Inter-individual variation in weaning among rhesus macaques (Macaca mulatta): Serum stable isotope indicators of suckling duration and lactation
Abstract
Weaning is a transition in early development with major implications for infant survival and well-being, and for maternal lifetime reproductive success. The particular strategy a primate mother adopts in rearing her offspring represents a negotiation between her ability to invest and her need to invest, and can be considered adaptive and influenced by biological and social factors. Any investigation into how and why maternal weaning strategies differ among non-human primates is limited by the precision of the measurement tool used to assess infants' weaning ages. Stable carbon and nitrogen isotope analysis of soft tissues (e.g., hair, nails, feces, urine, blood) offers an objective means of monitoring the weaning status of infants. In this study, we assess stable isotope ratios in blood serum from 14 captive rhesus macaque dyads (Macaca mulatta) at infant ages 2, 5, 6, 7, 8, and 10 months to estimate the timing of weaning events. Male infants wean earlier than female infants. Infants with the lowest birth weights wean latest. Most infants wean upon reaching 2.5 times their birth weights, sooner than when weaning elsewhere has been predicted for captive cercopithecine primates. The longest weaning periods (ca. 10 months) are observed among infants of small mothers. The shortest weaning period, between 2 and 5 months, was among the lowest ranking dyad. Parity and mothers' ages had no discernible effect on the timing of weaning events. The stable carbon and nitrogen isotope values of dams during lactation are significantly different than those of a non-lactating adult female outgroup, raising questions about the suitability and selection of adult comparative baselines in studies where lactating mothers cannot be sampled longitudinally (e.g., bioarchaeology; paleontology). Am. J. Primatol. 78:1113–1134, 2016. © 2015 Wiley Periodicals, Inc.
INTRODUCTION
Weaning is a critical period during which an infant makes the transition from complete dependence on its mother to independence [Martin, 1984]. How long a mother nurses and weans her infant can influence infant health outcomes [Dettwyler & Fishman, 1992; Hinde & Capitanio, 2010] and the behavioral phenotype of the infant [Vandeleest & Capitanio, 2012]. From an infant's perspective, gaining resources for growth and healthy immunological function via nursing is paramount during early development. It is not as simple for mothers. Closely spaced births can reduce a mother's energy supply and jeopardize infant survival, whereas spacing births too far apart can lead to reduced lifetime reproductive success. Variation in investment in dependent young is expected both across and within taxonomic groups, owing to phylogenetic, ecological, and social factors. We explore variation in the timing of weaning events among captive rhesus macaques (Macaca mulatta) to demonstrate the potential of stable carbon and nitrogen isotope analysis of blood serum for estimating the timing of weaning events with greater precision than what is possible using observational data. This study contributes to a growing body of literature in ecology and primatology advancing isotopic methodology in the study of weaning [Austin et al., 2013; Fahy et al., 2014; Habran et al., 2010; Macho & Lee-Thorp, 2014; Newsome et al., 2006; Oelze, in press; Polischuk et al., 2001; Reitsema, 2012; Smith et al., 2010]. Importantly, with increased precision in estimating weaning age, a greater range of hypothesis-testing concerning maternal investment is possible.
Mothers differ in their infant rearing strategies and “maternal strategy” may refer to a wide range of evolved behavioral and physiological investments in offspring. Maternal investment may be regarded as a rate of units of maternal resources invested per unit of time [Martin, 1984], varying in association with evolutionary trade-offs between investing in current offspring versus future, anticipated offspring [Trivers, 1972]. Henceforth in this paper, we narrow our use of the term maternal strategy to refer to the length of time an infant receives milk, in recognition of the fact that milk production is an example of high maternal investment with consequences for life-time fitness, while acknowledging that the transition is not purely relegated by the mother, but also by the infant and even other group members [Gomendio, 1991; Maestripieri, 2001, 2002].
Following Bowman and Lee [1995: 172–173], we make a distinction between two problems facing mothers of altricial young: ability to invest, and necessity to invest. A mother's ability to invest nutritional resources in her offspring via lactation effort is influenced by her body size and condition, her environment and resource availability, her rank, her age and parity, and her infant's size relative to her own. A mother's need to invest has to do with her infant's sex, size, and post-natal growth. The strategy a mother adopts in nursing and weaning her offspring ideally represents a best fit solution to these challenges and constraints.
Weaning can be defined narrowly as the period when an infant switches from mother's milk to a solid food diet, or more broadly as the whole array of behavioral, nutritional, morphological, and physiological changes that define independence [Martin, 1984]. For the sake of this study, we define weaning as a process that begins with the introduction of solid foods to the infant's diet, and ends when milk is no longer transferred to the infant. This definition excludes comfort nursing following the cessation of lactation. We adopt this definition because the introduction of solid foods and the eventual cessation of lactation are two important stages in the alleviation of the energetic burden of lactation, a critical factor in the trade-off between a mother's allocation of finite time and resources to her current infant versus her future offspring.
We employ stable carbon and nitrogen isotope analysis of mother and infant blood serum as a sensitive and physiologically meaningful method of assessing changes in weaning status, including the introduction of supplemental foods and the cessation of nutritive suckling. The purposes of this study are to illustrate the application of an isotopic method toward evaluating and reevaluating the weaning process of well-studied primates, and to provide preliminary results of weaning strategies of captive rhesus macaques. Blood serum offers a high level of precision in estimating weaning events appropriate for well-controlled captive populations, where maternal investment in lactation are still imperfectly understood. In wild studies, feces, hair, and urine are more feasible sampling substrates that yield similar isotopic evidence of weaning status and diet [e.g., Oelze, in press]. It is not our purpose to draw sweeping conclusions about rhesus monkey maternal investment in offspring or among primates in general, and this cannot be reduced to individual variables regardless of the method used to assess investment. However, we foresee that the broader application of soft tissue stable isotope analysis to questions of weaning and maternal investment has considerable promise in primatology.
Factors Influencing Weaning Strategies of Rhesus Macaques
Among primates, factors potentially related to a mother's investment strategy during nursing include her rank, her infant's sex, her experience including number of previous births and age, her health, the size of her young, the social environment, the resource environment, and maternal style [Bercovitch et al., 2000; Berman, 1990; Bowman & Lee, 1995; Drickamer, 1974; Gomendio, 1990; Gomendio, 1991; Hinde, 2007; Hopper et al., 2008; Jay, 1965; Maestripieri, 2001; Mas-Rivera & Bercovitch, 2008; Rajpurohit & Mohnot, 1990; Rosetta et al., 2011; Silk, 1988; Silk et al., 2006; Thompson et al., 2012; Valeggia & Ellison, 2009]. Rhesus macaques have a strong despotic matrilineal dominance hierarchy in which dominant females receive preferential access to food. Rank is related to body size and higher ranking mothers are typically larger [Small, 1981], being better able to deal with the burden of nutritional dependence of young. Rhesus macaques achieve sexual maturity before achieving skeletal maturity [Maestripieri, 2010], and for first-time mothers who are likely to be still growing, resources that could otherwise be allocated to growth are diverted to reproductive efforts, and young and primiparous females may struggle to support offspring [Bercovitch et al., 2000; Gomendio, 1990; Hinde, 2007; Hinde et al., 2009; Hopper et al., 2008; Mas-Rivera & Bercovitch, 2008; Nuñez et al., 2015]. Experienced mothers may wean their offspring earlier [Berman, 1992; Dolhinow et al., 1979; Gomendio, 1991; Hooley & Simpson, 1981; Nguyen et al., 2012; Schino et al., 1995; Silk, 1988]. Senescence, too, is a factor in maternal investment: among rhesus macaques, older mothers tend to have lower body mass indices than younger mothers and are more socially withdrawn, and infants of older mothers tend to be smaller, all of which hamper infants' abilities to thrive and survive [Hoffman et al., 2010]. However, it should also be noted that older mothers may invest more in their offspring because their residual reproductive value has declined [Clutton-Brock, 1991; Gagliardi et al., 2007; Hinde et al., 2009; Trivers, 1972; Williams, 1966].
Parental investment should be skewed toward infants of the sex that will benefit most from the additional investment. Among sexually dimorphic mammals, including rhesus macaques, males will benefit from greater body mass achieved through greater parental investment during growth and development [Clutton-Brock, 1991; Trivers & Willard, 1973]. However, male rhesus macaques disperse and lose their natal rank [Maestripieri, 2010], which may attenuate the advantages of investing in sons. Among macaques, there is some evidence that infant sex is related to duration of weaning: inter-birth intervals for mothers of daughters are reported longer in some studies [Simpson et al., 1981], shorter in others [Bercovitch et al., 2000], and similar in others [Hinde, 2009; Silk, 1988]. Hinde [2009] reports that milk of macaque mothers of sons differs in composition from mothers of daughters, but that overall available milk energy is similar [Hinde, 2009: 516]. Expectations for variation in how mothers wean sons versus daughters are thus mixed.
