in preparation). MAT is expected to have the smaller effect across the chimpanzee sites because of the relatively narrow range of MAT across within the tropical latitudes inhabited by chimpanzees. Additionally, although altitude can vary within sites, the magnitude is small relative to that observed affecting soil δ15N values [Amundson et al., 2003] and chimpanzees feeding is not limited to a single altitude.
Analyses from precipitation gradients across Australia, Africa, and in Israel find that δ15N values in plants and animals consistently show higher δ15N values in environments with lower water availability indices or with lower MAP, than in wetter zones [Ambrose, 1991; Grocke et al., 1997; Hartman, 2011; Heaton et al., 1986; Murphy & Bowman, 2006, 2009; Pate & Anson, 2008; Sealy et al., 1987]. This pattern occurs because water is a limiting nutrient in dryer systems resulting in a relative excess of soil nitrogen, which “... leaves the system more readily through fractionating pathways” [Murphy & Bowman, 2009:1046] that enrich the remaining soil nitrogen in 15N. The relationship, however, is not linear in either plants or in animals. Studies on Australian macropods [Murphy & Bowman, 2006; Pate & Anson, 2008] and eutherian mammals in Africa [Heaton et al., 1986] found the largest increases in bone δ15N values in regions with less than 400 MAP with smaller increases in regions with >400 and <1,000 MAP and little to no effect above 1,000 MAP. There is a marked range of MAP across the chimpanzee sites that could affect chimpanzee hair δ15N values.
METHODS
Our study analyzed samples from Gombe National Park in western Tanzania, which has been the location of Jane Goodall and colleagues’ ongoing research since 1960 [see Goodall, 1986; Wilson, 2012 for overviews]. It covers an area of about 35.4 km2, and is a mosaic of evergreen forest, open woodland, and grassland [Clutton-Brock & Gillett, 1979; Goodall, 1986; Wilson, 2012]. The dry season lasts from May to October [Wallis, 1995], average yearly rainfall has been variously reported from 750 to 1250 mm [Teleki et al., 1976] to 1546 [Clutton-Brock & Gillett, 1979]. Our study included samples from the Kasekela community [Goodall, 1986], which is located centrally in the park.
Stable isotope analysis was performed on hair from 13 known individuals (see Table I) collected in 1989 from night nests by J.J.M. and collaborators as part of another project. The hair collection involved no invasive techniques nor did it involve any animals directly; that is these were naturally shed hairs. The project received approval from IACUC and followed the American Society of Primatologists’ principles for the ethical treatment of nonhuman primates. The Tanzanian Commission on Science and Technology (COSTECH) permitted data collection on the Gombe chimpanzee community.
| Spec # | Name | Sex | δ13C (‰) | δ15N (‰) | C:N |
|---|---|---|---|---|---|
| MS-6174 | Evered | M | −23.6 | 3.2 | 3.7 |
| MS-6175 | Fifi | F | −23.1 | 3.1 | 3.7 |
| MS-6177 | Faustino | M | −22.7 | 3.4 | 3.7 |
| MS-6179 | Frodo | M | −22.7 | 3.8 | 3.4 |
| MS-6181 | Gimble | M | −23.1 | 4.0 | 3.5 |
| MS-6182 | Goblin | M | −23.5 | 3.6 | 3.5 |
| MS-6186 | Prof | M | −22.8 | 3.0 | 3.6 |
| MS-6190 | Pax | M | −23.0 | 3.9 | 3.5 |
| MS-6191 | Sandi | F | −23.7 | 3.1 | 3.6 |
| MS-6192 | Spindle | M | −23.2 | 3.6 | 3.7 |
| MS-6193 | Sparrow | F | −23.2 | 3.8 | 3.5 |
| MS-6194 | Tubi | M | −22.7 | 3.4 | 3.6 |
| MS-6197 | Wilkie | M | −23.0 | 3.4 | 3.7 |
In addition to the new data in our study, we compiled published data from eight other study sites to evaluate the association between habitat characteristics and rainfall levels (MAP, an indirect proxy for vegetation) with carbon and nitrogen stable isotope ratios across regions and researchers (Table II). These sites include Ishasha, Zaire and Ugalla, Tanzania [Schoeninger et al., 1999]; Fongoli, Senegal [Sponheimer et al., 2006]; Kibale, Uganda [Carter, 2001]; a restricted area within central Cameroon [Macho & Lee-Thorp, 2014]; Taï, Cote d'Ivoire [Fahy et al., 2013]; Ganta, Liberia [Smith et al., 2010]; and Loango, Gabon [Oelze et al., 2014] (Fig. 1).
