Systemic and ocular manifestations of a patient with mosaic ARID1A-associated Coffin-Siris syndrome and review of select mosaic conditions with ophthalmic manifestations
Abstract
Mosaic genetic mutations may be somatic, germline, or “gonosomal” and have the potential to cause genetic syndromes, disorders, or malformations. Mutations can occur at any point in embryonic development and the timing determines the extent of distribution of the mutation throughout the body and different tissue types. The eye and visual pathway offer a unique opportunity to study somatic and gonosomal mosaic mutations as the eye consists of tissues derived from all three germ layers allowing disease pathology to be assessed with noninvasive imaging. In this review, we describe systemic and ocular manifestations in a child with mosaic Coffin-Siris syndrome. The patient presented with a significant medical history of accommodative esotropia and hyperopia, macrocephaly, polydactyly, global developmental delay, hypotonia, ureteropelvic junction (UPJ) obstruction, and brain MRI abnormalities. The ophthalmic findings in this patient were nonspecific, however, they are consistent with ocular manifestations reported in other patients with Coffin-Siris syndrome. We also review ophthalmic findings of select mosaic chromosomal and single-gene disorders. Ophthalmic assessment alongside clinical genetic testing may play an important role in diagnosis of genetic syndromes as well as understanding disease pathology, particularly when mosaicism plays a role.
1 INTRODUCTION
Genetic disorders may be inherited, such as autosomal and X-linked dominant and recessive disorders, chromosomal, many of which are inherited and sporadic. Sporadic conditions, defined as disorders arising in the absence of obvious heritable or environmental etiology, may be secondary to de novo mutations occurring during gametogenesis or to somatic mutations during embryonic development. Mosaicism occurs when a genetic mutation is found in some, but not all cells in a person's body, and these cells all originated from the same zygote. In contrast, chimerism occurs when two zygotes merge. Mosaicism can result due to loss of a chromosome in some cells, such as when trisomic rescue occurs in some but not all cells resulting in two or more distinct cell lines (Campbell, Shaw, Stankiewicz, & Lupski, 2016). During the prenatal period, chromosomal mosaicism may be limited to the placenta (confined placental mosaicism) or may also be found in the fetus. Common examples of chromosomal mosaicism are mosaic Down syndrome and mosaic Turner syndrome. Chromosomal mosaicism may result due to postzygotic nondisjunction or meiotic nondisjunction that is followed by postzygotic chromosome loss or duplication (Conlin et al., 2010; Papavassiliou et al., 2009).
In contrast to chromosomal mosaicism, single gene mosaicism occurs when a mutation occurs in a gene and is then found in some but not all of the cells in the body. Mosaicism may be germline, in which the mutation is found in an unaffected individual's germ cells and gametes and can be transmitted to progeny or may be somatic, and only found in cell lines outside of the gametes. Occasionally, both somatic and germ cells may be mosaic, referred to as “gonosomal.” Some somatic mosaicism may be harmless or may be lost over time due to a growth disadvantage or apoptosis. In other cases, somatic mosaicism may create a cell line with malignant potential or may cause other genetic syndromes, disorders, or malformations. Mutations can occur at any time during embryonic development. The timing of somatic mutations determines spatial distribution of the mutation throughout the body and in different tissue types. Somatic mutations that occur at earlier developmental time points are more likely to be wide spread throughout the body. Some somatic mutations are severe enough that if they were found in all parts of the body during early development, they would be lethal during the prenatal period (Happle, 1987). Late post-zygotic somaticism may lead an apparently isolated congenital malformation of a single tissue or organ or a segmental distribution of disease.
Ocular involvement can occur in mosaic chromosomal disorders such as trisomy 21, multi-systemic single gene syndromes such as segmental neurofibromatosis I (NF1), and ocular malformations such as mosaic PAX6 related disease. Somatic mosaicism impacts laterality and age of onset in ocular malignancies, especially retinoblastoma. Unilateral involvement and later age of diagnosis are more likely to be caused by somatic mutation. In some cases, loss of function of a specific gene is lethal unless mosaic, such as GNAQ in non-syndromic port wine stain (PWS) and Sturge Weber Syndrome (SWS).
The eye and visual pathway represent a unique system in which to study mosaic conditions as the eye is derived from neural ectoderm, surface ectoderm, neural crest cells, and mesoderm. Therefore, the ocular manifestations may reflect the timing of the mosaic event in embryonic development. In addition, the eye can be readily examined clinically, and imaging studies such as optical coherence tomography (OCT) and adaptive optics may be used to non-invasively characterize the ocular phenotype at the histologic and cellular resolution, respectively.
We present the systemic and ocular manifestations of a child with mosaic Coffin-Siris syndrome and review the ophthalmic manifestations in select mosaic chromosomal and single gene disorders.