Dams respond to cues from their infants that affect how they budget time and resources for lactation. Eruption of dentition, changes in infant appearance such as pelt color changes in langurs and colobus monkeys, and infant growth point to infant independence and serve as visual indicators of when an infant is able to feed itself. Regarding body size, [Lee et al., 1991] conducted a comparative analysis of mammalian weaning ages and report that across multiple taxa, infants are weaned upon reaching nearly four times their birth weights. Bowman and Lee [1995], following up on this study, report that, more narrowly among cercopithecine primates and most specifically, including captive rhesus macaques, infants appear to be weaned at 3.2 times their birth weights.
In light of these observations, we explore the utility of serum stable isotope ratio analysis to assess primate weaning. We test the null hypotheses that high-ranking mothers will wean their offspring earlier than low-ranking mothers [e.g., Bowman and Lee, 1995], males will be weaned before females [e.g., Hinde, 2009], and infants will be weaned by the time they reach 3.2 times their birth weight [threshold weight hypothesis; Bowman and Lee, 1995]. We also explore whether experienced mothers wean offspring faster [relaxed parenting: Gomendio, 1991; improved lactational performance with parity: Hinde et al., 2009] and whether older mothers, having less to lose in terms of future investment than do younger mothers [residual reproductive value hypothesis; Williams, 1966], wean their offspring later.
Stable Isotope Analysis in Primate Weaning Ecology
Any investigation into how and why maternal weaning strategies differ among non-human primates is limited by the precision of the measurement tool used to assess the infant's weaning age. The lengths of weaning periods across primate populations are typically assessed using behavioral observations, which can be imprecise [Scanlon et al., 2002]. Suckling without actual milk transfer or “comfort nursing” is one complication to observational data [Harlow & Harlow, 1965]. Night nursing is difficult to detect, as is nursing among nocturnal species. Where observations are made, time on the nipple may be used as a proxy for suckling, or, for more cryptic species, mother–infant proximity may be the proxy. Estimations of mother–infant contact, including non-nutritive suckling with no actual milk transfer, are part of the bigger picture of weaning transitions and indicative of its social components [Maestripieri, 2002], but they are not specifically informative of the physiological ramifications of weaning, such as the cessation of lactational amenorrhea and infant nutritional status. Attempts at estimating nutritive suckling include weighing an infant immediately before and after a nursing episode [Meier et al., 1996], providing a mother with doubly labeled water and measuring an infant's urine throughout the day [Holleman et al., 1975], monitoring jaw movements and the number of sucks per second [Tanaka, 1992], and contextualized assessment of last nipple contact [Borries et al., 2014].
Another approach to assessing nutritive suckling is stable carbon and nitrogen isotope analysis of body tissues [Fogel et al., 1989; Fuller, 2003; Fuller et al., 2006]. Applied in some cases among non-primate mammals [e.g., Dalerum et al., 2007; Habran et al., 2010; Jenkins et al., 2001; Newsome et al., 2006; Polischuk et al., 2001], soft tissue stable isotope analysis was first reported among non-human primates using fecal samples from captive langurs [Reitsema, 2012]. Elemental evidence from mineralized tissues (dentine and enamel) has since been reported for wild chimpanzees (stable carbon and nitrogen isotope ratios [Fahy et al., 2014]) and rhesus macaques in captivity (barium–calcium ratios) [Austin et al., 2013]. Stable isotope ratios in consumer tissues provide researchers with a record of which types of food were eaten by an individual during its life [Koch, 2007]. Stable isotopes are atoms of the same element with the same number of electrons and protons, but different numbers of neutrons, giving different isotopes slightly different atomic masses that can be measured via mass spectrometry. Different types of foods exhibit characteristically different stable isotope signatures, and these differences are passed up to the tissues of consumers. One of the fundamental differences in the stable isotope signatures of foods is that between plants using different photosynthetic pathways. C3 plants use the Calvin–Benson photosynthetic pathway, and they exhibit very low δ13C values. C4 plants use the Hatch–Slack photosynthetic pathway, and exhibit higher δ13C values that do not overlap with C3 plants. Plants using Crassulacean acid metabolism (CAM plants) exhibit intermediate values [e.g., Godfrey et al., in press]. Apart from the C3/C4 distinction, δ13C values have been shown to vary in plants with forest canopy height [e.g., Carlson and Crowley, this issue].
Another fundamental difference between the isotopic signatures of organisms is that between herbivores, omnivores, and carnivores. In general, with every step up the food chain, δ15N becomes enriched by approximately 3–5‰, due to in vivo discrimination against the lighter isotope, 14N [Bocherens & Drucker, 2003; Minawaga & Wada, 1984]. From the overall isotopic composition of foods, relatively more 14N is excreted with urea while relatively more 15N is used for tissue building. In general, the stable carbon and nitrogen isotope signatures of a consumer's tissues represent a bulk average of the isotopic ratios of that consumer's foods, plus an anticipated element- and tissue-specific diet-tissue offset, which is the product of in vivo fractionation [Koch, 2007].
Nutritive suckling and weaning may be assessed isotopically because milk is a food that differs isotopically from most other foods in a mammal's diet. Because of in vivo fractionation and routing of nitrogen isotopes in the body, a mother's milk is higher in 15N in comparison to her diet. An infant's tissue δ15N signature reflects consumption of milk when it is higher than the δ15N ratio of its mother. As the infant begins to consume solid foods, most of which can be expected to exhibit lower δ15N ratios than milk, the offset between a mother and her infant declines. As such, δ15N values of infant tissues and excreta are indicative of the duration of nutritive suckling.
The cessation of suckling is one important element of the overall weaning process. Another important step is the introduction of solid foods to the infant's diet, which occurs while the infant is still suckling. Like δ15N values, δ13C values also become higher moving up between trophic levels in the food web. The diet-tissue increase is approximately 1‰ for δ13C in animal tissues [Bocherens & Drucker, 2003; Chisholm, 1989]. However, weaning studies conducted among animals whose milk have high, low-13C lipid contents, such as marine mammals, show depletion in 13C of suckling infants (e.g., Ursus maritimus; Mirounga angustirostris) [Habran et al., 2010; Polischuk et al., 2001]. While δ15N ratios track the duration of nutritive suckling, δ13C ratios more closely monitor the introduction of solid foods. This is in part because plants comprise large amounts of carbon (approximately 40–45% carbon by weight) and small amounts of nitrogen (just 1–3% nitrogen by weight); thus, plants as dietary components have a strong capacity to skew the δ13C signature of tissues, and a weak capacity to affect δ15N signatures. Compared to milk, most supplemental foods in non-human primate diets are lower in the heavy isotope 13C. Thus, as an infant begins to consume plant-based solid foods, its δ13C is expected to quickly drop [e.g., Fuller et al., 2006; Reitsema, 2012].
Stable isotope signatures of consumer tissues are continually “updated” as tissues grow and are replaced or repaired by atoms incorporated from the more recent food and drink. Depending on the tissue type, sampling strategies for isotopic estimations of weaning may yield snapshots of an infant's weaning status in daily, weekly, monthly, or biannual increments (using excreta, blood components, hair, and dentine serial sections, respectively). In archaeological applications, bone is the most commonly analyzed tissue, but studies of living organisms may capitalize on refined time slices afforded by analyzing tissues with quicker turnover times. Altogether, the refined snapshots of weaning status obtained isotopically from tissues such as hair, nails, blood, and excreta permit previously impossible hypothesis-testing in primate ecology.
In this study, we begin exploring the weaning strategies of rhesus macaques (M. mulatta) for the first time using more precise technique, addressing variability in weaning ages with known information from both the mothers and their infants. The weaning period of captive rhesus macaques has been estimated through behavioral observations at approximately seven months [Bowman & Lee, 1995]. The goals of this study are to use a potentially more precise method to assess the introduction of solid foods and the length of the weaning process among captive rhesus macaques, and to evaluate the timing of these events in relation to factors known or suspected to influence a mother's weaning strategy: maternal dominance rank, number of previous births, mother's age, infant sex, and infant size and growth rate. We assess whether infants are weaned at 3.2 times their birth weight, as predicted by the threshold weight hypothesis developed by Bowman and Lee [1995]. Finally, we compare the estimated age of weaning for each infant to its current group rank and health status, to explore later-life effects of variation in the timing of weaning events.