| δ13C (‰) | δ15N (‰) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Site | Biomea | Ecoregion | MAP (mm) | n | Mean | SD | n | Mean | SD |
| Ishashab | TSGSS | Sahelian Acacia Savanna | 750 | 8 | −23.1 | 0.2 | 7 | 5.9 | 0.8 |
| Fongolic | TSGSS | West Sudanian Savanna | 950 | 36 | −22.2 | 0.4 | 36 | 2.9 | 0.3 |
| Ugallab | TSGSS | Central Zambezian Miombo Woodlands | 1050 | 12 | −22.0 | 0.3 | 8 | 2.3 | 0.8 |
| Gombed | TSGSS | Central Zambezian Miombo Woodlands | 1250 | 13 | −23.1 | 0.3 | 13 | 3.5 | 0.3 |
| Kibalee | TSMBF | Albertine Rift Montane Forest | 1500 | 15 | −23.5 | 0.5 | 15 | 7.0 | 0.8 |
| Cameroonf | TSMBF | Northwestern Congolian Lowland Forests | 1700 | 39 | −24.9 | 0.9 | 39 | 9.1 | 1.7 |
| Taїg | TSMBF | Western Guinean Lowland Forest | 1800 | 52 | −24.9 | 0.5 | 52 | 7.4 | 0.9 |
| Gantah | TSMBF | Western Guinean Lowland Forest | 1956 | 37 | −24.6 | 0.5 | 21 | 6.2 | 0.7 |
| Loangoi | TSMBF | Atlantic Equatorial Coastal Forest | 2215 | 14 | −24.6 | 0.5 | 14 | 4.8 | 0.4 |
- a Biome types. TSGSS, Tropical and subtropical savannas, grasslands, and shrublands; TSMBF, Tropical and subtropical moist broadleaf forests. Both designations follow the nature conservancy nomenclature.
- b Schoeninger et al. [1999].
- c Sponheimer et al. [2006].
- d This study.
- e Carter [2001].
- f Macho and Lee-Thorp [2014].
- g Fahy et al. [2013].
- h Smith et al. [2010] with MAP data from Harley [1939].
- i Oelze et al. [2014].
When the original data were derived from bone collagen samples [e.g., Smith et al., 2010 from Ganta], we converted the values to δ13Chair values. In doing so, we chose not to follow Fahy et al. [2013] in using the value of 2‰ because the source they cite is a paper on nitrogen stable isotopes [DeNiro & Epstein, 1981], and the earlier paper on carbon [DeNiro & Epstein, 1978] reported on chitin and bone collagen, but not keratin. Instead, we applied the conversion (−1.4‰ for δ13C values and −0.86‰ for δ15N values) following O'Connell et al. [2001] even though the data are limited to modern British people and the Δ13C values show a large amount of variation (i.e., S.D. = 0.45 for δ13C values and 0.17 for δ15N values). We chose not to follow Crowley et al. [2010] in using apparent enrichment values (ϵ*) because while they are necessary when comparing materials with vastly different 13C/12C ratios relative to the PDB or SMOW standards (e.g., geochemical versus biological materials) [Hoefs, 2009] we compare only biological materials (e.g., hair versus bone collagen) that are relatively similar to one another. In addition, the majority of ecological and biological papers use delta values. The reader can convert the δ13Chair values to apparent enrichment values using the following equations: (1) ϵ* = (α−1) and (2) αcollagen-keratin = (1,000 + δ13Ccollagen)/(1,000+ δ13Chair) where ϵ* is the apparent enrichment value and α is the apparent fractionation factor [see Crowley et al., 2010 for apparent enrichment and alpha values]. In any case, within this study any errors resulting from these choices will be constants (with the exception of the Ganta data) and will not affect the assessments of relationships between the variables.