2 CASE PRESENTATION
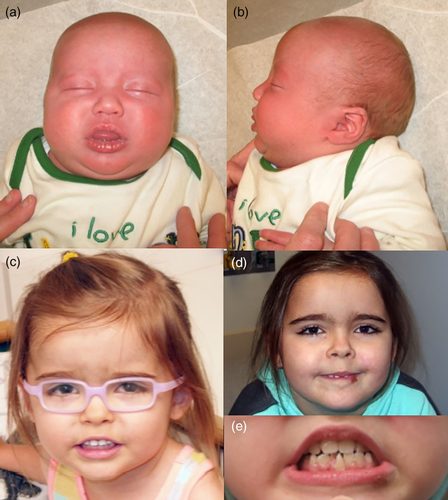
A 7-month-old Caucasian girl presented to Abrahamson Pediatric Eye Institute at Cincinnati Children's Hospital for infantile-onset esotropia. She was the product of a 37 weeks gestation pregnancy by spontaneous vaginal delivery to a 27-year-old G1P0 woman and her 45-year-old unrelated husband. Her birth weight was 3.11 kg (z = −0.59), length 47.5 cm (z = −0.98) and head circumference 35.5 cm (z = 0.47). Her prenatal history was significant for by polyhydramnios prompting fetal MRI demonstrating left ureteropelvic junction obstruction, mild right renal pelviectasis, mild right lateral ventriculomegaly, and bilateral post-axial polydactyly. No prenatal genetic testing was performed. The infant underwent nephrostomy tube placement at 3 days of life and open left dismembered reduction pyeloplasty, left stent insertion at 3 months of life, and bilateral supernumary digit excision. Brain MRI at 5 months of age revealed abnormal sulcation in the occipital and parietal lobes, deficient corpus callosum, gray matter heterotopia, abnormal sulcation posterior sylvian fissure with possible closed lip schizencephaly on the right, and dysplastic left cerebellum. MRI spectroscopy showed low NAA and myoinositol for age (Figure 1a,b).
At 7 months of age she was referred to Ophthalmology for esotropia. Her visual behavior was central, steady, and maintained in each eye without fixation preference. She had an alternating esotropia measuring 35–40 PD by Krimsky with full motility. Pupils, anterior and posterior segment examinations were unremarkable. Cycloplegic refraction was +3.75 sphere in each eye and glasses were prescribed initially. Bilateral medical rectus recession of 5.5 mm for the non-accommodative component of esotropia was performed at age 17 months. She was seen jointly by ophthalmology and human genetics in a multi-disciplinary Eye Genetics Clinic given her constellation of structural and developmental abnormalities. Of note, 1 month earlier, her weight was 8.60 kg (z = 1.41), length 66.4 cm (z = 0.26), but she had macrocephaly with head circumference 47.2 cm (z = 3.34).
Genetics consultation was performed at 17 months. Her motor development was delayed, she was not walking, and she had a 20-word vocabulary but was not combining words. The most pertinent finding was the macrocephaly. Follow up at 23 months showed that she had started walking by 20 months and speech and language was progressing. She still had fine motor delays. At the time considered in the differential diagnoses was megalencephaly-polydactyly-polymicrogyria-hydrocephalus syndrome and research testing was planned, but the family was lost to follow up. Examination was negative except for the macrocephaly and a supernumerary mandibular central incisor. She was placed in bifocals at age 3.5 years (Figure 3) for high accommodative convergence to accommodation (AC/A) ratio and treated for amblyopia of the right eye (Figure 1c). At the age of 6 years, the parents expressed concern about her depth perception and visual attention. Although she had a diagnosis of autism, she was referred to occupational therapy for a cortical visual impairment evaluation given the known CNS structural abnormalities. She was diagnosed with mild CVI (Roman-Latsky range 9/10 [Roman-Lantzy, 2007], indicating Phase III visual impairment). At her last follow-up at 7 years of age, her visual acuity was 20/25 in each, orthophoric in bifocal correction with subnormal stereoacuity (200 s arc by Randot testing [Adler, Scally, & Barrett, 2012; Kulp & Mitchell, 2005]).
The patient was seen for Genetics follow up at 6 years 5 months. Development was delayed and a psychoeducational evaluation had been performed documenting autism spectrum disorder. Growth parameters were normal except for the macrocephaly with head circumference 56.5 cm (z = 4.0). At that visit a SNP chromosomal microarray was performed (Cincinnati Children's Laboratory of Genetics and Genomics) and was normal. Intellectual disability-Autism spectrum disorder panel was performed (GeneDx) and showed mosaicism for a pathogenic variant of ARID1A (c.169 G>T; E57X). This variant is diagnostic for Coffin-Siris syndrome. This is a nonsense variant is predicted to result in protein truncation or nonsense mediated decay. This variant has not been described in large population cohorts (GnomAD). In addition, it was not reported in the Human Gene Mutation Database, dbSNP, or ClinVar. The variant was present in 22% of 77 sequencing reads indicating it was underrepresented in comparison to the reference allele in the specimen tested. Follow up MRI scan at 6 years 8 months showed hypogenesis of the corpus callosum, cerebellar dysplasia, and cleft in the ventral brainstem and scattered foci of periventricular gliosis with a posterior predominance.