METHODS
Subjects
This research adhered to the American Society of Primatologists principles for the ethical treatment of primates. All procedures were approved by The Emory University Institutional Animal Care and Use Committee, in accordance with the Animal Welfare Act and the US Department of Health and Human Services “Guide for the Care and Use of Laboratory Animals.” Rhesus macaques were housed in groups of up to 125 individuals, living in up to 13 family units with a mean size of 9–10 individuals each, in large, semi-free ranging environments at the Yerkes National Primate Research Center. Animal subjects were provisioned with two different diets. Eight mothers were fed a standard or “normal” low fat, high fiber diet supplied by Purina® (Lab Diets 5038) and six mothers were fed a high fat, high sugar diet (HCD) supplied by Research Diet® (Diet D07091204). These diets proved to be isotopically distinct, and henceforth are given separate consideration. Each diet was in the form of a biscuit and was available to both mothers and infants via automated feeders [Wilson et al., 2008]. Subjects also were given supplemental foods including vegetables, fruits, sunflower seeds, and popcorn. Dominance rank of family units was assessed by recording submissive gestures by individuals elicited by the approach or directed threat of another animal in encounters between all possible combinations of families. The ranks of families sampled for this study ranged from 2 to 12, and within-family ranks are unknown. No more than one dam was sampled from one family unit.
Blood Serum
To provide an isotopic estimation of infant nutritional dependence on dams, blood serum of mothers and their infants was sampled. Blood serum is blood plasma with the clotting factors, specifically fibrinogens, removed. The main component of blood serum is water, yet other components include proteins, non-protein nitrogen, lipids, and carbohydrates, along with electrolytes, hormones, and carbon dioxide [Krebs, 1950]. Stable isotope values in blood serum reflect dietary intake over the preceding 7–10 days [Jenkins et al., 2001], allowing up to weekly snapshots of an infant's weaning status.
Sampling Regime
Dams' serum samples were collected when infants were 2 and 5 months of age. For mothers fed the standard lab diet throughout the entire study period, maternal δ13C and δ15N values were not expected to fluctuate significantly owing to homogenous diets. Mothers who began the study period on the HCD were switched to a standard diet at varying time points during the study period, and so their isotopic ratios were expected to shift during the sampling period. Only in cases when HCD mothers and their infants could be said to have been consuming exclusively the HCD (start of the collection period) or exclusively the standard diet (end of the collection period) do we report their isotopic values. In general, for the mothers, this restricts discussion of the HCD cohort to infant ages 2 and 10 months.
Infants were born in 2011 and infant serum samples were collected in 2011–2012. Isotopic analysis occurred in 2013–2014. Eleven non-lactating adult females, fed the standard monkey diet, were sampled after results of the mother–infant dyads were assessed. The non-lactating adult females provide a comparative outgroup.
Follow-Up Study of Infant Outcomes
In 2014, subjects were all approximately 3 years old, and, excepting male infants who had left their natal groups and for whom detailed health information was not available, family rank and health of the study subjects were assessed as a follow-up to the isotopic assessment of their weaning ages.
Sample Preparation
From blood that was frozen after collection, 50 μl of serum and pieces of commercial feed were subsampled, freeze-dried, and homogenized using an agate mortar and pestle in the University of Georgia Bioarchaeology and Biochemistry Laboratory. Samples were weighed into tin capsules at the Center for Applied Isotope Studies (CAIS) at the University of Georgia to reach a range of 1.2–1.5 mg for mother and infant serum, and 2.4–2.6 mg for biscuit samples. A revised amount of just 0.5–0.7 mg was used for the non-lactating adult females, on the basis of the carbon and nitrogen concentrations (%C, %N) in serum from the mothers and infants in the initial runs. Eight duplicates of mother and infant samples were randomly chosen to estimate intra-sample variation and efficacy of homogenization.
Isotopic Analysis of Samples
Combustion of CO2 for carbon stable isotope ratios and reduction of N2 for nitrogen stable isotope ratios were on a Costech elemental analyzer at CAIS. Acetanilide and an internal protein standard were used. Samples were analyzed for δ13C and δ15N values using a Thermo Delta plus XL isotope ratio mass spectrometer. Results were reported according to the equation: δ = [(Rsample/Rstandard) −1]*1,000. Results of repeat analyses of the acetanilide standard were ±0.06‰ for δ13C and ±0.18‰ for δ15N. Results of repeat analyses of the protein standard were ±0.05‰ δ13C and ±0.33‰ for δ15N. Replicate analyses of samples differed by 0.08‰ for δ15N and by 0.04‰ for δ13C.
Statistical Analysis
Kruskal–Wallis and Mann–Whitney U tests, both appropriate for small samples whose distributions deviate from normality, are used to compare groups. Linear regressions and Pearson's correlations explore relationships between variables. These statistical tests were performed using SYSTAT software. Values are considered significant when P < 0.05.
RESULTS
Commercial Diet Isotopic Analysis
The standard, low-calorie biscuit exhibits a δ15N value of 2.4 ± 0.3‰ (mean ± 1 SD) and a δ13C value of −18.5 ± 0.4‰ (analyzed in triplicate). The high-calorie biscuit exhibits a δ15N value of 6.1 ± 0.2‰ and a δ13C value of −26.5‰.
Stable Isotope Ratios of Dams’ Blood Serum