In addition, museum samples (Ganta and Cameroon) collected in the last century had to be corrected as adjustment for the lower δ13C values in today's atmospheric CO2. We chose not to follow [Kohn, 2010] who applied a correction of 0.023‰/year to plant samples collected within the last 10–15 years because the museum samples were collected in the early 1940's. The massive changes in the δ13C values of atmospheric carbon dioxide (Keeling Curve at Scripps Institution of Oceanography, https://scripps.ucsd.edu) have occurred since 1960 so atmospheric δ13C values in the years of museum collecting would be virtually identical to 1960. For the Ganta and Cameroon specimens, we subtracted an additional 1.1‰ from the bone collagen δ13C values [following Smith et al., 2010] who relied on Hoppe's estimate from prehistoric bison tooth enamel samples standardized against ice core data [Hoppe et al., 2006]. This figure is almost identical to the 1.2‰ used by Crowley [Crowley et al., 2011a] who relied on Chamberlain's estimate from prehistoric condors that was also standardized against ice core data [Chamberlain et al., 2005].
The different Pan sites were characterized using The Nature Conservatory's (TNC) online spatial dataset for the terrestrial ecoregions and biomes of the world (see http://maps.tnc.org/). Categories of the TNC system were designated through collaborations with the World Wildlife Fund and over 1,000 scholars from diverse fields, including ecologists, biogeographers, taxonomists, and conservationists. The categories provide highly accurate spatial categorization of different environmental regions [Olson et al., 2001]. The freely available georeferenced maps provide a standard reference for scholars around the globe. All chimpanzee sites included in the present meta-study fall within two broad biomes: Tropical and Subtropical Moist Broadleaf Forests (TSMBF) and Tropical and Subtropical Savannas, Grasslands, and Shrublands (TSGSS). Within these categories, seven separate terrestrial ecoregions are represented and are identified in Table II.
Rainfall figures (MAP) in the literature can vary greatly as a function of sampling period and methodological issues (as noted above for Gombe). We use the values reported for individual sites by the authors of the papers reporting the stable isotope data. In the case of Gombe, we chose the high end of the range reported by Teleki and colleagues [750–1250 mm; Teleki et al., 1976], which covered a 10-year period, because the value presented by Clutton-Brock & Gillett [1979; 1546 mm] fell so far outside the 10-year report. For Ganta, we follow Smith et al. [2010] who base their estimate on a 6 year average of values recorded between 1927 and 1936 [Harley, 1939].
To test for differences between males and females at Gombe, we used the non-parametric Mann–Whitney U test. Comparisons of chimpanzee δ13C and δ15N hair values between the two biomes were made with independent samples t-tests. Simple linear regressions determined the relationship between MAP and stable isotope ratios. A one-way analysis of variance (ANOVA) and Tukey's post hoc tests of significance assessed the differences in stable isotope ratios between the individual sites. All statistical analyses were conducted with SPSS v. 22.
All hair samples were washed in sequential ultra-sonic baths of double-distilled de-ionized water and acetone to remove environmental contaminants and extraneous body oils. Hairs were dried at 50°C overnight in a laboratory oven. To homogenize bulk samples, hairs were finely cut with a stainless steel scalpel into small (∼1 mm) pieces and mixed. Between 0.5 and 1.0 mg were added directly to tin capsules for combustion. Samples were analyzed in an automated fashion on a Thermo-Finnigan Delta XP Plus, Conflow and Costect EA in the Analytical Laboratory at Scripps Institute for Oceanography (SIO). Data are expressed using the standard formula δ = ((Rsample/Rstandard)−1) × 1,000 where R = 13C/12C or 15N/14N. The carbon sample was standardized relative to relative to the Pee Dee Belemnite standard (PDB) while the nitrogen sample was standardized relative to the ambient inhalable reservoir (AIR). Repeated analysis of an internal laboratory standard over the last 5 years indicates a precision of 0.12‰ and 0.15‰ for δ13C and δ15N, respectively. All hair samples had C:N ratios falling within the acceptable range of 2.9–3.8 [O'Connell et al., 2001].
