A diagnostic approach to syndromic retinal dystrophies with intellectual disability
Ian M. MacDonald and A. Micheil Innes shared equally to the senior and corresponding authorship.
Abstract
Inherited retinal dystrophies are a group of monogenic disorders that, as a whole, contribute significantly to the burden of ocular disease in both pediatric and adult patients. In their syndromic forms, retinal dystrophies can be observed in association with intellectual disability, frequently alongside other systemic manifestations. There are now over 80 genes implicated in syndromic retinal dystrophies with intellectual disability. Identifying and accurately characterizing these disorders allows the clinician to narrow the differential diagnosis, evaluate for relevant associated features, arrive at a timely and accurate diagnosis, and address both sight-threatening ocular manifestations and morbidity-causing systemic manifestations. The co-occurrence of retinal dystrophy and intellectual disability in an individual can be challenging to investigate, diagnose, and counsel given the considerable phenotypic and genotypic heterogeneity that exists within this broad group of disorders. We performed a review of the current literature and propose an algorithm to facilitate the evaluation, and clinical and mechanistic classification, of these individuals.
1 INTRODUCTION
Inherited retinal dystrophies (IRDs) comprise a group of clinically diverse ocular disorders with varied molecular causes. These disorders are relatively common, with an incidence of approximately 1/2,000 (Rattner, Sun, & Nathans, 1999). Intellectual disability (ID) is another, more frequent presentation, seen daily in the genetics clinic. While both may be independently common, IRD and ID also co-occur in select classes of disorders, with classic examples being the ciliopathies and inborn errors of metabolism (Werdich, Place, & Pierce, 2014). The advent of next-generation sequencing has spurred the discovery of many additional disorders that combine retinal dystrophy and ID (IRD-ID), emphasizing the genetic heterogeneity underlying this clinical presentation and adding to the diagnostic complexity of these patients.
IRDs commonly involve dysfunction at the level of the outer retina. Abnormalities of the choroid, retinal pigment epithelium (RPE), or cone and rod photoreceptors may exist together or in isolation as part of the retinal dystrophy spectrum (Berger, Kloeckener-Gruissem, & Neidhardt, 2010). Individuals with syndromic IRD-ID disorders often present with additional systemic manifestations; for example, in Joubert syndrome, Bardet–Biedl syndrome, and Cohen syndrome. Evaluating patients for these additional extraocular features prove useful in narrowing the differential, and guides further diagnostic workup.
Recognizing the complexity of this clinical presentation, we performed a literature and database review, using Medline and Scopus, of the syndromes associated with IRD-ID, expanding the list of causative genes to over 80 (Table 1). This number rises yet higher when the inborn errors of metabolism are considered. We present an approach to the diagnostic evaluation, and clinical and mechanistic classification, of such individuals.
| Gene | Disorder | MIM# | Inheritance | Ocular phenotype | Systemic features | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Microcephaly (refer to Table 2) | Neurologic or structural brain (refer to Table 3) | Obesity (refer to Table 4) | Renal (refer to Table 5) | Short stature or skeletal (refer to Table 6) | Endocrine (refer to Table 7) | Hearing (refer to Table 8) | Hematologic (refer to Table 9) | |||||
| ACBD5 | AR | CRD, RCD, nystagmus, ptosis | + | + | ||||||||
| ACO2 | Infantile cerebellar retinal degeneration | 614559 | AR | IRD, OA, nystagmus, strabismus | + | + | + | |||||
| ADIPOR1 | AR | RP, myopia | + | |||||||||
| AHI1 | Joubert syndrome | 608629 | AR | RP, LCA, OMA, strabismus, nystagmus, ptosis, chorioretinal coloboma, OA | + | + | + | + | ||||
| ALMS1 | Alström syndrome | 203800 | AR | CRD, nystagmus | + | + | + | + | + | |||