At 7 years of age she still had mild to moderate developmental delay, a loquacious personality and liked conversing (Figure 1d). She was entering first grade with special education resource assistance and speech, physical and occupational therapies. She was easily distracted, but able to learn. Physical examination showed a healthy girl, normal height (z–z = 0.81) and weight (z = 1.59) and macrocephaly (57 cm, z = 4.4). Of note was a central midline mandibular incisor, which was conical in shape (Figure 1e).
3 REVIEW OF SELECTED MOSAIC CONDITIONS WITH OCULAR MALFORMATIONS
We selected several categories of syndromes where somatic or gonosomal mosaicism should be suspected. Chromosomal mosaic trisomies allow survival of the fetus. The eye exam can play a key role in the diagnostic work-up. The genes NF1, RB1, GNAQ, and IKBKG were selected because failure of the ophthalmologist to recognize the systemic associations in a patient with an atypical presentation may lead to visual morbidity and/or death. A high index of suspicion is required. Finally, PAX6 is the master regulator of the eye with a wide array of clinical manifestations, and salient example cases of variable expressivity related to gonosomal mosaicism (Table 1).
| Chromosome | Periorbital/orbital | Anterior segment | Posterior segment | Refractive error | Electrophysiology | Sensorimotor |
|---|---|---|---|---|---|---|
| Mosaic ring 1 trisomy (Kjessler et al., 1978) |
|
None reported | None reported | Unknown | Unknown | Unknown |
| Partial mosaic trisomy 5p (Schlegel et al., 2009) | Iridolenticular adhesions (Schlegel et al., 2009) | None reported | High hypermetropia (Schlegel et al., 2009) |
Unknown | Strabismus (accommodative esotropia) | |
| Mosaic trisomy 8 |
|
Axenfeld Reiger spectrum Corneal opacity (Stark et al., 1987; Stone & Siatkowski, 2005) (Welsh et al., 2018) | Foveal hypoplasia Pigmentary retinopathy (Stone & Siatkowski, 2005) |
|
|
|
| Mosaic trisomy 21 (Motley 3rd & Saltarelli, 2011) |
|
Cataracts | None reported | -Significant Ametropia | Unknown |
|
| Mosaic trisomy 22 (Thomas et al., 2004) |
|
None reported | Chorio-retinal coloboma | Refractive error | Unknown |
|
- Abbreviation: DRS, Duane retraction syndrome.
3.1 Chromosomal mosaic trisomies and select mosaic chromosomal syndromic disorders
3.1.1 Trisomy 1 mosaicism
Complete trisomy of chromosome 1 is incompatible with life and results in fetal death (Van den Berghe, Van Eygen, Fryns, Tanghe, & Verresen, 1973). Several authors have reported mosaic ring 1, but most of the children in these reports had additional chromosomal abnormalities (Gordon & Cooke, 1964; Kjessler, Gustavson, & Wigertz, 1978; Wolf, Peterson, LoGrippo, & Weiss, 1967). Wolf and Gordon did not report any ocular abnormalities (Gordon & Cooke, 1964; Wolf et al., 1967). Kjessler reported a child with small palperal fissures, epicanthal folds, downslanting eyelids, with normal examination of the eye otherwise (Kjessler et al., 1978). In this particular patient, some cells had a normal karyotype, while others had one or more ring 1 chromosome (Kjessler et al., 1978).
3.1.2 Trisomy 5p mosaicism
The ocular findings of partial Trisomy 5p mosaicism (46, XY/47, XY + mar) was reported by Schlegel and colleagues (Schlegel, Valent, & Hirsch, 2009). Previous reports of trisomy 5p syndrome describe an atypical Peters' anomaly (D'Amato Sizonenko, Ng, Oei, & Winship, 2002; Velagaleti, Morgan, & Tonk, 2000), however, corneal opacification in this patient was absent. This patient did, however, have anterior segment dysgenesis with iridolenticular adhesions (Schlegel et al., 2009). Other periocular features in this patient included epicanthal folds and hypertelorism. Accommodative esotropia was present, but resolved with refractive error correction. The patient was +9.00 spherical equivalent at 14 months of age.