Isotopic data as well as maternal and infant characteristics varied across subjects (Table I; Fig. 1). The mean δ15N value of all standard diet mothers is 6.9 ± 0.3‰, and the mean δ13C value is −19.3 ± 0.4‰. These standard diet mothers' values together make up what we refer to as the maternal, mothers', or dams' baseline value. The biscuit-tissue space for dams on the standard diet is +4.5‰ for δ15N and −0.8‰ for δ13C.
| Infants | Dams | Mother-infant offsets | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mother ID; traits | Infant | Sex | Sample month | δ15N (‰) | δ13C (‰) | %N | %C | CN | Weight (kg) | δ15N (‰) | δ13C (‰) | %N | %C | CN | Weight (kg) | Δ15N (‰) | Δ13C (‰) |
| Normal diet subjects | |||||||||||||||||
| RZh8 | RHs14 | F | 2 | 7.5 | −18.0 | 11.2 | 43.1 | 4.5 | 0.8 | 6.5 | −19.1 | 11.7 | 42.1 | 4.2 | 7.16 | 1.2 | 1.4 |
| Rank = 2 | 5 | 8.2 | −18.5 | 11.2 | 45.5 | 4.7 | 1.4 | 6.1 | −19.7 | 11.7 | 42.2 | 4.2 | 7.78 | 1.9 | 0.9 | ||
| Parity = 3 | 6 | 7.8 | −19.0 | 10.0 | 39.1 | 4.6 | 1.5 | – | – | – | – | – | – | 1.5 | 0.4 | ||
| Age = 10 y | 7 | 8.0 | −19.1 | 10.5 | 40.6 | 4.5 | 1.6 | – | – | – | – | – | – | 1.6 | 0.3 | ||
| Initial wt = 7.53 kg | 8 | 7.4 | −19.3 | 10.9 | 40.0 | 4.3 | 1.8 | – | – | – | – | – | – | 1.0 | 0.1 | ||
| 10 | 6.8 | −19.9 | 11.4 | 43.9 | 4.5 | 2.1 | – | – | – | – | – | – | 0.5 | −0.5 | |||
| RFm6 | RCt14 | M | 2 | 7.9 | −18.4 | 10.7 | 41.7 | 4.6 | 0.7 | 7.2 | −19.1 | 12.0 | 44.1 | 4.3 | 7.68 | 0.9 | 1.3 |
| Rank = 9 | 5 | 7.4 | −19.4 | 10.9 | 42.2 | 4.5 | 1.1 | 6.9 | −20.1 | 12.7 | 43.7 | 4.0 | 8.19 | 0.4 | 0.3 | ||
| Parity = 7 | 6 | 6.8 | −19.6 | 10.7 | 40.7 | 4.5 | 1.2 | – | – | – | – | – | – | −0.3 | 0.0 | ||
| Age = 13 y | 7 | 6.6 | −20.1 | 10.5 | 39.4 | 4.4 | 1.4 | – | – | – | – | – | – | −0.4 | −0.5 | ||
| Initial wt = 7.86 kg | 8 | 6.8 | −19.7 | 10.5 | 39.0 | 4.4 | 1.6 | – | – | – | – | – | – | −0.3 | −0.1 | ||
| 10 | 6.7 | −20.0 | 11.0 | 42.3 | 4.5 | 2.1 | – | – | – | – | – | – | −0.3 | −0.4 | |||
| RTr6 | RZt14 | M | 2 | 7.4 | −18.0 | 10.7 | 42.6 | 4.6 | 0.7 | 6.8 | −19.1 | 11.7 | 42.8 | 4.3 | 8.42 | 0.5 | 1.2 |
| Rank = 12 | 5 | 6.8 | −19.2 | 11.2 | 43.3 | 4.5 | 1.1 | 6.9 | −19.3 | 11.7 | 42.2 | 4.2 | 6 | 0.0 | 0.0 | ||
| Parity = 6 | 6 | 6.5 | −19.8 | 11.1 | 40.7 | 4.3 | 1.3 | – | – | – | – | – | – | −0.3 | −0.6 | ||
| Age = 13 y | 7 | 6.8 | −19.6 | 10.3 | 39.6 | 4.5 | 1.2 | – | – | – | – | – | – | −0.1 | −0.4 | ||
| Initial wt = 7.32 kg | 8 | 6.3 | −20.7 | 10.5 | 41.4 | 4.6 | 1.3 | – | – | – | – | – | – | −0.5 | −1.5 | ||
| 10 | 6.5 | −20.0 | 11.1 | 42.8 | 4.5 | 1.4 | – | – | – | – | – | – | −0.3 | −0.8 | |||
| RDr10 | RJu14 | M | 2 | 7.8 | −18.2 | 10.7 | 42.4 | 4.6 | 0.8 | 6.9 | −19.2 | 11.7 | 42.4 | 4.2 | 9.55 | 0.9 | 1.2 |
| Rank = 2 | 5 | 7.6 | −18.9 | 10.7 | 41.8 | 4.5 | 1.3 | 6.9 | −19.5 | 11.5 | 41.1 | 4.2 | 9.55 | 0.7 | 0.5 | ||
| Parity = 3 | 6 | 7.1 | −19.7 | 10.3 | 41.0 | 4.7 | 1.3 | – | – | – | – | – | – | 0.2 | −0.3 | ||
| Age = 7 y | 7 | 6.9 | −19.5 | 9.9 | 37.8 | 4.4 | 1.5 | – | – | – | – | – | – | 0.0 | −0.1 | ||
| Initial wt = 9.06 kg | 8 | 6.6 | −19.6 | 10.3 | 38.4 | 4.3 | 1.7 | – | – | – | – | – | – | −0.3 | −0.2 | ||
| 10 | 7.1 | −19.6 | 12.6 | 45.3 | 4.2 | 2.0 | – | – | – | – | – | – | 0.2 | −0.2 | |||
| RRg11 | RFw14 | F | 2 | – | – | – | - | - | 0.8 | 7.4 | −19.3 | 11.5 | 44.6 | 4.5 | 10.49 | – | |
| Rank = 5 | 5 | 7.9 | −18.6 | 9.4 | 37.4 | 4.7 | 1.3 | 7.2 | −19.8 | 12.0 | 44.0 | 4.3 | 11.09 | 0.7 | 0.9 | ||
| Parity = 2 | 6 | 7.8 | −19.7 | 10.7 | 40.5 | 4.4 | 1.4 | – | – | – | – | – | – | 0.5 | −0.1 | ||
| Age = 6 y | 7 | 7.7 | −19.2 | 10.5 | 40.5 | 4.5 | 1.5 | – | – | – | – | – | – | −0.3 | 0.4 | ||
| Initial wt = 10.45 kg | 8 | 7.4 | −19.9 | 10.0 | 39.4 | 4.6 | 1.6 | – | – | – | – | – | – | −0.6 | – | ||
| 10 | 7.0 | −20.9 | 10.1 | 38.0 | 4.4 | 1.8 | – | – | – | – | – | – | −0.1 | −1.4 | |||
| REs6 | RLm14 | F | 2 | 8.3 ± 0.1 | −17.5 ± 0 | 10.1 | 38.7 | 4.5 | 0.9 | 7.3 ± 0 | −18.8 ± 0 | 11.5 | 43.1 | 4.4 | 10.65 | 1.1 | 1.5 |
| Rank = 3 | 5 | 7.5 | −18.5 | 11.6 | 44.7 | 4.5 | 1.5 | 7.1 | −19.3 | 11.7 | 43.1 | 4.3 | 12.04 | 0.3 | 0.5 | ||
| Parity = 9 | 6 | 7.0 | −19.8 | 10.2 | 39.5 | 4.5 | 1.7 | – | – | – | – | – | – | −0.3 | −0.7 | ||
| Age = 13 y | 7 | 6.7 | −19.6 | 10.4 | 39.3 | 4.4 | 2.0 | – | – | – | – | – | – | −0.5 | −0.5 | ||
| Initial wt = 11.41 kg | 8 | 6.9 | −19.7 | 10.2 | 39.2 | 4.5 | 1.9 | – | – | – | – | – | – | −0.3 | – | ||
| 10 | 7.0 | −19.4 | 11.8 | 44.9 | 4.4 | 2.3 | – | – | – | – | – | – | −0.2 | −0.3 | |||
| RQq10 | RCr14 | F | 2 | 7.8 | −18.2 | 10.4 | 41.9 | 4.7 | 0.8 | 7.0 ± 0 | −19 ± 0.1 | 11.8 | 43.1 | 4.3 | 8.66 | 0.9 | 1.1 |
| Rank = 4 | 5 | 7.4 | −19.1 | 10.9 | 42.1 | 4.5 | 1.2 | 6.9 ± 0.1 | −19.6 ± 0 | 11.8 | 43.1 | 4.3 | 9.52 | 0.5 | 0.2 | ||
| Parity = 3 | 6 | 7.2 | −19.5 | 10.3 | 39.7 | 4.5 | 1.4 | – | – | – | – | – | – | 0.2 | −0.3 | ||
| Age = 7 y | 7 | 7.2 | −20.0 | 10.6 | 39.9 | 4.4 | 1.5 | – | – | – | – | – | – | 0.3 | −0.7 | ||
| Initial wt = 8.42 kg | 8 | 7.1±0 | −20.0 ± 0 | 10.5 | 39.8 | 4.4 | 1.6 | – | – | – | – | – | – | 0.1 | −0.7 | ||
| 10 | 7.1 | −19.9 | 11.0 | 41.3 | 4.4 | 1.8 | – | – | – | – | – | – | 0.2 | −0.6 | |||
| RFq9 | RRr14 | F | 2 | 7.5 | −17.9 | 11.0 | 44.2 | 4.7 | 0.7 | 6.9 ± 0.06 | −18.9 ± 0.00 | 11.7 | 43.5 | 4.4 | 7.52 | 0.5 | 1.2 |
| Rank = 7 | 5 | 7.7 | −18.7 | 11.7 | 45.3 | 4.5 | 1.1 | 6.9 | −19.2 | 11.5 | 41.7 | 4.2 | 7.73 | 0.8 | 0.3 | ||
| Parity = 3 | 6 | 7.4 | −19.2 | 10.4 | 39.8 | 4.5 | 1.2 | – | – | – | – | – | – | 0.5 | −0.2 | ||
| Age = 8 | 7 | 7.4 | −19.4 | 10.3 | 39.8 | 4.5 | 1.3 | – | – | – | – | – | – | 0.5 | −0.4 | ||
| Initial wt = 7.32 kg | 8 | 7.3 | −19.6 | 10.0 | 38.5 | 4.5 | 1.4 | – | – | – | – | – | – | 0.4 | −0.5 | ||
| 10 | 6.4 | −19.8 | 11.1 | 44.7 | 4.7 | 1.6 | – | – | – | – | – | – | −0.5 | −0.8 | |||
| High-calorie diet subjects | |||||||||||||||||
| VXW | RA114 | F | 2 | 9.5 | −20.1 | 10.8 | 41.2 | 4.5 | 0.8 | 8.9 | −21.9 | – | – | 4.3 | 9.36 | 2.1 | −1.3 |
| Rank = 2 | 5 | 7.6 | −19.8 | 10.9 | 41.8 | 4.5 | 1.2 | – | – | – | – | – | 9.07 | 1.5 | 0.3 | ||