RESULTS
There are no statistically significant differences in hair stable isotope values between males and females within the Kasekela community at Gombe (δ13Chair females = −23.2‰ and males = −23.0‰; P = 0.573, δ15Nhair females = 3.3‰ and males; = 3.6‰ P = 0.371). We note that the sample size for females is small (N = 3 versus 10 males), and more samples are needed to be sure of the apparent lack of difference. Based on the lack of significant differences, however, we combined the samples when comparing with the published data from other sites.
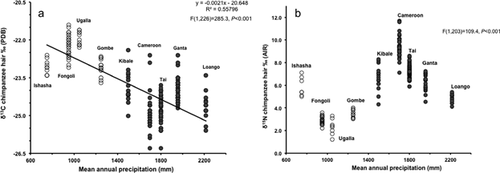
When the Gombe isotope data are combined with data from the other Pan sites (Fig. 2), some patterns emerge. Highly significant differences exist between the individual chimpanzee sites in both carbon (P < 0.001; Table III) and nitrogen (P < 0.001; Table IV) stable isotope values. There is a statistically significant negative regression equation (r2 = 0.5579, df = 285.3, P < 0.001) between MAP and δ13Chair values at the various study sites (Fig. 2a) although two sites fall off the line. Ishasha, which has the lowest MAP value, falls below the line and Ugalla with 250 mm more MAP per year than Ishasha falls above the line. In addition, most of the Cameroon samples fall below the line and there are two outliers. The simple linear regression assessing the relationship between δ15Nhair and MAP found a significant positive regression equation (r2 = 0.35012, df = 109, P < 0.001). But the relationship is not entirely clear because MAP explains only 35% of the variation in δ15Nhair values. For that reason, we have plotted the data without a regression line (Fig. 2b).
| Fongoli | Ugalla | Gombe | Kibale | Cameroon | Taї | Ganta | Loango | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ishasha | 0.00 | ** | 0.00 | ** | 1.00 | 0.01 | * | 0.00 | ** | 0.00 | ** | 0.00 | ** | 0.00 | ** | |
| Fongoli | 0.00 | ** | 0.86 | 0.00 | ** | 0.00 | ** | 0.00 | ** | 0.00 | ** | 0.00 | ** | |||
| Ugalla | 0.00 | ** | 0.00 | ** | 0.00 | ** | 0.00 | ** | 0.00 | ** | 0.00 | ** | ||||
| Gombe | 0.01 | * | 0.00 | ** | 0.00 | ** | 0.00 | ** | 0.00 | ** | ||||||
| Kibale | 0.00 | ** | 0.00 | ** | 0.99 | 0.00 | ** | |||||||||
| Cameroon | 0.63 | 0.00 | ** | 0.63 | ||||||||||||
| Taї | 0.00 | ** | 1.00 | |||||||||||||
| Ganta | 0.00 | ** | ||||||||||||||
- Bold values indicate statistical significance between the sites compared.
- ** P < 0.05.
- * P < 0.001.
| Fongoli | Ugalla | Gombe | Kibale | Cameroon | Taї | Ganta | Loango | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ishasha | 0.00 | ** | 0.00 | ** | 0.00 | ** | 0.233 | 0.00 | ** | 0.00 | ** | 0.92 | 0.03 | * | ||
| Fongoli | 0.41 | 0.14 | 0.00 | ** | 0.00 | ** | 0.00 | ** | 0.00 | ** | 0.00 | ** | ||||
| Ugalla | 0.00 | ** | 0.00 | ** | 0.00 | ** | 0.00 | ** | 0.00 | ** | 0.00 | ** | ||||
| Gombe | 0.00 | ** | 0.00 | ** | 0.00 | ** | 0.00 | ** | 0.00 | ** | ||||||
| Kibale | 0.00 | ** | 0.09 | 0.76 | 0.00 | ** | ||||||||||
| Cameroon | 0.00 | ** | 0.00 | ** | 0.00 | ** | ||||||||||
| Taї | 0.00 | ** | 0.00 | ** | ||||||||||||
| Ganta | 0.00 | ** |
- Bold values indicate statistical significance between the sites compared.