3.1.3 Trisomy 8 mosaicism
Trisomy 8 (Warkany Syndrome) mosaicism is characterized by intellectual disability, dysmorphic facies, skeletal anomalies, congenital heart disease, gastrointestinal anomalies, and deep furrows in the palms and soles. Ophthalmologic findings include canthal abnormalities and abnormal slanting, as well as cases of blepharophymosis syndrome. Patients appear hyperteloric because of the enlarged nasal root. Strabismus is observed in approximately half of patients (Riccardi, 1977), including craniodysinnervation syndromes such as Duane Syndrome (Anwar, Bradshaw, & Vivian, 1998). Anterior segment abnormalities include corneal opacities (Frangoulis & Taylor, 1983; Stark, Gilmore, Vance, & Pearn, 1987; Stone & Siatkowski, 2005; Welsh, Khalili, Hazrati, & Mireskandari, 2018), Axenfeld-Reiger Spectrum malformations, and microcornea (Anwar et al., 1998). The corneal opacity is distinguished from Peters anomaly histologically as the lesion described in this syndrome involves fibrovascular proliferation in the superficial (anterior) layers of the cornea. In contrast, Peters anomaly is characterized by posterior defects including endothelial attenuation, immature or absent Descemet's membrane, attenuated Bowman's layer with or without corneo-lenticular or iridocorneal adhesions. In some cases, the corneal opacity may regress without intervention (Stone & Siatkowski, 2005), whereas other may require keratoplasty to prevent deprivation amblyopia (Stark et al., 1987). Merdassi and colleagues reported a case of a corneal choristoma, composed of fibrovascular and glandular tissue (Merdassi et al., 2004). Therefore, the phenotypic spectrum of congenital corneal opacities is broad. Posterior segment manifestations include foveal hypoplasia (Anwar et al., 1998; Riccardi, 1977), optic disc coloboma with chorioretinal defects (Taban, Marcotty, & Traboulsi, 2006), pigmentary retinopathy have been infrequently reported (Riccardi, 1977; Stone & Siatkowski, 2005). Electrophysiologic findings range from normal (Stark et al., 1987), to attenuated photopic responses (Abu-Amero et al., 2013), to extinguished scotopic and photopic responses (Stone & Siatkowski, 2005). Myopic refractive error is associated with trisomy 8 (Abu-Amero et al., 2013). Thus, varied ophthalmic manifestations are frequently present in patients with partial or complete trisomy 8 mosaicism. Importantly, trisomy 8 mosaicism is seen frequently in patients with myelodysplastic disease such as acute promyelocytic leukemia (Kwong, Wong, & Chan, 1995).
3.1.4 Trisomy 21 mosaicism
Down syndrome (DS) is one of the most prevalent chromosomal abnormalities, occurring in ~14 in 10,000 births (Besser, Shin, Kucik, & Correa, 2007; Canfield et al., 2006; Parker et al., 2010). Mosaicism in chromosome 21 results in at least two distinct cell lines, displaying both trisomic, and euploid cell lines in the individual (Constance, Clarke, & Smallpeice, 1961; Fitzgerald & Lycette, 1961). Mosaic DS occurs in an estimated 2–4% of individuals with DS, however the true incidence of mosaic DS may be higher as low-level mosaicism may go unrecognized, and undiagnosed (Gibson, 1973; Pangalos et al., 1994; Papavassiliou et al., 2009). Generally, individuals with mosaic DS present with fewer clinical traits associated with DS than those with complete trisomy-21 DS, often presenting with nearly normal phenotype and cognition (Bull, 2011; Devlin & Morrison, 2004; Papavassiliou et al., 2009; Shin, Siffel, & Correa, 2010). Phenotypically, ocular manifestations in complete trisomy-21 DS are known to include cataracts, severe refractive error, strabismus, nystagmus, and lacrimal duct obstruction (Berk, Saatci, Erçal, Tunç, & Ergin, 1996; Bull, 2011; Coats et al., 2003). Characteristic periocular findings such as epicanthal folds and up-slanting palpebral fissures are present. To date, there is little literature on specific clinically significant ocular findings in individuals with mosaic DS. The reported incidence of severe refractive error, accommodative insufficiency, strabismus, nystagmus, and cataracts in individuals with mosaic DS suggests that ocular manifestations in mosaic DS and complete trisomy-21 DS occur at similar rates of incidence and severity (Motley 3rd & Saltarelli, 2011). Therefore, screening for ocular manifestations of mosaic DS should mirror those of complete trisomy 21.
3.1.5 Trisomy 22 mosaicism
Children with mosaicism for trisomy 22 may be severely affected with systemic findings of dysmorphic facial features and asymmetry, microcephaly, pulmonary stenosis, hypotonia, developmental delay, and abnormal dentition (Antle, Pantzar, & White, 1990). Ocular manifestations include hypertelorism, down-slanting palpebral fissures, epicanthal folds, ptosis, strabismus, and microphthalmia (Thomas, Parker, Tan, Duckett, & Woodruff, 2004). Thomas and colleagues report a case of a child with mosaic trisomy 22 who presented with unilateral ocular features including esotropia, ptosis, and monocular elevation deficiency (Antle et al., 1990). The child had myopic anisometropia in that eye and a choroidal coloboma involving the macula.