| Parity = 5 | 6 | – | – | – | – | – | 1.4 | – | – | – | – | – | – | – | – | ||
| Age = 19 y | 7 | – | – | – | – | – | 1.4 | – | – | – | – | – | – | – | – | ||
| Initial wt = 9.09 kg | 8 | – | – | – | – | – | 1.5 | – | – | – | – | – | – | – | – | ||
| 10 | 6.1 | −20.3 | 11.1 | 41.6 | 4.4 | 1.6 | – | – | – | – | – | – | – | – | |||
| XBA | RTk14 | F | 2 | 9.7 | −20.1 | 11.7 | 45.0 | 4.5 | 0.8 | 9.3 ± 0 | −22.0 ± 0 | 10.8 | 42.5 | 4.6 | 8 | 0.4 | 1.8 |
| Rank = 1 | 5 | 10.2 | −20.6 | 11.7 | 45.4 | 4.5 | 1.1 | 9.1 | −21.7 | 11.4 | 41.8 | 4.3 | 8.32 | 1.1 | 1.2 | ||
| Parity = 5 | 6 | – | – | – | – | – | 1.2 | – | – | – | – | – | – | – | – | ||
| Age = 13 y | 7 | – | – | – | – | – | 1.4 | – | – | – | – | – | – | – | – | ||
| Initial wt = 8.03 kg | 8 | – | – | – | – | – | 1.5 | – | – | – | – | – | – | – | – | ||
| 10 | 8.1 | −19.3 | 11.6 | 44.9 | 4.5 | 1.7 | – | – | – | – | – | – | – | – | |||
| RHh11 | RKk14 | M | 2 | 9.6 ± 0.1 | −20.3 ± 0 | 11.6 | 44.9 | 4.5 | 0.8 | 9.1 | −22.4 | 10.2 | 46.1 | 5.2 | 8.59 | 0.5 | 2.1 |
| Rank = 3 | 5 | 10.0 | -21.1 | 10.9 | 44.5 | 4.8 | 1.2 | 8.7 | −22.3 | 10.4 | 45.8 | 5.1 | 8.82 | 1.3 | 1.2 | ||
| Parity = 1 | 6 | – | – | – | – | – | 1.4 | – | – | – | – | – | – | – | – | ||
| Age = 5 y | 7 | – | – | – | – | – | 1.5 | – | – | – | – | – | – | – | – | ||
| Initial wt = 7.39 kg | 8 | – | – | – | – | – | 1.6 | – | – | – | – | – | – | – | – | ||
| 10 | 8.0 | −19.8 | 11.3 | 44.1 | 4.5 | 1.8 | – | – | |||||||||
| 438 | RNp14 | F | 2 | 9.2 | −20.5 | 10.8 | 44.4 | 4.8 | 0.8 | 8.4 | −21.7 | 11.4 | 43.2 | 4.4 | 6.57 | 1.2 | 1.2 |
| Rank = 5 | 5 | 8.3 | −20.4 | 11.1 | 44.9 | 4.7 | 1.3 | 6.8 | −20.9 | 11.2 | 41.5 | 4.3 | 6.56 | 1.5 | 0.5 | ||
| Parity = 6 | 6 | – | – | – | – | – | 1.4 | – | – | – | – | – | – | – | – | ||
| Age = 13 y | 7 | – | – | – | – | – | 1.5 | – | – | – | – | – | – | – | – | ||
| Initial wt = 6.9 kg | 8 | – | – | – | – | – | 1.6 | – | – | – | – | – | – | – | – | ||
| 10 | 7.1 | −20.2 | 11.6 | 45.0 | 4.5 | 1.8 | – | – | – | – | – | – | – | – | |||
| 458 | RJs14 | F | 2 | 9.8 | −20.3 | 10.8 | 43.0 | 4.6 | 0.8 | 9.4 | −22.0 | 11.1 | 42.6 | 4.5 | 10.6 | 0.4 | 1.7 |
| Rank = 6 | 5 | 8.5 | −20.5 | 10.8 | 43.9 | 4.7 | 1.1 | 7.2 | −20.8 | 11.4 | 42.8 | 4.4 | 8.84 | 1.4 | 0.3 | ||
| Parity = 6 | 6 | – | – | – | – | – | 1.3 | – | – | – | – | – | – | – | – | ||
| Age = 13 y | 7 | – | – | – | – | – | 1.4 | – | – | – | – | – | – | – | – | ||
| Initial wt = 10.97 kg | 8 | – | – | – | – | – | 1.5 | – | – | – | – | – | – | – | – | ||
| 10 | 6.7 | −19.9 | 10.6 | 41.2 | 4.5 | 1.7 | – | – | – | – | – | – | – | – | |||
| RFd11 | RBv14 | M | 2 | – | – | – | – | – | 0.7 | 8.6 | −21.5 | 11.7 | 42.6 | 4.3 | 5.13 | – | – |
| Rank = 4 | 5 | 7.9 | −20.1 | 10.2 | 40.8 | 4.7 | 1.0 | 6.6 | −20.5 | 11.6 | 42.2 | 4.3 | 5.85 | 1.2 | 0.4 | ||
| Parity = 2 | 6 | – | – | – | – | – | 1.1 | – | – | – | – | – | – | – | – | ||
| Age = 5 y | 7 | – | – | – | – | – | 1.3 | – | – | – | – | – | – | – | – | ||
| Initial wt = 6.29 kg | 8 | – | – | – | – | – | 1.5 | – | – | – | – | – | – | – | – | ||
| 10 | 7.0 | −20.1 | 10.5 | 38.7 | 4.3 | 1.9 | – | – | – | – | – | – | – | – | |||
- All data from mother-infant dyads. Descriptive information from dams is presented in the first column, including weights of dams following birth. Stable nitrogen (δ15N) and carbon (δ13C) isotope data, carbon (%C) and nitrogen (%N) content in serum, the carbon/nitrogen ratio (CN) of serum, and weights are presented at sampling time points for which these data are available (2–10 months of infant age). Results of duplicate isotopic analyses are presented as the mean ± 1 standard deviation. Mother–infant offsets (Δ) are the result of subtracting an infant's dam's mean value from the infant's value. Offsets of the high-calorie (HCD) cohort compare mothers and infants at each of two individual months, rather than averaging the dams' values. Months preceding the diet switch (HCD to standard) are shown in bold for the HCD cohort.
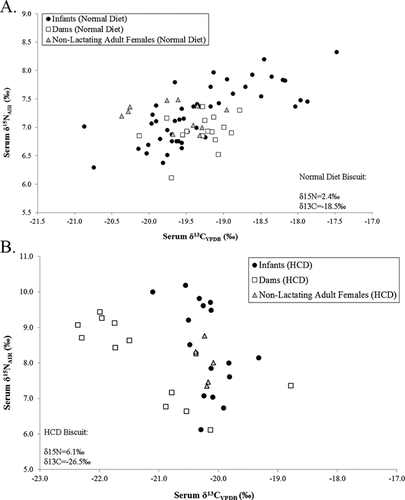
For dams eating the HCD, prior to transitioning to the standard diet, the mean serum δ15N value is 9.0 ± 0.3‰ and the mean δ13C value is −22.0 ± 0.3‰ (n = 7 time points) (Fig. 1). The biscuit-tissue space for dams on the high-calorie diet is, therefore, +2.9‰ for δ15N and +4.5‰ for δ13C.
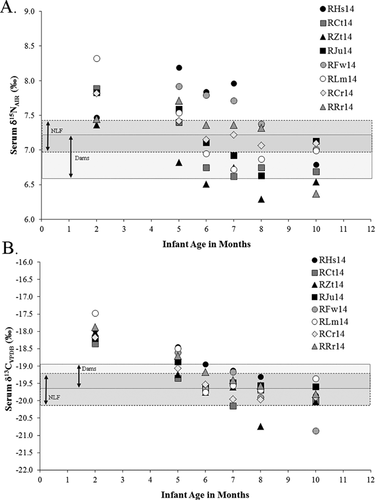
Eleven non-lactating adult females (all fed the standard diet) exhibit a mean δ15N value of 7.2 ± 0.2‰ and a mean δ13C value of −19.7 ± 0.5‰ (Table II; Fig. 1). The δ15N values of dams also fed the standard diet are significantly lower than those of non-lactating adult females (one-tailed Mann–Whitney U, P = 0.008). The δ13C values of dams are significantly higher than those of non-lactating adult females (P = 0.015). The %N and %C values of standard diet lactating and non-lactating adult females differ significantly (%C P < 0.001; %N P = 0.007), with NLF exhibiting higher values.
| ID | Rank | δ15N (‰) | δ13C (‰) | %N | %C | C:N | ||
|---|---|---|---|---|---|---|---|---|
| Standard diet | Sm8 | 1 | 6.9 | −19.7 | 11.8 | 45.9 | 4.5 | |
| Standard diet | Lo9 | 2 | 7.5 | −19.8 | 12.4 | 44.9 | 4.2 | |
| Standard diet | Ed8 | 4 | 7.4 | −20.3 | 12.3 | 45.2 | 4.3 | |
| Standard diet | Ai10 | 5 | 7.3 | −19.0 | 12.3 | 45.5 | 4.3 | |
| Standard diet | Bi10 | 5 | 7.0 | −19.3 | 12.3 | 46.1 | 4.4 | |
| Standard diet | Tm13 | 6 | 7.0 | −19.4 | 11.9 | 44.9 | 4.4 | |
| Standard diet | Gi6 | 1 | 7.5 | −19.6 | 11.1 | 47.3 | 5.0 | |
| Standard diet | Cj9 | 2 | 7.3 | −20.3 | 12.5 | 46.1 | 4.3 | |
| Standard diet | Iv8 | 3 | 7.4 | −19.4 | 12.3 | 45.5 | 4.3 | |
| Standard diet | Rn9 | 4 | 7.2 | −20.4 | 12.5 | 45.8 | 4.3 | |
| Standard diet | Lm8 | 5 | 6.9 | −19.3 | 11.9 | 45.9 | 4.5 | |
- Data from non-lactating adult females (NLF) including dominance rank, stable nitrogen (δ15N) and carbon (δ13C) isotope data, carbon (%C) and nitrogen (%N) content of serum, and the carbon–nitrogen ratio (CN) of serum.