- ** P < 0.05.
- * P < 0.001.
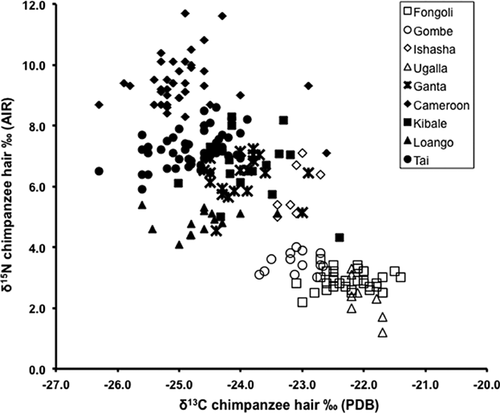
As seen in the plot of δ15Nhair against δ13Chair values (Fig. 3), most of the samples from the Tropical and Subtropical Moist Broadleaf Forests (TSMBF) biomes (Kibale, Cameroon, Taï, Ganta, and Loango) exhibit δ13Chair values between −24.0‰ and −26‰ whether they are from Montane or Lowland Forest Ecoregions. In contrast, most of the samples from the Tropical and Subtropical Savannas, Grasslands, and Shrublands (TSGSS) biomes (Ishasha, Fongoli, Ugalla, and Gombe) have δ13Chair values between −23.0‰ and −21.5‰ whether they are Savanna or Miombo Woodland ecoregions. There is some overlap of samples from Gombe, Fongoli, and Ishasha (TSGSS biomes) with those from Ganta and Kibale (TSMBF biomes). Independent sample t-tests found a highly significant difference in δ13Chair hair values between TSGSS (N = 71, Mean = −22.5‰, SD = 0.57) and TSMBF (N = 157, Mean = −24.6‰, SD = 0.7) biomes; t(226) = 21.6, P < 0.001. The majority of the δ15Nhair values from the TSMBF biomes fall between 5‰ and 10‰, and those from TSGSS biomes fall between 2‰ and 4‰. Ishasha is an exception: its δ15Nhair values fall well within the values for the TSMBF biomes even though it is located within a Savanna ecoregion of the TSGSS Biome. Even so, independent sample t-tests found a highly significant difference in δ15Nhair values between TSGSS (N = 64, Mean = 3.3‰, SD = 1.1) and TSMBF (N = 141, Mean = 7.5‰, SD = 1.6) biomes; t(173.75) = −18.73, P < 0.01.
DISCUSSION
The correlation between MAP and δ13Chair values follows the predicted pattern of higher δ13C values in animal tissues from drier regions with some exceptions although the chimpanzee δ13Chair values at Ishasha are, on average, almost 1‰ lower than expected. These chimpanzees live in the gallery forest that lines a perennially flowing river with their night nests situated within the gallery forest and most feeding takes places within this more humid region [Sept, 1992]. In contrast, the chimpanzees from Ugalla have δ13Chair values that are higher than expected based on reported MAP (Fig. 2a) although they are well within expected values for eating a complete C3 diet. Ugalla has leguminous trees that form a continuous but thin canopy, which allows enough light for C4 grasses in the understory (the botanical identifier for a TSGSS biome). It is possible that Ugalla is a case where the MAP in an unreliable indicator of canopy cover or that the lacey leaves of leguminous trees have relatively high δ13C values. A large project is collecting vegetation samples at present, and the stable isotope analyses of the samples should further our understanding of this site [Piel et al. unpublished data].