3.2 Single-gene somatic mosaicism
3.2.1 Retinoblastoma
Retinoblastoma (RB) is the most common ocular cancer observed in children, usually occurring before age 7. The prevalence is estimated to be 1 out of 15,000–28,000 live births (Gallie, Campbell, Devlin, Duckett, & Squire, 1999; McLean, 1996). Tumor initiation requires loss of function in biallelic copies of the RB1 gene, and accompanying additional mutational events are common (Dimaras et al., 2008; Knudson, 1971; Sampieri et al., 2009). Next-generation sequencing has revealed that somatic mutant mosaic RB with a high variant frequency occurs in an estimated 14% of bilateral patients and 38% of unilateral patients (Rodríguez-Martín et al., 2020). Somatic mutant mosaic RB with a low variant frequency occurs in ~10–19% patients (Amitrano et al., 2015; Rodríguez-Martín et al., 2020). The prevalence of somatic mutant mosaic RB may be higher than currently reported as less sensitive techniques were used previously and may not have detected lower variant frequency mosaics. Presentation of RB appears to differ between high-level somatic mutant mosaics, low-level somatic mutant mosaics, and patients with true heterozygous RB. It is speculated that high-level somatic mutant mosaics may have a lower probability of producing retinal cells with loss of function of both RB1 alleles than patients with true heterozygous RB. This may increase the likelihood of high-level somatic mutant mosaics to develop unilateral rather than bilateral retinoblastomas (Rodríguez-Martín et al., 2020). Additionally, age at diagnosis is older in low-level somatic mutant mosaics with unilateral retinoblastomas than in unilateral retinoblastomas in high-level somatic mutant mosaics or true heterozygous RB (Rodríguez-Martín et al., 2020). Identification of mosaicism and variant frequency in RB patients is relevant to treatment management and genetic counseling.
Occasionally germline mosaicism can lead to a later presentation of ocular disease. Astudillo and colleagues describe an 8-year-old who presented with bilateral retinoblastoma (Group A in the right eye (best-corrected visual acuity, BCVA 20/20) and Group B in the left eye (BCVA 20/200) (Astudillo, Chan, Heon, & Gallie, 2014). Despite 96% sensitivity of genetic testing for RB1, no mutation was identified in the blood. Salvage therapy was attempted in both eyes, but the tumor was not controlled in the left eye. The patient elected for enucleation and the mutant alleles were identified from vitreous seeds. By mutation-specific PCR, one of the mutations was detected in 10% of blood lymphocytes. A precise genetic diagnosis of germline mosaicism enabled accurate genetic counseling on risk to antecedent relatives and for the patient's future offspring.
3.2.2 Neurofibromatosis type 1
Neurofibromatosis type 1 (NF1) is neurocutaneous disorder resulting from a mutation in the NF1 gene. NF1 is reported to occur in 1 in 522–7,800 live births (Rasmussen & Friedman, 2000). There is a large variability in reported NF1 prevalence across various ethnic groups. Mosaic neurofibromatosis type 1 (MNF1), or segmental neurofibromatosis type 1, is estimated to occur in 1 in 36,000–40,000 live births (Ingordo, D'Andria, Mendicini, Grecucci, & Baglivo, 1995; Pascual-Castroviejo, Pascual-Pascual, & Viaño, 2008; Rasmussen & Friedman, 2000; Ruggieri et al., 2004; Ruggieri & Huson, 2001; Wolkenstein, Mahmoudi, Zeller, & Revuz, 1995). The prevalence of MNF1 is thought to be higher than what is reported, notably, patients with MNF1 do not meet the minimum diagnostic criteria for NF1 and therefore a MNF1 diagnosis may be overlooked. Ocular manifestations of NF1 include Lisch nodules, optic pathway gliomas, choroidal hamartomas, eyelid, and/or conjunctival neurofibromas, and plexiform neurofibromas of the orbit. Reports of MNF1 ocular manifestations have largely been restricted to bilateral and unilateral Lisch nodules (Adams, Stewart, Borges, & Darling, 2011; Lal, Leavitt, Lindor, & Mahr, 2003; Nicita et al., 2013; Poornimambaa et al., 2017). Additionally, irregular pupil shape and optic pathway glioma have been noted in MNF1 (Garcia-Romero, Parkin, & Lara-Corrales, 2016; Poornimambaa et al., 2017). Patients with MNF1 generally present with a milder phenotype than their counterparts with NF1, however, 28% have clinically relevant findings, and 13% are at risk for malignancy (Garcia-Romero et al., 2016). Due to this risk, patients with suspected or confirmed MNF1 should undergo health surveillance, including regular comprehensive ophthalmic exams.
3.3 Genes where loss of function is lethal
3.3.1 GNAQ: Sturge weber syndrome (Encephalotrigeminal Angiomatosis)
Sturge Weber Syndrome (Encephalotrigeminal Angiomatosis) (SWS) is a sporadic, congenital neurocutaneous disorder with the classic triad of nevus flammeus (port-wine stain, PWS), leptomeningeal angiomatosis, and choroidal hemangioma. Vascular stasis from the leptomeningeal angiomatosis leads to intraparenchymal dystrophic calcification and cortical necrosis. The clinical course and complications are variable, but may include glaucoma, seizures, stroke, contralateral hemiparesis, and intellectual disability (Thomas-Sohl, Vaslow, & Maria, 2004). Approximately, 10% of those with a congenital PWS will have the SWS, and the risk increases when the PWS is in the distribution of the ophthalmic branch of the trigeminal nerve (Enjolras, Riche, & Merland, 1985; Tallman et al., 1991).
