Investigations of an individual with a Marfanoid habitus, mild intellectual disability, and severe social anxiety identifies PCDHGA5 as a candidate neurodevelopmental disorder gene
Henri Margot and Adrien Pizano should be considered joint first author.
Abstract
Marfanoid habitus and intellectual disability (MHID) co-occur in multiple neurodevelopmental disorders (NDD). Among those, Lujan-Fryns, an X-linked genetic disorder associated with variants in MED12 was the first such syndrome identified. Accurate molecular diagnosis for these MHID syndromes remains a challenge due to significant clinical and genetic heterogeneity. We present a case report of a 20-year-old male patient with MHID and severe social anxiety. A comprehensive clinical evaluation, including morphotype assessment, cognitive, and psychometric and genetic testing, was conducted to provide a detailed understanding of the patient's complex clinical presentation. Psychometric assessments revealed severe social anxiety and various cognitive and emotional challenges. Despite some autism-like symptoms, the patient's clinical presentation was more aligned with mild intellectual disability. Exome sequencing was inconclusive but identified a heterozygous de novo missense variant in the PCDHGA5 gene. This gene is not known in human pathology yet, but we also report a second patient with a syndromic neurodevelopmental disorder and a rare de novo variant which leads us to propose this as a candidate gene. Our findings emphasize the importance of multidisciplinary approach in the diagnosis and management of MHID. This case report underscores the need for objective clinical evaluations and standardized tools to better understand the complex clinical profiles of patients with NDDs. The identification of novel PCDHGA5 gene variants adds this gene's candidacy to the genetic landscape of MHID-NDD, warranting further investigation to determine its potential contribution.
1 INTRODUCTION
The accurate diagnosis and classification of neurodevelopmental disorders (NDDs) remains a significant challenge due to the extensive range of clinical presentations and the intricate genetic landscape involved. NDDs comprise a diverse array of conditions characterized by atypical development, resulting in various degrees of cognitive, behavioral, and emotional impairment. Such disorders include autism spectrum disorder (ASD), attention deficit hyperactivity disorder, or intellectual disability.
Among these NDDs, Marfanoid habitus with intellectual disability (MHID) syndromes are a heterogeneous group with the genetic basis largely still unknown (Chevarin et al., 2020). Currently recognized MHID syndromes include Lujan–Fryns syndrome (LFS) (#309520), which is a rare genetic disorder characterized by Marfanoid habitus, mild to moderate intellectual disability, behavioral abnormalities, and other neurodevelopmental concerns. Initially a distinction between LFS and MHID was not routinely considered, but with the genetic basis of LFS partially elucidated, the diagnosis of this condition cannot apply to all MHID patients, as the diagnosis of LFS implies the presence of specific facial features, nasal speech. An X-linked segregation is often observed, while some individuals have a de novo variant (Hackmann et al., 2016). A comprehensive clinical evaluation, including physical examination, cognitive and psychometric assessment, and genetic testing is therefore essential.
Patients with LFS typically exhibit tall stature, long hyper-extensible fingers. They present with mild to moderate intellectual disability, and craniofacial features that include a long forehead, long narrow face, maxillary hypoplasia, small mandible, long nose with a high and narrow nasal bridge, short and deep philtrum, thin upper lip, and a highly arched palate. The Marfanoid habitus becomes more apparent after puberty. In adulthood, patients are tall, but their height falls within the normal range. A hypernasal voice and generalized hypotonia are often present. Secondary sexual development and testicular size are typically normal (Clark et al., 2009).
Molecular diagnosis of LFS was first established by Schwartz et al. (2007), based on the initial family description by Lujan et al. (1984) and two other patients. They identified pathogenic variants in MED12, located on the X chromosome (Schwartz et al., 2007). Schwartz et al. noted that one family member originally thought to be affected was later found not to carry the pathogenic MED12 variant and was not affected by the same disorder. In some cases, pathogenic variants in UPF3B (#300676) and ZDHHC9 (#300799) have been reported in X-linked MHID phenotype. One author also described a case of MHID phenotype associated with a terminal deletion of Chromosome 5p (Stathopulu et al., 2003).