| ARL13B | Joubert syndrome | 612291 | AR | RP, LCA, OMA, strabismus, nystagmus, ptosis, chorioretinal coloboma, OA | + | + | + | + | ||||
| ARL3 | Joubert syndrome | 618161 | AR | RP, LCA, OMA, strabismus, nystagmus, ptosis, chorioretinal coloboma, OA | + | + | + | + | ||||
| ARL6 | Bardet Biedl syndrome | 600151 | AR | RCD, maculopathy, OA | + | + | + | + | + | + | ||
| ARMC9 | Joubert syndrome | 617622 | AR | RP, LCA, OMA, strabismus, nystagmus, ptosis, chorioretinal coloboma, OA | + | + | + | + | ||||
| B9D1 | Joubert syndrome | 617120 | AR | RP, LCA, OMA, strabismus, nystagmus, ptosis, chorioretinal coloboma, OA | + | + | + | + | ||||
| B9D2 | Joubert syndrome | 614175 | AR | RP, LCA, OMA, strabismus, nystagmus, ptosis, chorioretinal coloboma, OA | + | + | + | + | ||||
| BBIP1 | Bardet Biedl syndrome | 615995 | AR | RCD, maculopathy, OA | + | + | + | + | + | + | ||
| BBS1 | Bardet Biedl syndrome | 209900 | AR | RCD, maculopathy, OA | + | + | + | + | + | + | ||
| BBS10 | Bardet Biedl syndrome | 615987 | AR | RCD, maculopathy, OA | + | + | + | + | + | + | ||
| BBS12 | Bardet Biedl syndrome | 615989 | AR | RCD, maculopathy, OA | + | + | + | + | + | + | ||
| BBS2 | Bardet Biedl syndrome | 615981 | AR | RCD, maculopathy, OA | + | + | + | + | + | + | ||
| BBS4 | Bardet Biedl syndrome | 615982 | AR | RCD, maculopathy, OA | + | + | + | + | + | + | ||
| BBS5 | Bardet Biedl syndrome | 615983 | AR | RCD, maculopathy, OA | + | + | + | + | + | + | ||
| BBS7 | Bardet Biedl syndrome | 615984 | AR | RCD, maculopathy, OA | + | + | + | + | + | + | ||
| BBS9 | Bardet Biedl syndrome | 615986 | AR | RCD, maculopathy, OA | + | + | + | + | + | + | ||
| C2CD3 | Joubert syndrome | 615948 | AR | RP, LCA, OMA, strabismus, nystagmus, ptosis, chorioretinal coloboma, OA | + | + | + | + | ||||
| C8orf37 | Bardet Biedl syndrome | 617406 | AR | RCD, maculopathy, OA | + | + | + | + | + | + | ||
| CC2D2A | Joubert syndrome | 612285 | AR | RP, LCA, OMA, strabismus, nystagmus, ptosis, chorioretinal coloboma, OA | + | + | + | + | ||||
| CEP104 | Joubert syndrome | 616781 | AR | RP, LCA, OMA, strabismus, nystagmus, ptosis, chorioretinal coloboma, OA | + | + | + | + | ||||
| CEP120 | Joubert syndrome | 617761 | AR | RP, LCA, OMA, strabismus, nystagmus, ptosis, chorioretinal coloboma, OA | + | + | + | + | ||||
| CEP164 | Nephronophthisis 15 | 614845 | AR | IRD, LCA | + | + | + | |||||
| CEP290 | Bardet Biedl syndrome | 615991 | AR | RCD, maculopathy, OA | + | + | + | + | + | + | ||
| Joubert syndrome | 610188 | AR | RP, LCA, OMA, strabismus, nystagmus, ptosis, chorioretinal coloboma, OA | + | + | + | + | |||||
| CEP41 | Joubert syndrome | 614464 | AR | RP, LCA, OMA, strabismus, nystagmus, ptosis, chorioretinal coloboma, OA | + | + | + | + | ||||
| CPLANE1 | Joubert syndrome | 614615 | AR | RP, LCA, OMA, strabismus, nystagmus, ptosis, chorioretinal coloboma, OA | + | + | + | + | ||||
| CSPP1 | Joubert syndrome | 615636 | AR | RP, LCA, OMA, strabismus, nystagmus, ptosis, chorioretinal coloboma, OA | + | + | + | + | ||||
| CWC27 | RP with or without skeletal anomalies | 250410 | AR | RP, LCA | + | |||||||
| EXOSC2 | Short stature, hearing loss, RP, and distinctive facies | 617763 | AR | RP, myopia, corneal dystrophy, glaucoma | + | + | + | |||||
| FAM149B1 | Joubert syndrome | 618763 | AR | RP, LCA, OMA, strabismus, nystagmus, ptosis, chorioretinal coloboma, OA | + | + | + | + | ||||
| FLVCR1 | Posterior column ataxia with RP | 609033 | AR | RP, bull's eye maculopathy | + | |||||||
| HYLS1 | Joubert syndrome | AR | RP, LCA, OMA, strabismus, nystagmus, ptosis, chorioretinal coloboma, OA | + | + | + | + | |||||
| IFT140 | Mainzer–Saldino syndrome/short-rib thoracic dysplasia 9 with or without polydactyly | 614620 | AR | LCA, IRD | + | + | + | + | ||||
| IFT172 | Bardet Biedl syndrome | AR | RCD, maculopathy, OA | + | + | + | + | + | + | |||
| Joubert syndrome | AR | RP, LCA, OMA, strabismus, nystagmus, ptosis, chorioretinal coloboma, OA | + | + | + | + | ||||||
| IFT27 | Bardet Biedl syndrome | 615996 | AR | RCD, maculopathy, OA | + | + | + | + | + | + | ||
| IFT81 | AR | RCD | + | + | + | |||||||