The average values for the rest of the sites fall on or close to the regression line, although there is a lot of scatter in individual δ13Chair values around the line. Some of this scatter is probably the result of chimpanzee dietary selectivity. For example, although the average δ13Chair value at Loango falls on the regression line, many individuals fall above the line, which may reflect the elevated δ13C values in some fruits eaten by chimpanzees as discussed by Oelze et al. [2014]. To evaluate the possibility that the δ13Chair values reflect fruit-eating rather than MAP, we compiled published data on percent of feeding time spent on fruit, leaves, flowers, bark, stem/pith, seeds, and other (Table V). Such data are not available for the majority of the sites included in the present study and we decided that there was not a way to compare feeding information taken from fecal material directly with percent of feeding time so we list those sites as not having available feeding data and report only on sites with observations of feeding. Semliki, Uganda [Hunt & McGrew, 2002], in a TSGSS biome with a permanently flowing river, shows the lowest amount of feeding time spent on fruit (39%) of all the sites for which we have data. Fongoli, Senegal [Pruetz, 2006] another TSGSS biome site reports 62.5% feeding time devoted to fruit based on a limited sample size. We include three different dietary estimates for Gombe, Tanzania (also a TSGSS biome), all of which cite Wrangham [1977]. Two of these report amounts of feeding time spent on fruit (59.4% and 63%) that are relatively similar to each other [Newton-Fisher, 1999; Watts et al., 2012] and to Fongoli; but the third [Morgan & Sanz, 2006] reports a much lower amount of time (43%). Based on personal observations, JMM thinks that the lower value is unlikely; but we include it because it is often cited.
| Site | Fruit (%) | Leaves (%) | Flowers (%) | Bark (%) | Other (%) | Stem/pith (%) | Seeds (%) | Total |
|---|---|---|---|---|---|---|---|---|
| Fongolia | 62.5 | 16 | 11 | 2.5 | 3 | 95 | ||
| Gombeb | 43 | 27 | 10 | 0 | 7 | 8 | 7 | 102 |
| Gombec | 63 | 19 | 82 | |||||
| Gombed | 59.4 | 21.2 | 19.4 | 100 | ||||
| Kibale- Kanyawarae | 79 | 2.6 | 16.9 | 98.5 | ||||
| Kibale-Ngogof | 70.7 | 19.6 | 2.5 | 2.2 | 4 | 99 | ||
| Taїg | 77 | 17.5 | 1 | 4 | 99.5 | |||
| Semlikih | 39 | 30 | 3 | 12 | 84 | |||
| Budongoi | 64.5 | 19.7 | 8.8 | 3.2 | 96.2 | |||
| Not available | ||||||||
| Cameroonj | ||||||||
| Gantaj | ||||||||
| Loangok | ||||||||
| Ugallaj | ||||||||
| Ishashaj |
- a Pruetz [2006].
- b Morgan and Sanz [2006] citing Wrangham [1977].
- c Newton-Fisher [1999] citing Wrangham [1977].
- d Watts [2012] citing Wrangham [1977].
- e Wrangham et al. [1996].
- f Watts [2012].
- g Based on Doran [1997] and Watts [2012].
- h Hunt and McGrew [2002].
- i Newton-Fisher [1999].
- j No collection or no systematic collection.
- k Fecal prevalence, Oelze et al [2014].
For the TSMBF biome sites, Kanyawara [Wrangham et al., 1996] and Ngogo [Watts et al., 2012] at Kibale, Uganda (a TSMBF biome) are similar to each other (>70% of feeding time spent on fruit), and also Taï, Cote d'Ivoire (77%) [based on Doran, 1997; Watts et al., 2012]. But, Budongo, Uganda, another TSMBF biome reports 64.5% of feeding time spent on fruit [Newton-Fisher, 1999], which is similar to that reported for Fongoli and Gombe (TSGSS biome sites). Overall the wetter sites (Kibale and Taï; but not Budongo) report greater percent feeding time focused on fruit and have lower δ13Chair values than do the dryer sites (Fongoli and Gombe) suggesting a negative correlation between fruit eating and δ13Chair value whereas the higher δ13C values in some fruits at Loango suggest that a higher fruit intake would associate with higher, not lower, δ13Chair values [Oelze et al., 2014]. In other words, the relationship (if there is one) is the opposite of that expected based on assumptions that fruit δ13C values would be higher than leaf δ13C values. Our results, however, compare individuals from multiple populations over a large geographic scale, and do not necessarily negate isotopic patterning across different foods within single sites where data are available on the δ13C values of individual foods. But, they serve to underscore the possibility of error if using high δ13C values in primate tissues to indicate fruit-eating.