Happle suggested somatic mosaicism as the mechanism for disease in 1987 (Happle, 1987). However, the genetic cause of SWS remained elusive until Shirley and colleagues performed whole-genome sequencing of DNA from samples of visibly affected and nonaffected tissue of individuals with SWS and isolated PWS. A single nucleotide variant (c.548 G>A, p.R183Q) in the G-α q gene (GNAQ, 9q21.2, OMIM 185300) was found in a majority of affected tissue, supporting the role of somatic activating mutations in GNAQ in the development of nonsyndromic PWS and SWS. The GNAQ gene encodes Gαq, an alpha-subunit of heterotrimeric G protein involved in cell signaling in endothelial cells in the vessels of skin and brain (Huang et al., 2017; Tan et al., 2016). The mutation leads to Gαq in its GTP-bound, active state (O'Hayre et al., 2013). The extent of tissue involvement depends on the location and timing of the somatic mutation in embryonic development. Shirley and colleagues proposed that nonsyndromic PWS results from late origin of the somatic GNAQ mutation in vascular endothelial cells, whereas early occurrence in progenitor cells leads to SWS (Shirley et al., 2013).
The most common ocular manifestations of SWS include choroidal hemangiomas and glaucoma. Glaucoma occurs in ~50–70% of patients, with a bimodal distribution of onset.(Sujansky & Conradi, 1995a, 1995b) Approximately 60% of patients will present with SWS-associated glaucoma in the first year of life (Sujansky & Conradi, 1995b). Developmental abnormalities of the anterior chamber angle or trabeculodysgenesis contribute to the pathogenesis in this early-onset group (Lavaju & Mahat, 2015; Phelps, 1978). The clinical presentation is similar to primary congenital glaucoma with buphthalmos and corneal enlargement; and the management is largely with angle-based surgery (Olsen, Huang, & Wright, 1998; Sujansky & Conradi, 1995b). In general, congenital-onset glaucoma is almost always associated with PWS involvement of both upper and lower eyelids (V1 and V2 trigeminal nerve distribution) (Sujansky & Conradi, 1995b; van Emelen, Goethals, Dralands, & Casteels, 2000). Glaucoma can also occur in the setting of an extensive PWS without SWS or CNS involvement (Stevenson, Thomson, & Morin, 1974). In the remaining 40% of patients, glaucoma develops later in childhood or early adulthood and is hypothesized to be secondary to elevated episcleral pressure and creating resistance to aqueous outflow from the eye (Phelps, 1978; Shiau, Armogan, Yan, Thomson, & Levin, 2012; Sujansky & Conradi, 1995b; Sullivan, Clarke, & Morin, 1992; Weiss, 1973). This is supported by blood in Schlemm's canal and more pronounced glaucoma in patients with episcleral hemangiomas (Phelps, 1978; Weiss, 1973). Less frequently, later onset disease is associated with a closed angle glaucoma mechanism secondary to ectopia lentis, choroidal detachment or neovascularization of the anterior chamber angle (Lee et al., 2015; Moore, Reck, & Chen, 2011; Mwinula, Sagawa, Tawara, & Inomata, 1994; Su, 2018). Medical management with aqueous suppressants may be utilized, but patients often require surgical intervention (Pavlenko, Scovpen, & Vitovska, 2018). Other findings include vascular malformations of the conjunctival, episcleral, retinal and choroidal vessels, retinal detachment, ectopia lentis, and strabismus (Sullivan et al., 1992). Visual field abnormalities may be present secondary to CNS disease and or surgical intervention for medical treatment-refractory, seizure disorder. Therefore, patients require frequent ophthalmic follow-up to monitor intraocular pressure, optic nerve for cupping, and other complications of disease.