The historic LFS presentation, is today defined with presence specific p.Asn1007Ser variant in MED12. FG (#305450), Ohdo (#300895) or Hardikar (#301068) syndromes are caused by other variants located in MED12, and impairs the regulation of transcription machinery (van de Plassche & de Brouwer, 2021). No clear patterns differentiate those syndromes, and some patients show overlap between those academic descriptions, creating a spectrum of possible symptoms, named mediatorpathy (Caro-Llopis et al., 2016). The emergence of new candidate genes, involved in other pathway, broaden the field of MHID diagnosis possibility.
To date, 45 cases of LFS have been reported in the literature. The psychiatric expression in LFS appears to be heterogeneous (see Figure 1). Most case reports described mild to moderate intellectual disability as the primary neurodevelopmental disorder. Alongside with this deficiency, two general profiles can be discerned: The first and more prevalent profile consists of individuals with extreme shyness or autism-like syndrome (van de Plassche & de Brouwer, 2021). The second profile centers on ADHD core symptoms, including hyperkinesis, emotional lability, and aggressiveness, occasionally accompanied by defiant or oppositional behaviors. The only review about psychological functioning in LFS was published in 2006 (Lerma-Carrillo et al., 2006) and focused rather on psycho-affective than neurodevelopmental presentation. Psychiatric disorders, such as psychotic disturbances with hallucinatory visions and sounds, and more broadly, schizophrenia, are found in 17% of cases. The last case report with psychiatric description dates from 2020 and reported delusion symptoms (Elyasi et al., 2019). Consequently, some clinicians have proposed LFS as a differential diagnosis for schizophrenia (Lerma-Carrillo et al., 2006). In this paper, they estimated a 9.6% prevalence of schizophrenia in this population. Other reported cases include an eating disorder (Alonso et al., 2006), two cases of behavior disorders with pyromania (Lerma-Carrillo et al., 2006), and a single patient described as having no psychiatric expression of LFS syndrome (Rivera et al., 1992).
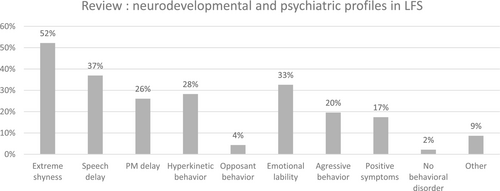
In total, targeted approaches for MHID affected individuals with MED12, ZDHHC9, UPF3B sequencing and the addition of Marfan genes not conventionally associated with intellectual disability: FBN1 (#154700), TGFBR1 (#609192), and TGFBR2 (#610168) allowed to diagnose around 20% patients with MHID (Callier et al., 2013). Further consideration can be given to Shprintzen-Goldberg syndrome (#182212), which unifies MHID and craniosynostosis and is now known to be associated with coding variants typically involving exon 1 of SKI (Carmignac et al., 2012). However, most patients with MHID still lacked a molecular diagnosis.
The development of large-scale genomic sequencing, applied to MHID patients now allows the diagnosis of more than half of patients. Some of these new variants were found in genes previously associated with overgrowth syndromes, suggesting an overlap between those entities (Chevarin et al., 2020). New genes are also candidates for such phenotype as truncating variants of DLG4 (#618793) (Moutton et al., 2018).
In this case report, we present the detailed clinical evaluation of a 20-year-old male patient with a clinical MHID and severe social anxiety. By employing a multidisciplinary approach, including cognitive and psychometric assessment, genetic testing, and a thorough review of the patient's medical history, we aim to provide a comprehensive understanding of this patient's complex clinical presentation and explore the potential contribution of a novel genetic variant in the PCDHGA5 gene.
2 METHODS
The patient underwent a comprehensive clinical evaluation, encompassing medical history review, and physical examination to gain a thorough understanding of the patient's clinical presentation and any relevant medical conditions. Following suitable informed consent, genetic analyses were performed on an accredited clinical basis according to standard protocols.
2.1 Cognitive and psychometric assessment
The patient's cognitive abilities were evaluated using Wechsler Adult Intelligence Scale | Fourth Edition (WAIS IV) and adaptative profile using Vineland 2. The psychiatric symptoms were evaluated using clinical interview and a variety of standardized scales. These included the Mini International Neuropsychiatric Interview (MINI), Liebowitz Social Anxiety Scale (LSAS), Autism Diagnosis Observation Schedule-2 (ADOS-2), Beck Depression Inventory (BDI), Toronto Alexithymia Scale (TAS-20), Social Responsive Scale-2 (SRS-2), Sensory Glasgow Scale, Attention deficit and Hyperactivity disorder-rating scale (ADHD-RS), and the Edinburgh Handedness Inventory (EHI). These assessments provided insight into the patient's cognitive function and mental health status.