Therefore at this time, we can say only that the δ13Chair values correlate with MAP as expected based on a worldwide survey of C3 plants [Kohn, 2010], and not with fruit-eating. The average δ13Chair values demonstrate a range in δ13Chair values over 4‰ where all of the animals eat C3 foods. Therefore, the general pattern strongly supports the need to take aspects of habitat ecology (biome and ecoregion) into consideration when interpreting the δ13C values in tissues from extant primates and also fossil primates, including hominins.
In a simple example, an early paper on δ13Chair values in tooth enamel of Australopithecus africanus reports an average value of −8.2‰, concluding “this early hominid…ate large quantities of carbon-13 enriched foods…” [Sponheimer & Lee-Thorp, 1999:368]. Corrected for differences in atmospheric carbon dioxide δ13C values (−1.5‰) and the offset between diet and tooth enamel δ13C values (approximately 10‰ for a species with a simple gastrointestinal tract) yields a diet value of −19.7‰. This value is approximately 2‰ higher than that expected for C3 plants in an open, but not desert, environment [Hartman & Danin, 2010]. Diagenetic alteration of approximately 1‰ [following Lee-Thorp, 2000] is expected to enrich the tooth enamel in 13C for a C3 feeder [see Schoeninger et al., 2003]. Subtracting the diagenetic alteration of 1‰, lowers the estimated diet δ13C value to −20.7‰ or approximately 1‰ higher than that expected for a C3-feeding early hominin feeding in the dryer portion of a Tropical and Subtropical Savanna, Grassland, and Shrubland biome and indicates a diet around 5% C4 foods. Using the lowest Australopithecus africanus value reported by Sponheimer & Lee-Thorp [1999], that is −11.3‰, the estimated diet value (−22.8‰) falls within the range for C3 plants in this biome. Using the highest value −5.6‰ corrected in the same manner as presented above, gives a diet estimate of −17.1‰, which would include upwards to 25% C4 foods. If, on the other hand, Australopithecus africanus had a more complex gastrointestinal tract as suggested by the Expensive Tissue Hypothesis [Aiello & Wheeler, 1995]. A diet to enamel offset of 14‰ as reported for modern hindgut and foregut fermenters [Passey et al., 2005] results in a diet estimate of −21.1‰ or 5% C4 plants in the diet. We do not purport to know the specifics of the digestive tract in Australopithecus africanus; but this simple exercise suggests that the overall calculation of diet in fossil species is more complicated than is often presented.
In contrast, the δ15Nhair values do not follow the predicted pattern of higher values in regions of lower rainfall. In fact, our regression analysis found a weak, but significant, correlation of increasing δ15Nhair values with higher levels of MAP, which is the exact opposite of the expected relationship. The overall scatter indicates that although the relationship between δ15Nhair values and MAP is statistically significant, it is probably meaningless biologically. More likely, chimpanzee δ15Nhair values reflect diet selectivity within individual sites as suggested previously [Fahy et al., 2013; Oelze et al., 2014; Schoeninger et al., 1999] rather than any direct effect of MAP. Some unexpected and interesting relationships appear in the data, however. With one exception (Ishasha), the chimpanzees from the TSGSS biomes have δ15Nhair values are significantly lower than those from the TSMBF biomes. Again, we considered the possibility that the explanation might be different amounts of fruit eating in TSGSS and TSMBF biomes; but we lack a clear pattern of association of fruit-eating with specific biomes. Another possibility is that the chimpanzees in savanna and woodland ecoregions may be eating more flowers, pods, and leaves of leguminous trees that are engaged in N2-fixation. Such a relationship was shown for mantled howler monkeys (Alouatta palliata) in a similar environment [Schoeninger et al., 1997] and in sportive lemurs (Lepilemur leucopus) [Schoeninger et al., 1998]. The consumption of leguminous species by chimpanzees is suggested by fecal data from Ugalla [see Schoeninger et al., 1999 for original references] and by species lists noting that leguminous plants are eaten throughout the year at Fongoli [Pruetz, 2006]. We look forward to more detailed publications including dietary information at TSGSS sites [e.g., Bogart & Pruetz, 2011; Hernandez-Aguilar et al., 2007; Stewart & Piel, 2014; Webster et al., 2014] and also truly comparative data from TSMBF sites.