3.3.2 Incontinentia pigmenti
Incontinentia pigmenti (IP) is a rare X-linked ectodermal disorder that affects the skin, hair, teeth, nails, eyes, and central nervous system. Characteristic skin lesions evolve through four stages consisting of blistering, verrucous rash, swirling hyperpigmentation, and linear hypopigmentation. Other clinical manifestations include intellectual disability, seizures, alopecia or hair thinning, abnormally shaped, or small teeth, high arched or cleft palate, and dystrophic nails (Minic, Trpinac, & Obradovic, 2014). IP is caused by mutations in the IKBKG gene (NEMO, Xq28, OMIM 308300), and approximately 80% of patients carry a common deletion mutation involving exons 4–10. The estimated prevalence of IP is 0.7 in 100,000, live births (Mullan, Barbarian, Trakadis, & Moroz, 2014). Generally, IP is considered to be lethal in male embryos with few reported cases of males surviving. Surviving males with loss of function alleles occur as the result of three mechanisms: a 47, XXY karyotype (Buinauskaite, Buinauskiene, Kucinskiene, Strazdiene, & Valiukeviciene, 2010; Fowell, Greenwald, Prendiville, & Jampol, 1992; Kenwrick et al., 2001; Prendiville, Gorski, Stein, & Esterly, 1989), low-level germline and somatic mosaicism with recurrence risk to future female offspring (Fusco et al., 2017; Rashidghamat et al., 2016), and segmental forms secondary to post-zygotic mosaicism of the IKBKG/NEMO gene mutation (Hull et al., 2015; Matsuzaki et al., 2018). Ocular manifestations occur in one-third of patients with IP and include an avascular peripheral retina which leads to peripheral ischemia, neovascular and fibrovascular proliferation (Holmstrom & Thoren, 2000; Peng et al., 2019; Rosenfeld & Smith, 1985). FIbrovascular proliferation may lead to retinal detachment and eventually, phthisis bulbi. Retinopathy can categorized into stages, with Stage 1 consisting of retinal pigment epithelium (RPE) changes only, Stage 2: Retinal vascular abnormalities without neovascularization, Stage 3: Neovascularization or retinal exudation, Stage 4 retinal detachment (RD) without endstage changes, subtype 4a is partial RD and subtype 4b is total RD, Stage 5 is Endstage with severe complications such as phthisis bulbi or neovascular glaucoma. Additional associated findings include strabismus, cataracts, secondary glaucoma, optic atrophy, severe myopia, retinal pigmentary abnormalities, and microphthalmia (Ehrenreich, Tarlow, Godlewska-Janusz, & Schwartz, 2007; Fusco, Fimiani, Tadini, Michele, & Ursini, 2007; Meuwissen & Mancini, 2012; Minić, Novotny, Trpinac, & Obradović, 2006; Scheuerle & Ursini, 1993-2020). Ophthalmic manifestations in somatic mosaic surviving males are known to include cataracts, retinal detachment, retinal neovascularization, and abnormal iris pigmentation (Fusco et al., 2007). The clinical presentation of somatic mosaic IP surviving males is similar to female patients with IP, therefore screening for ocular manifestations in somatic mosaic IP surviving males should mirror screening in females with IP. Screening should begin in the neonatal period with fluorescein angiography to detect subtle peripheral vascular changes (Swinney, Han, & Karth, 2015).
3.4 Specific genes involved with ocular development
3.4.1 Paired box 6 gene (PAX6)
Mutations in PAX6 are involved in several developmental eye disorders, with considerable phenotypic variability ranging from isolated findings in the anterior or posterior segment to panocular involvement. Aniridia is the classic PAX6-related phenotype and is a panocular disorder characterized by absent or hypoplastic irides (aniridia), limbal stem cell deficiency and corneal pannus, trabeculodysgenesis with early-onset glaucoma, developmental and adult-onset angle closure glaucoma due iris mal-rotation and aqueous outflow obstruction, birth/developmental or pre-senile cataracts, optic nerve hypoplasia, foveal hypoplasia (Hingorani, Williamson, Moore, & van Heyningen, 2009; Moosajee, Hingorani, & Moore, 1993). Nystagmus is commonly observed secondary to visual deprivation from foveal hypoplasia and occasionally concomitant optic nerve hypoplasia. PAX6 is also associated with isolated anterior segment anomalies such as anterior segment dysgenesis or Peters' anomaly (Hanson et al., 1994). Glaucoma is the major cause of progressive vision loss in patients with aniridia or those with isolated anterior segment dysgenesis, with a 50% lifetime risk (Moosajee et al., 1993). As an example of isolated posterior segment PAX6-related anomalies, foveal hypoplasia can also occur in isolation with resultant nystagmus (Azuma, Nishina, Yanagisawa, Okuyama, & Yamada, 1996). In addition, PAX6 also may be causal for MAC (microphthalmia, anophthalmia, coloboma) spectrum disorders along with other transcription factors, SOX2, OTX2, and RX (Williamson et al., 2020; Williamson & FitzPatrick, 2014).
The biology of the PAX6 gene itself explains some of the phenotypic heterogeneity. The PAX6 gene encodes a highly conserved homeobox gene and is regarded as the “Master” switch for eye development. The PAX6 gene includes two DNA-binding domains (bipartite paired domain and homeodomain), which can independently and cooperatively bind target molecules and the Proline-serine–threonine-rich C terminal transactivation domain (Hall, Williamson, & FitzPatrick, 2019). In addition, alternative splicing results in the canonical PAX6 and PAX6 (5a) isoform (Manuel, Mi, Mason, & Price, 2015). The wide range of target specificity is afforded by the two DNA binding domains, alternative splicing, cooperative binding to other transcriptional factors such as SOX2, WNT signaling molecules, SIX3 and FOXC1 (Cvekl, Sax, Bresnick, & Piatigorsky, 1994; Wang, Shan, & Gregory-Evans, 2017). Therefore, the diverse ocular pathology depends on location and type of mutation. Gene-disruptive variants such as nonsense mutations, frameshift deletions/insertions/duplications and splice-site mutations are the most common and often lead to a panocular phenotype (Hingorani, Hanson, & van Heyningen, 2012; Lima Cunha, Arno, Corton, & Moosajee, 2019). With large deletions, syndromic phenotypes result such as WAGR±O (Wilms tumor, Aniridia, Genitourinary anomalies, Intellectual disability, and Obesity) with deletions involving 11p13 The ophthalmology and genetics team much be vigilant for screening to Wilms tumor when the WT1 gene is deleted or the genetic mutation or deletion is unknown. Missense mutations in PAX6 tend to cause aniridia or non-aniridic phenotypes.