2.2 Genetic testing
Array-comparative genomic hybridization (array CGH) with a 60 K Agilent array (median resolution of 400 kb), FMR1 locus analysis and exome sequencing (WES) were performed. We performed WES on the DNA extracted from the blood of probands and parents in trio with the SureSelect Human All Exon V8; Agilen (Agilent, Santa Clara, CA, USA) according to manufacturer's instructions. We generated 75-bp paired-end reads on an Illumina NextSeq550Dx. Alignment to the reference sequence (GRCh38), annotation, and variant filtering were conducted using Alissa Align and Call and Alissa Interpret software (Agilent). The Alamut software (Interactive biosoftware) was used to study retained variant sites.
3 RESULTS
The individual, a 20-year-old man living with his parents, consults in specialized services for people with intellectual disabilities. He has recently begun internships in specialized employment settings, which he seems to enjoy. He received a second assessment at the Autism and Neurodevelopmental Disorders Specialized Center in Bordeaux.
He is the second child of a non-consanguineous couple. His sister has mosaic Turner syndrome, 45,X. No other neurodevelopmental or genetic syndromes are known in the family over three generations.
He experienced a mild global developmental delay, he walked at 22 months and speech was limited to few words at 24 months. At age 3, he developed severe social and separation anxiety, resulting in sleep disorders and co-sleeping with his parents for 5 years.
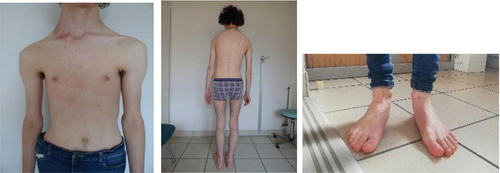
The clinical assessment identified specific elements of facial morphology such as a flat face, prominent nasal ridge, hypernasal voice, high narrow palate, retro and micrognathia. Height is −0.5 SD (173 cm), weight −2.4 SD (49 kg), and head circumference −0.6 SD (56 cm). Additionally, the patient exhibits Marfanoid habitus with pectus excavatum, dolichostenomelia, arachnodactyly of hands and feet, little subcutaneous fat, muscle hypotonia, and pes planus (Photos: Figure 2).
As shown in Table 1, psychometric assessments revealed very severe social anxiety, along with other cognitive and emotional challenges. Despite presenting some autism-like symptoms, his clinical presentation aligned more with his cognitive level, assessed as mild intellectual disability. The Vineland-2 assessment in 2019 was heterogenous and revealed various strengths and weaknesses in communication, daily living skills, and socialization.
| Psychometric scales | Results |
|---|---|
| MINI | Social Phobia |
| LSAS | Performance Anxiety 28; Performance Avoidance 24 Social Anxiety 30; Social Avoidance 29 Total Anxiety 58; Total Avoidance 53 Total 111 (very severe) |
| ADOS-2 | Communication 0; Affect Social 1 Out of Autism Spectrum |
| BDI | 6 (minor) |
| TAS 20 | 62 > 56 (in favor of Alexithymia) |
| STAI-B | 53 moderate Anxiety |
| Sensory Glasgow Scale | Total Score 60 |
| ADHD-RS | 26 (under threshold) |
| SRS-2 (patient) | Awr 8; Cog 24; Com 49; Mot 28; RRB 28; Total Raw score 137; SCI Raw Score 109; Total Raw Score 137; T-score 84 (severe range) |
| SRS-2 (parents school age male 4,5-18y/o) | Awr 9; Log 19; Com 39; Mot 26; RRB 18; Total Raw score 111; T-score 81 (severe range) |
| Vinland-22,019 | Communication: reception: adapted (<18y), expression: weak (4y7m), written: rather weak (12y2m) Daily living skills: personal assessment: weak (8y10m), domestic: Adapted (17y3m), community: weak (7y4m) Socialization: interpersonal relationships: weak (2y9m), play/leisure time: weak (2y5m), adaptation: weak (7y10m) |
| Cognitive level | VCI/POI/WMI/PSI/FSIQ |
| WISC III 2010 | 72/66/NC/NC/65 |
| WISC IV 2011 | 76/73/73/71/66 |
| WISC IV 2017 | 84/60/76/50/NC |
| WAIS IV 2020 | 110/72/71/58/NC |
- Abbreviations: ADOS-2, Autism Diagnosis Observation Schedule; BDI, Beck Depression Inventory; EHI, Edinburgh Handedness Inventory; LSAS, Liebowitz Social Anxiety Scale; MINI, Mini International Neuropsychiatric Interview; TAS-20, Toronto Alexithymia Scale; SRS-2, Social Responsive Scale 2nd Edition; STAY-B: Spielberg Test anxiety.