Stable Isotope Ratios of Infant Blood Serum
Infant δ13C and δ15N values are reported for ages 2, 5, 6, 7, 8, and 10 months. The mean infant δ13C value is −19.3 ± 0.7‰, and the mean δ15N value is 7.2 ± 0.5‰. Over time, the mean mother–infant δ15N and δ13C spaces decrease. Infants are shown in relation to their particular mothers in Figures 2 (females) and 3 (males).
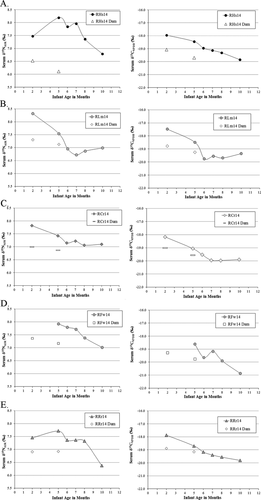
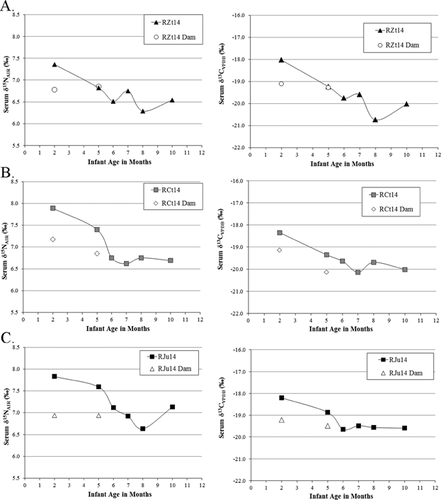
Translating Stable Isotope Ratios into Weaning Ages
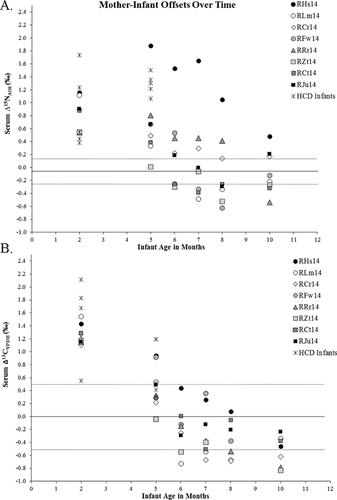
To translate δ15N values into weaning ages for the infants, we calculated the mean of each mother's 2- and 5-month values, which differed by 0.2‰, on average, and used this as a dyad-specific baseline for her infant. A dam's single baseline was subtracted from her infant's values at each of the six sampling months, effectively scaling all individuals from absolute isotopic values, to “mother–infant offset” values (Δ15N) specific to each dyad. We use this number to represent a range of error surrounding zero, zero being the point at which infants and mothers in the same dyad have identical values. The Δ13C range of error for dams' values is 0.5‰. In Figure 4, infants are all scaled against their particular mother's baseline in this manner, and plotted over time. An infant is estimated as weaned (no longer consuming milk) when its Δ15N value drops to 0.2‰ or lower 0.2‰, being the mean difference between dams' 2- and 5-month values. All dyad Δ values appear in Table I. Isotopically estimated weaning ages are presented in Table III.
| Infant | Infant sex | Isotopically-estimated weaning age (months) | Age infant reached 3.2x birth mass (months) | Mass at weaning (kg) | Mass at weaning (% of birth mass) | ||||
|---|---|---|---|---|---|---|---|---|---|
| RHs14 | F | > 10 | 5 | 2.12 | 4.82 | ||||
| RCt14 | M | 6 | 10 | 1.24 | 2.14 | ||||
| RZt14 | M | 5 | 8 | 1.29 | 2.24 | ||||
| RJu14 | M | 6 | 8 | 1.34 | 2.58 | ||||
| RFw14 | F | 7 | 9 | 1.50 | 2.28 | ||||
| RLm14 | F | 6 | 10 | 1.70 | 2.54 | ||||
| RCr14 | F | 8 | 9 | 1.56 | 2.89 | ||||
| RRr14 | F | 10 | 8 | 1.63 | 3.89 | ||||
- Isotopically derived weaning ages (individuals from the standard diet group) are calculated by subtracting the mean value of a dam's 2- and 5-month samples from her infant's value at each month. Ages at which infants reached 3.2 times their birth weight are shown, after by Bowman and Lee [1995]. Weight at weaning is reported as the weight of an infant at its isotopically estimated weaning age, divided by its birth weight. Infants of dams fed the high-calorie diet are excluded because they were sampled less frequently owing to a diet switch mid-way through the sampling period (see text).
Maternal Characteristics (Mass, Rank, and Parity)
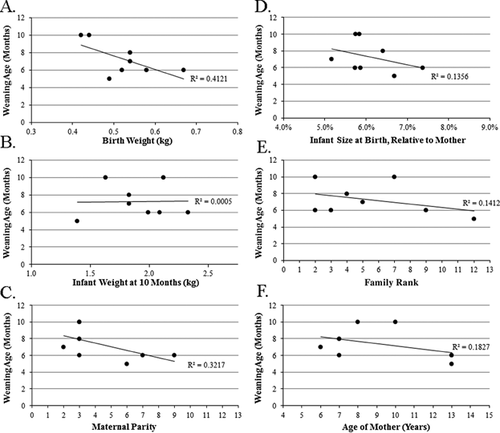
Body mass is an important consideration in the context of weaning because a dam's mass may impact her ability to invest resources in young, and her infant's mass may impact her need to invest, as well as the age at which the infant becomes independent. Dams' mass and their infants' calculated weaning ages are not significantly related (linear regression, R2 = 0.109; P = 0.424).
Family rank and weaning age are not significantly related (R2 = 0.1412; P = 0.359) (Fig. 5E). Prior to giving birth to the infants in this study, dams had given birth to between two and nine infants previously. Parity and weaning age inversely related, albeit not significantly so (R2 = 0.3217; P = 0.071) (Fig. 5C).
Growth of Infants
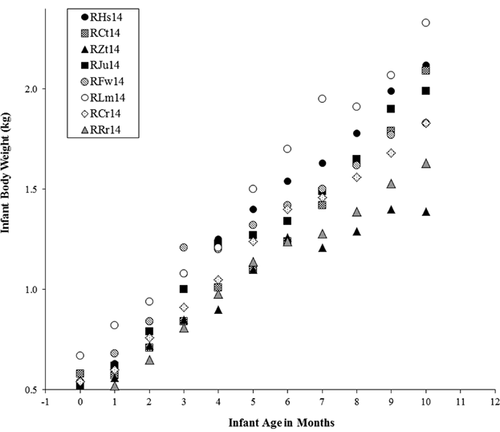
Infants vary in growth velocity and absolute body size throughout the sampling period (Fig. 6). Between male and female infants, there are no significant differences in growth velocity (Mann–Whitney U, P = 0.733), or in absolute body mass (P = 0.136). Growth faltering is exhibited by some infants, including infant RZt14, whose dam was in the lowest-ranking family (12) and lost nearly 2.5 kg in body mass during lactation. Weaning age is inversely related to infant birth mass in kg (R2 = 0.4121; Fig. 5A) and to infant size relative to mother's size, albeit not significantly so (R2 = 0.1356; Fig. 5D). Weaning age is unrelated to infant mass at 10 months of age (R2 = 0.0005; Fig. 5B).
Stable Isotope Data: Male Versus Female Infants
Pooling all time points together into one sample, female infants exhibit higher δ15N values than do male infants (Mann–Whitney U, P = 0.006). Separating sampling months, sex-based differences in δ15N values are greatest during month six (Mann–Whitney U, P = 0.071) and month eight (P = 0.036). There are no significant sex-based differences in δ13C values, neither when time points are pooled (Mann–Whitney U, P = 0.279) nor when time points are considered separately, excepting month five, when lower δ13C values of males approach significance (P = 0.053).
DISCUSSION
Dietary Baselines and Diet-Serum Spaces of Dams
Consistent with expectations that adult stable isotope values would not vary considerably, owing to monotonous diets, the ranges of dams' isotopic values are small: the range of standard diet dams is 1.3‰ for δ15N and 1.4‰ for δ13C. The ranges of non-lactating adult female values are even smaller, at 0.6‰ for δ15N and 1.4‰ for δ13C. We present additional differences between lactating and non-lactating adult female values below.