Interestingly, even within families with the autosomal dominant PAX6-related aniridia, variable expressivity is a prominent feature. Tissue mosaicism has been hypothesized to contribute to some of this familial variability. Recently, Tarilonte and colleagues describe three families with variable inter-generational ocular phenotypes including iris coloboma, microphthalmia, and aniridia (Tarilonte et al., 2018). The mutations were found to be gonasomal in the affected father, with mutant allele fraction of 12–29% depending on cell-type, rather than the expected 50%. Thus, tissue-specific mosaicism likely contributes to the ocular manifestations of disease. This also has implications for cases of seemingly “sporadic” aniridia, as parental mosaicism should be explored to determine recurrence risk in future offspring (Tarilonte et al., 2018). Gonadal chimerism has also been reported as a mechanism for disease. The phenomenon of mosaicism for developmental eye genes if further being exploited in developmental models using CRISPR technology to knock out PAX6 and other transcription factors in a tissue-specific manner (Ohuchi, Sato, Habuta, Fujita, & Bando, 2019). This will likely provide further insight into the pathogenesis of ocular development and malformations.
4 DISCUSSION
In this manuscript, we describe an interesting patient with mosaic Coffin Siris Syndrome. The molecular cause of Coffin Siris Syndrome in the order of frequency includes ARID1B (OMIM 135900) (37%), SMARCA4 (OMIM 614609) (7%), SMARCB1 (OMIM 614608) (7%), ARID1A (OMIM 614607) (5%), PHF6 (OMIM 301900) (5%), SMARCE1 (OMIM 616938) (2%), SOX11 (OMIM 615866) (2%), SMARCA2 (OMIM 601358). In about 40%, the genetic cause is unknown (Santen et al., 2013; Tsurusaki et al., 2012; Tsurusaki et al., 2014; Wieczorek et al., 2013).
While strabismus is a nonspecific ophthalmic finding, it is reported in up to 50% of patients with Coffin-Siris syndrome (Pallotta, 1985; Vergano, van der Sluijs, & Santen, 1993). Other ocular findings associated with Coffin-Siris syndrome include myopia and ptosis, both of which were not present in our patient. Our patient, in contrast, was moderately hypermetropic. Cortical visual impairment has not previously been reported in association with this condition. Because symptoms may be mild or vague and visual acuity can be normal as in our patient, the diagnosis requires a high level of suspicion and referral to an occupational therapist for a comprehensive evaluation. The parents noted poor visual attention span, poor visual memory, and problems with visuo-spatial integration. The diagnosis is important to make as specific interventions can promote both visual development and support academic performance.
Somatic mosaicism may occur in single gene syndromic disorders as in the present case of mosaic Coffin-Siris syndrome, chromosomal disorders, and specific developmental genes involved in organogenesis of a specific end-organ, or organs. In addition, mosaicism in the setting of dominant, X-linked disorders allows survival in males. The review provides important information on the spectrum of gonosomal or somatic mosaicism of selected disorders with ophthalmic manifestations. A multi-disciplinary approach to patients with complex disorders may help to characterize phenotype. Importantly, even if mosaicism is present, medical screening and follow-up should proceed as in nonmosaic patients.
The eye offers a unique window into gonosomal and somatic mosaic disorders. The ophthalmic exam may become especially important in mosaic disorders where patients do not meet all of the systemic diagnostic criteria. Key ocular findings such as unilateral or bilateral Lisch nodules may help to support a diagnosis of mosaic NF-1. In addition, the ocular tissue is derived from all three germ layers, thus the eye is subject to a host of epithelial, neurologic, vascular, connective tissue, inflammatory, and other disorders specific to each tissue subtype. Further research involving molecular methods such as CRISPR combined with advanced imaging such as OCT and confocal microscopy may further the understanding of the structure and functional changes of ocular development and malformations.
DISCLOSURE OF INTERESTS
None related to this manuscript; Dr. Miraldi Utz's affiliation, Cincinnati Children's Hospital Medical Center, has received research grants from Retrophin.
Open Research
DATA AVAILABILITY STATEMENT
Data sharing is not applicable to this article as no new data were created or analyzed in this study.