It is unlikely that the variation in δ15Nhair values is due to differences in soil baseline δ15N values. The difference between the average of the four sites within TSGSS biomes (3.3‰) and the five sites within the TSMBF biomes (7.5‰) is 4.2‰. If Ishasha is dropped because it is a riverine environment (2.9‰ average of Fongoli, Ugalla, and Gombe) the average difference between biome types is 4.6‰. In contrast, the anticipated variations in soil values in this region of Africa are on the order of 2‰ [Amundson et al., 2003]. We look forward to additional studies on δ15N values in plants and soils from across Africa since the expected relationship between MAP and δ15Nhair values is unsupported in our study. The data thus far do not support the use of nitrogen stable isotope data in habitat reconstructions; but could be extremely useful in identifying specific aspects of diet choice in chimpanzees from different sites.
In summation, we show that the carbon stable isotope data in chimpanzees correlate with environmental variation in ecology (forest vs savanna) and with Mean Annual Precipitation (MAP) across most of the nine chimpanzee habitats for which we have stable isotope data, and can serve as indirect estimators of those habitats. The only exception is the chimpanzee habitat within a riverine gallery forest that lies within a larger region of low rainfall. Where similar regions occur within the fossil record (e.g., the Turkana basin in northern Kenya at 3.9 my with sites along a perennially flowing Omo River), regional habitat reconstructions [Cerling et al., 2011] may not capture the local areas in which fossil hominins lived and fed. For example, if the δ13C values in tooth enamel of fossil hominin material collected along a perennial river are at the lower end of the expected range for eating C3 foods, it would suggest that they are feeding and sleeping in a forest even though the overall ecoregion might look to be a grassland or savanna based on associated faunal species or soil δ13C values. Those with values that are high for eating C3 foods such as Australopithecus anamensis [Cerling et al., 2013] could be feeding away from the forest on C3 foods rather than including small amounts of C4 foods in their diet as is often suggested [Codron et al., 2008; Sponheimer & Lee-Thorp, 1999].
Most importantly, researchers can use stable isotope data to address scales of space and time that are impractical by direct observation over the course of long periods of time and between distant ecological regions. The stable isotope values can, in turn, provide the basis for modeling aspects of the habitats of animals whose behavior is unobservable such as mouse lemurs [e.g., Crowley et al., 2011b] or chimpanzees where the specimens were collected without habitat information. Indeed, we demonstrate that we must consider that the carbon stable isotope values of extinct hominins and other nonhuman primate species contain more information than a diet positioned somewhere along a C3 to C4 continuum.
ACKNOWLEDGMENTS
We thank the editors of this volume for the opportunity to participate in a session at the American Association of Physical Anthropologists and to submit a manuscript as well as the efforts of two reviewers whose comments greatly improved the article. We also thank the funding sources, which supported this project and the ones in which the hairs were collected (The Regents of the University of California to MJS and a CARTA Fellowship to CAM). Dr. Bruce Deck oversaw the stable isotope analyses at the Analytical Facility of The Scripps Institution of Oceanography at UCSD, and Kristen Snodgrass assisted with sample preparation. This research compiles with all policies of ethical research and treatment of non-human primates and adheres to the American Society of Primatologists (ASP) Principles for the Ethical Treatment of Non-Human Primates, with all protocols of the Institutional Animal Care Committee at UCSD, and with all legal requirements of the host country, that is Tanzania.