Stable isotope signatures of HCD dams differ significantly from standard diet dams, reflecting isotopically dissimilar biscuits. HCD dams were switched to the standard diet between infant ages one and five months. It is interesting to note that, even after the dietary switch, the δ13C values of HCD and standard diet mothers differ significantly, indicating serum retains the δ13C signal of a different diet even months after a dietary transition. In contrast, δ15N values are similar between the HCD and standard diet mothers following the diet switch, indicating δ15N values in serum update more quickly. An interpretation of this finding is that the origin of serum carbon may be more from endogenous sources (body reserves), whereas the origin of serum nitrogen may be more from exogenous sources (foods).
Significant Stable Isotope Differences Between Lactating and Non-Lactating Dams
While adult inter-individual isotopic variation is low overall, there are significant differences between values of dams and non-lactating females. Variation in stable isotope values of mothers' tissues associated with pregnancy and lactation have been reported in a limited number of studies with humans and other animals [Fuller et al., 2004, 2005; Koch, 1997; O'Connell, 2001, cited in Fuller et al., 2004; Polischuk et al., 2001].
One explanation for lower δ15N values among lactating (and pregnant) females has to do with nitrogen retention and urea salvage [Fuller et al., 2004]. When nitrogen from ingested protein is broken down, urea may be excreted, or broken down by bacteria in the gut into ammonia, which is used and converted by those bacteria into amino acids and peptides that the host organism may re-use [Stewart & Smith, 2005]. Urea salvage allows more nitrogen to be retained than would otherwise be excreted. More nitrogen from foods is retained in pregnant and lactating females' bodies. Because excretion of 15N-depleted urea is the reason why consumers exhibit higher δ15N values than their diets, urea salvage results in a net decrease of the diet-tissue space for δ15N (i.e., lower tissue δ15N values for urea-salvaging individuals). Interestingly, neonates also salvage urea nitrogen [Waterlow, 2006; Wheeler et al., 1993: p. 114]. For adults, approximately 39–46% of urea produced in the body is salvaged, whereas for healthy infants, approximately 76–80% is salvaged. This may be a contributing factor to δ15N “dips” occasionally reported among infants in weaning studies [see also Reitsema & Muir, 2015].
Another explanation for lower δ15N values among lactating females is that more dietary amino acids are routed directly to tissue-building when nutritional demands are higher, bypassing the fractionating steps involved in urea excretion [Fuller et al., 2004; Hobson et al., 1993; Reitsema, 2013]. Finally, lower δ15N values among dams may be due to milk serving as an outlet for 15N during lactation, leaving maternal tissues relatively depleted in 15N compared to non-lactating individuals [Koch, 1997]. The idea that heavy or light isotopes are preferentially excreted in milk may equally apply in the case of δ13C values in the present study. Lipids, which are low in 13C, are excreted in milk among lactating females, but retained for tissue-building among non-lactating females. This would account for lower δ13C values among non-lactating adult females in the present study.
Differences in lactating and non-lactating females as isotopic baselines for infants are illustrated in Figure 7, where shaded areas represent the means ± 1 SD of both the dams, and the non-lactating females. Infants are overlaid as individual data points and plotted through time. This figure shows how the weaning age estimated for infants changes depending on the baseline used for comparison. Methodologically, in stable isotope studies of weaning, an infant may be considered weaned when its stable isotope values are indistinguishable from its mother's. This study demonstrates that lactation induces changes in the isotopic composition of maternal tissues in macaques, and that maternal baselines are therefore moving targets that ideally should be tracked throughout the entire weaning period. It should be noted that bioarchaeologists, many ecologists, and many primatologists will not have the luxury of longitudinally following mothers throughout lactation. Bioarchaeologists, for example, estimate weaning by comparing the isotopic values of subadults to means of adults (ideally, adult females) from the same population. However, bone collagen of adult female skeletons may not isotopically represent the breast milk of lactating adult females, and evidence is accumulating that this is the case. Tissues of lactating and non-lactating mammals may differ by as much as 1‰ (both δ15N and δ13C), according to the present study. If isotopic changes of body tissues among humans were explored systematically among living subjects, a correction factor could be devised, and applied among skeletal samples.
Estimating Infant Weaning Ages From Isotopic Data
Infant δ13C and δ15N values show anticipated trophic enrichment above their mothers' values, demonstrating consumption of mothers' milk. In Figure 4, infants are scaled to their respective mothers, and offsets in isotopic values between infants and their respective dams are shown with all individuals pooled. In general, supplemental foods appear to be consumed in increasing amounts from two to six or seven months, judging from declining Δ13C values. In general, suckling appears to cease at between five and 10 months of age (or later, in the case of RHs14, see below), judging from infant Δ15N values.
Introduction of solid foods: infant δ13C ratios
δ13C values are sensitive to the introduction of high-carbon supplemental foods, such as plants and commercial feed. As expected, we observe the highest infant δ13C (and Δ13C) values at 2 months of age. This time period offers the closest isotopic approximation of a diet based exclusively on mother's milk. Although we cannot be sure that supplementation did not begin prior to 2 months, previous research using barium–calcium ratios in captive rhesus macaque dental tissues indicates exclusive suckling persists until approximately 3 months of infant age [Austin et al., 2013]. Month 5 is the next available sampling point, and the decline in δ13C values between months two and five indicates increased supplementation by solid foods in this period.
In month five, males exhibit lower δ13C values than do females (P = 0.053), suggesting males are consuming more supplemental foods at younger ages, perhaps to meet greater energetic requirements [see Hinde, 2009: 517]. Consumption of supplemental foods increases for all infants throughout the remainder of the sampling period. Consistent with other isotopic studies of weaning [Fuller et al., 2006; Reitsema, 2012], infant δ13C values decline faster and reach maternal values more quickly than do infant δ15N values (Figs. 5 and 6). As previously discussed, δ13C values, in comparison to δ15N values, are disproportionately affected by supplemental foods, which are carbon-rich and nitrogen- poor.
Cessation of suckling: infant δ15N ratios
To assess how infants are meeting their protein requirements via milk, we look to infant δ15N values, which more particularly represent the duration of suckling. δ15N values show prolonged suckling in spite of adding supplemental foods to infants' diets. Milk is still part of some infants' diets as late as 10 months, but for most infants, weaning occurred between five and 10 months. This is consistent with other estimations of captive rhesus macaque weaning periods at 166–257 days [Austin et al., 2013; Bowman & Lee, 1995], but provides greater resolution toward understanding variation in timing of weaning among individual infants. Some of the individual variations in our sample are discussed below.
First to wean: outlier RZt14
Looking to δ15N values, the first infant to be weaned is male infant RZt14, at five months. This infant's family group was lowest-ranking among the sample (12 of 13). His dam was one of the three oldest in the sample (all three aged 13 years), lost more than 2 kg during lactation, and died shortly after the study period. The old age and low family rank of RZt14's dam could have contributed to his early weaning, especially if his dam lacked the available energy reserves to devote to lactation. Infant RZt14 remained at a low mass throughout the sampling period and experienced periods of growth faltering, further suggesting his mother was unable to provide adequate nutritional resources.
Last to wean: RHs14 and RRr14
Other infants began to reduce their dependence on milk at 6 months of age. Infants RHs14 and RRr14, both females, are the only two individuals to be weaned as late as 10 months (RHs14 still was apparently suckling at 10 months of age). Both infants had low birth weights. During the sample period, infant RHs14's growth velocity was among the highest at most time points, and it is likely that lactation effort from her mother supported her growth and development, but resulted in a later weaning age. Infant RRr14 was born at the lowest weight in the sample and appears to have ceased suckling at 10 months, implicating prolonged lactation effort to support catch-up growth. Both dams were relatively small themselves: among the entire sample of standard diet dams, the dam of RRr14 was smallest, at 7.30 kg, and the dam of RHs14 was third smallest, at 7.53 kg. These dams may have needed to spread their lactation effort over a longer period of time in light of limited body reserves available to supplement caloric intake in sustaining lactation.
Body size and early weaning
The first female infant to wean, RLm14 (Fig. 4), was also the largest at most time points (Fig. 6). She was born to the largest mother. For RLm14, her mother's body size and, speculatively, her high rank (third of 13) may have afforded her the ability to invest higher-quality milk, perhaps in longer, less-frequent bouts that encouraged infant independence and growth. Hinde et al. [2009: 154] showed among rhesus macaques that “The amount of milk that mothers produced was associated with infant weight… Mothers that produced greater quantities of milk had heavier infants at both 1 and 3–4 months of age.” The milk produced by RLm14's dam, though not analyzed for composition, could have been a factor in RLm14's early weaning and attainment of an ultimately large body size. The dam of RLm14 was one of four who lost weight during the sampling period, losing 0.76 kg between 0–2 months of infant age (Table I), which may indicate mobilization of maternal reserves to support lactation, infant growth, and early weaning of her young. However, the other three dams who lost weight weaned their young at later than 10 months (RHs14), at six months (RCt14), and at five months (RZt14), demonstrating no consistent relationship between weight loss during lactation and weaning age.