An autism spectrum disorder was first ruled out at age 10, and he was diagnosed with mild developmental intellectual disability associated with severe social anxiety disorder. The patient still benefits from psychiatric institutions, he works in a sheltered environment. He starts an individual cognitive behavioral therapy. We recommended, as suggested in literature the use of selective serotonin reuptake inhibitors drugs (Wang et al., 2017).
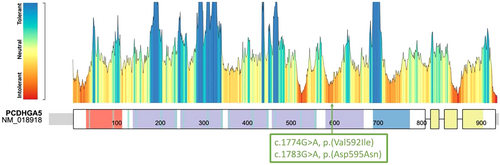
In line with suggestions from (Lerma-Carrillo et al., 2006; Lyons, 2021), a morphologic evaluation was conducted. Cardiac ultrasound, ophthalmologist assessment, hyperhomocysteinemia, and regular lab tests showed no abnormality. Cerebral MRI reveals a marked visibility of the vermis stripes indicative of vermian atrophy. Genetic analysis were carried out due to cognitive impairment and did not detect any abnormality (CGH array and FMR1 locus). We therefore completed these analyses with trio WES, which identified a heterozygous de novo missense variant in the PCDHGA5 gene (NM_018918.3:c.1783G > A, p.(Asp595Asn)). This variant is absent from healthy control database (GnomAD v3), in a highly conserved nucleotide with a predict pathogenicity by in silico tools (CADD Phred: 28.8; AlphaMisense: 0.993-Likely Pathogenic, REVEL 0.547 Damaging). Regarding the ACMG criteria (PM2, PS2, PP3) (Richards et al., 2015), this variant should be consider as likely pathogenic, however due to the lack of knowledge about this gene, we overall caution that clear causation of the described phenotype remains unclear.
By using GeneMatcher (Sobreira et al., 2015), we identified a second individual with a PCDHGA5 missense variant. He was born to a healthy 26-year-old mother at 37 weeks. Family history revealed the father having ADHD and anxiety. He was born full term with birth parameters weight 2.96 kg (20th), Length 53.5 (90th), and OFC 35.5 (40th).
He exhibited developmental delays and was not able to walk or talk by 18 months but began walking at 20 months. He faces various medical issues, including congenital esotropia, obstructive sleep apnea, and eustachian tube dysfunction, as well as notable overgrowth: at age 8.5, patient displayed significantly increased OFC 57.8 cm (>4SD), height 150 cm (+3.38), and weight 79.9 kg (+5.40). Physical features included a broad face, epicanthal folds, coarse hair, 5 cafe au lait macules and multiple freckles. Although Marfanoid habitus was not evident in the child, it is acknowledged that these signs may manifest later as, is often the case in MHID syndromes. Psycho-educational assessments indicated a Learning Disability (WISC 75[70–82]) and moderate ADHD. Clinically significant anxiety was also documented. Bone age, hip and pelvis Xray and MRI brain were normal. Array CGH, FMR1 analysis were without abnormality, but WES trio reveals a de novo NM_018918.3:c.1774G > A, p.(Val592Ile) PCDHGA5 missense variant. This variant is rare (absent from GnomAD v3) and has conflicting in silico predictions but is predicted pathogenic by different reputable in silico tools (CADD Phred: 24.9, intolerant Metadome). Interestingly, this amino-acid change was predicted to destabilize the protein structure by DynaMut2 with a ΔΔG (Stability change) = −0.278 kcal/mol. This structural impact is also predicted by ProtVar (based on AF2 structure) with a FoldX ΔΔG = −0.972. Overall, causation of the phenotype is unclear, but there is strong evidence from the ACMG classification (PS2, PM2, PP3) (Richards et al., 2015).
4 DISCUSSION
We report two individuals with MHID associated with severe social anxiety sharing common clinical features (neurocognitive, growth) and variants in PCDHGA5 located very close within the same domain, cadherin 6. This domain is one of the few of the protein to be intolerant according to metadome (Figure 3) and seems important to PCDHGA5 cell-adhesion function.
PCDHGA5 encodes one of member of the protocadherin gamma gene cluster, a subfamily of nonclassic cadherins. Each member has a specific extracellular variable region followed by a constant region, containing three exons shared by all genes in the cluster. Extracellular region includes six cadherin ectodomains and a transmembrane region. These neural cadherin-like cell adhesion proteins most likely play a critical role in the establishment and function of specific cell–cell connections in the brain (Wu & Maniatis, 1999). Little is known regarding this gene specifically in human disease, and in fact a Pubmed search (February 4, 2024) of this gene returns zero relevant publications.
This gene is also referenced in the neurodevelopmental disorder gene lists selected in Gene Trek (Leblond et al., 2021). In the literature, only one publication reported a frameshift variant in the PCDHGA5 gene possibly involved in a patient with developmental and epileptic encephalopathy (Takata et al., 2019), however the PLI of the gene is 0 and therefore it is not entirely clear whether haploinsufficiency would be associated with disease, or seizures.
PCDHGA5 tissue expression data from GTEx project showed a strong brain expression, especially in the cerebellum (The GTEx Consortium atlas of genetic regulatory effects across human tissues|Science, n.d.). This could be consistent with the vermian hypoplasia observed in MRI. The cerebellum has not only a motor role but also works in cognitive and psycho-affective processes (Koziol et al., 2014). Strong links have been made in the involvement of cerebellum development and autism (Roux & Bossu, 2016). Cerebellar impairment could explain the mild intellectual deficiency, or the central atypical treatment of sensorial signals (as vestibular or proprioception) as found in sensory Glasgow scale of our first patient.
The clinical presentation in both our patients is relatively mild, and as genomic approaches are still recent in clinical practice, they are more frequently used with more striking neurodevelopmental presentations or other suggestive clinical features. In patient 1, the Marfanoid phenotype was more distinguishable after puberty, as expected in literature (Donders et al., 2002; Fryns & Van Den Berghe, 1991). The second patient presented with mild generalized overgrowth as an additional reason to pursue genomic testing. The cognitive level in both was borderline, without formal intellectual deficiency. The diagnosis of autism is also not obvious, and mixed with social anxiety, a sensory inhibited profile, NDD and language delay. However, the two autism dimensions are represented (social difficulty and repetitive activities with sensory atypicality). These observations could be consistent with an association with a gene not yet linked to a highly penetrant human disorder.
MHID currently encompasses a broad group of conditions with rather non-specific distinctions and that may overlap strongly other phenotypical presentations such has overgrowth (Rubinato et al., 2020). Given the evolving phenotype and lack of a clearly defined clinical profile across studies, delineating a comprehensive clinical profile is difficult. Additionally, the frequency of de novo cases in the literature suggests a lack of pertinent genetic family history (Polla et al., 2021). Furthermore, limited medical access, particularly for patients residing far from hospitals, could contribute to this diagnostic odyssey/delay.
This work underlines the necessity to assess patients in psychiatry using objective tools to describe precise clinical profiles with international and widely used clinical scales to objectify and standardize our evaluation. By promoting access to specialized service, and fostering collaboration among clinicians, we can improve the diagnosis and management of patients with NDD and Marfanoid habitus.
5 CONCLUSION
In conclusion, our report highlights the complex nature of MHID, and proposes the candidacy of PCDHGA5 as a potential neurodevelopmental disorder gene. Despite the challenges in diagnosing MHID due to its variable clinical presentation, our multidisciplinary approach provided valuable insights into the clinical profiles of our patients.
Through comprehensive clinical evaluations, including cognitive and psychometric testing, and genetic analysis, we identified common clinical features and novel genetic variants in the PCDHGA5 in two individuals with neurodevelopmental disorders. This gene's involvement in neural cell adhesion and its strong expression in the brain, particularly in the cerebellum, suggest its potential role in neurodevelopmental processes and disorders. However, further studies will be necessary to confirm this statement.
The vast phenotype of MHID and the emergence of new candidate genes highlight the need for continued research and collaboration to improve diagnostic accuracy and patient care.
ACKNOWLEDGMENTS
We thank the patients and their families for their participation in this study.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.