Sex differences in weaning age: males wean first
Male infants in the present study wean earlier than female infants (Table III; Figs. 5 and 6). Males may be receiving milk of higher quality that supports an earlier weaning age. Hinde et al. [2013] and Hinde [2009] found that among rhesus macaques, male infants received milk higher in protein and fat content while females received more milk higher in sugar and calcium, and that this disparity was a likely cause of sexual dimorphism among infants [Hinde et al., 2013]. If mothers provide males with higher quality milk, males may grow more rapidly and wean sooner, while a mother of a female infant may be obliged to nurse longer before her infant becomes physically independent. We might expect males to weigh more at the time of weaning, but this is not the case: males weigh less in the month at which they are weaned, compared to females (Table III). Viewed in this context, the cessation of nutritive suckling for males may have more to do with social and behavioral aspects of gaining independence (i.e., spending more time away from mother) than with physiological necessity. Assessing milk composition alongside stable isotope evidence for weaning age would be a fruitful future direction for research.
Weaning precedes 3.2x birth weight
Weaning ages estimated isotopically are earlier than the age at which infants attain 3.2 times their birth weights (Table III). Excepting two “late-weaning” outliers, discussed below, infants weaned at 2.5 ± 0.3 times their birth weights. Two possible explanations that are not mutually exclusive for why the isotopic data do not support the threshold weight hypothesis include (1) rhesus macaques at the Yerkes Primate Research Center actually may wean earlier than the populations upon which the threshold weight hypothesis was based, and (2) the methods used to estimate weaning age in the studies upon which the threshold weight hypothesis is based overestimated weaning ages. This is certainly possible: non- nutritive suckling is common among mammals [Cameron, 1998], and may create the illusion of feeding when nutritive suckling has, in fact, ceased.
Two infants, RHs14 and RRr14, have isotopically-assessed weaning ages that are two to five or more months later than the age at which they reach their anticipated threshold weights (Table III). Their status as outliers may be explained by noting their small birth weights. These individuals were the smallest at birth (Table I), meaning the ages at which they were expected to attain 3.2 times their birth weight were skewed mathematically to be anomalously early.
Male and female weaning weights overlap. Considering males wean earlier than females, but at approximately the same body sizes, it seems likely that males either receive higher quality milk from their mothers compared to females, permitting faster growth, or males are genetically predisposed to be thriftier with food resources during development [e.g., Hinde, 2009: 517].
Rank, age, and parity
Figure 5E shows an inverse, non-significant (P = 0.359) relationship between family rank and infant weaning age in this sample. The mother who weaned her infant first (RZt14; at five months) was of a low-ranking family, which is inconsistent with an expected association between low rank and longer weaning periods [Bowman & Lee, 1995; Hinde et al., 2009; Nguyen et al., 2012]. The inverse relationship between rank and weaning age in our small sample is complicated by the fact that the lowest ranks are represented by mothers of sons, both of whom were of the oldest age in the sample (13 years). Thus, the weak relationship between early weaning and low rank may actually be tracking sex-differences in weaning age, or age-related differences in the sample. The fact that older mothers wean their young somewhat earlier (Pearson's one-tailed correlation, P = 0.145) (Fig. 5F) is contra expectations that older mothers will invest more in offspring at later years, perceiving diminishing returns for early weaning ages given their relatively low future reproductive potential [Williams, 1966]. In line with our expectations, as parity increases, weaning age decreases, albeit not significantly (Pearson's one-tailed correlation, P = 0.071) (Fig. 5C), suggesting experienced mothers are able to wean offspring earlier.
Three Years Later: Weaning Age and Later-Life Health, Rank, and IBI
How might weaning age affect infant and maternal outcomes? In 2014, when study subjects reached 3 years of age, we examined health status and rank, and maternal IBI, to gauge the possible impacts of weaning age variation on these variables. In general, there were no patterns in weaning age and subsequent infant rank (Table IV). Of the five female infants, all were in good health in 2014.
| Infant ID | Family rank in 2011 | Infant's family rank in 2014 | Weaning age | Dam IBI 2011–12 (days) | Infant's age at time of conception |
|---|---|---|---|---|---|
| RZt14 | Low | Low | 5 mos | – | – |
| RCt14 | Low | Mid | 6 mos | 385 | 7.3 mos |
| RJu14 | High | High | 6 mos | 305 | 4.7 mos |
| RLm14 | High | High | 6 mos | 310 | 4.8 mos |
| RFw14 | Mid | Low | 7 mos | 322 | 5.2 mos |
| RCr14 | Mid | High | 8 mos | 333 | 5.6 mos |
| RRr14 | Mid | Low | 10 mos | 317 | 5.1 mos |
| RHs14 | High | Mid | 10 + mos | 340 | 5.8 mos |
- Changes in subjects' family ranks from infancy to age three years are shown, along with weaning age. Dams' inter-birth intervals (IBI) are shown in days, excepting the dam of infant RZt14, who died. Infants' ages, in months, at the time of their mothers' conception are shown in the last column, calculated as each dam's IBI in days, minus 165 days (a 5.5 month gestation period), divided by 30 days. Only infants from the standard diet group are shown; infants from the high-calorie diet group (n = 7) are excluded owing to the diet shift this group experienced during the sample period.
Rhesus macaques have a gestation period of 5.5 months [Silk et al., 1993] and in captivity, tend to reproduce again in the following year [M. Wilson, personal communication; cf. Maestripieri, 2010]. Weaning periods much longer than 6.5 months may either extend into a subsequent pregnancy, inhibit a subsequent pregnancy [Maestripieri, 2010], or co-vary with other factors inhibiting a subsequent pregnancy, such as resource scarcity [Nuñez et al., 2015; Rosetta et al., 2011; Thompson et al., 2012]. Dams' 2011–2012 inter-birth intervals in days are known, and are presented in Table IV. All dams on the standard diet, excepting one who died, gave birth the following year, after between 305 and 385 days, regardless of the weaning age of their infants. There is no correlation between weaning age and inter-birth interval, in days (Pearson's two-tailed correlation: P = 0.337). Given a 5.5 month gestation period, suckling persisting past 165 days would have intruded into the subsequent pregnancies of these dams. Therefore, four dams became pregnant while nursing. Nursing has well-known effects on inter-birth intervals, and both suckling intensity and maternal energy balance have been implicated as proximate mechanisms [Mattison et al., 2015; Rosetta et al., 2011; Thompson et al., 2012; Valeggia & Ellison, 2009; Wilson et al., 1988; Wilson, 1992]. In the present study, nursing does not inhibit a mother's ability to conceive during the next available opportunity, supporting the latter hypothesis: that energy balance is a crucial variable in permitting or inhibiting conception. All dams in the present study were well-fed, multiparous adults, not faced with tradeoffs between maintenance, development, and reproduction.
CONCLUSION
This study explores the utility of serum stable isotope analysis among non-human primates for understanding variation in weaning. Stable isotope data show that captive rhesus macaque infants at the Yerkes National Primate Research Center cease to suckle between five and 10 months of age. The data begin to explain some of the variation within this five month range. Males, regardless of rank, weaned before females and at lower body weights. The earliest weaning infant, a male, experienced growth faltering and was born to the lowest-ranking female. The latest weaning individuals were among the smallest birth weights, but eventually attained body sizes commensurate with the rest of the subjects, suggesting mothers had a lengthy lactation effort to bring them up toward adult body sizes. The point at which infants reached 3.2 times their birth weight (posited by a threshold weight hypothesis) almost always post-date the isotopically estimated age of weaning: most rhesus macaque infants were weaned at 2.5 ± 0.3 times their birth weights. All dams conceived during the following breeding season, even when nursing was ongoing, pointing to energy availability, rather than suckling intensity, as a critical factor in the resumption of fecundity. The stable isotope ratios of lactating females differ significantly from those of non-lactating females. Understanding the underlying variables influencing a strategy with stable isotope evidence sheds light on the adaptive significance of early versus late weaning for both mothers and infants, and these preliminary results show some of the promise that application of stable isotope analysis will hold in the future among larger cohorts.
ACKNOWLEDGMENTS
We thank Mark Wilson and Natalie Brutto (Yerkes National Primate Research Center), Randy Culp and Jeff Speakman (University of Georgia Center for Applied Isotope Studies), and Brooke Crowley and Matt Sponheimer for organizing the 2013 American Association of Physical Anthropologists session upon which this special issue of American Journal of Primatology is based. We thank two anonymous reviewers for their feedback on the manuscript.