| INPP5E | Joubert syndrome | 213300 | AR | RP, LCA, OMA, strabismus, nystagmus, ptosis, chorioretinal coloboma, OA | + | + | + | + | ||||
| KIAA0556 | Joubert syndrome | 616784 | AR | RP, LCA, OMA, strabismus, nystagmus, ptosis, chorioretinal coloboma, OA | + | + | + | + | ||||
| KIAA0586 | Joubert syndrome | 616490 | AR | RP, LCA, OMA, strabismus, nystagmus, ptosis, chorioretinal coloboma, OA | + | + | + | + | ||||
| KIAA0753 | Joubert syndrome | AR | RP, LCA, OMA, strabismus, nystagmus, ptosis, chorioretinal coloboma, OA | + | + | + | + | |||||
| KIF7 | Joubert syndrome | 200990 | AR | RP, LCA, OMA, strabismus, nystagmus, ptosis, chorioretinal coloboma, OA | + | + | + | + | ||||
| KIF11 | Microcephaly with or without chorioretinopathy, lymphedema, or mental retardation | 152950 | AD | Chorioretinopathy, retinal detachment, myopia | + | |||||||
| LAMA1 | Poretti–Boltshauser syndrome | 615960 | AR | IRD, high myopia, eye movement abnormalities | + | |||||||
| LZTFL1 | Bardet–Biedl syndrome | 615994 | AR | RCD, maculopathy, OA | + | + | + | + | + | + | ||
| MKKS | Bardet–Biedl syndrome | 605231 | AR | RCD, maculopathy, OA | + | + | + | + | + | + | ||
| MKS1 | Bardet–Biedl syndrome | 615990 | AR | RCD, maculopathy, OA | + | + | + | + | + | + | ||
| Joubert syndrome | 617121 | AR | RP, LCA, OMA, strabismus, nystagmus, ptosis, chorioretinal coloboma, OA | + | + | + | + | |||||
| NPHP1 | Joubert syndrome | 609583 | AR | RP, LCA, OMA, strabismus, nystagmus, ptosis, chorioretinal coloboma, OA | + | + | + | + | ||||
| OFD1 | Joubert syndrome | 300804 | AR | RP, LCA, OMA, strabismus, nystagmus, ptosis, chorioretinal coloboma, OA | + | + | + | + | ||||
| PDE6D | Joubert syndrome | 615665 | AR | RP, LCA, OMA, strabismus, nystagmus, ptosis, chorioretinal coloboma, OA | + | + | + | + | ||||
| PIBF1 | Joubert syndrome | 617767 | AR | RP, LCA, OMA, strabismus, nystagmus, ptosis, chorioretinal coloboma, OA | + | + | + | + | ||||
| PLK4 | Microcephaly and chorioretinopathy, autosomal recessive, 2 | 616171 | AR | Chorioretinopathy, microphthalmia | + | + | ||||||
| PNPLA6 | Boucher-Neuhauser syndrome/Oliver–McFarlane syndrome/Laurence–Moon syndrome | 215470/275400/245800 | AR | Chorioretinopathy | + | + | + | |||||
| POC5 | AR | RP | + | + | + | |||||||
| PRPS1 | Arts syndrome | 301835 | XL | IRD, LCA, OA | + | + | + | + | ||||
| RCBTB1 | Retinal dystrophy with or without extraocular anomalies | 617175 | AR | RP, reticular dystrophy, chorioretinopathy | + | + | ||||||
| RDH11 | Retinal dystrophy, juvenile cataracts, and short stature syndrome | 616108 | AR | RP, juvenile cataracts | + | |||||||
| RPGRIP1L | Joubert syndrome | 611560 | AR | RP, LCA, OMA, strabismus, nystagmus, ptosis, chorioretinal coloboma, OA | + | + | + | + | ||||
| SCAPER | Intellectual developmental disorder and RP | 618195 | AR | RP | + | + | ||||||
| SDCCAG8 | Bardet Biedl syndrome | 615993 | AR | RCD, maculopathy, OA | + | + | + | + | + | + | ||
| SUFU | Joubert syndrome | 617757 | AR | RP, LCA, OMA, strabismus, nystagmus, ptosis, chorioretinal coloboma, OA | + | + | + | + | ||||
| TCTN1 | Joubert syndrome | 614173 | AR | RP, LCA, OMA, strabismus, nystagmus, ptosis, chorioretinal coloboma, OA | + | + | + | + | ||||
| TCTN2 | Joubert syndrome | 616654 | AR | RP, LCA, OMA, strabismus, nystagmus, ptosis, chorioretinal coloboma, OA | + | + | + | + | ||||
| TCTN3 | Joubert syndrome | 614815 | AR | RP, LCA, OMA, strabismus, nystagmus, ptosis, chorioretinal coloboma, OA | + | + | + | + | ||||
| TMEM107 | Joubert syndrome | 617562 | AR | RP, LCA, OMA, strabismus, nystagmus, ptosis, chorioretinal coloboma, OA | + | + | + | + | ||||
| TMEM138 | Joubert syndrome | 614465 | AR | RP, LCA, OMA, strabismus, nystagmus, ptosis, chorioretinal coloboma, OA | + | + | + | + | ||||
| TMEM216 | Joubert syndrome | 608091 | AR | RP, LCA, OMA, strabismus, nystagmus, ptosis, chorioretinal coloboma, OA | + | + | + | + | ||||
| TMEM231 | Joubert syndrome | 614970 | AR | RP, LCA, OMA, strabismus, nystagmus, ptosis, chorioretinal coloboma, OA | + | + | + | + | ||||
| TMEM237 | Joubert syndrome | 614424 | AR | RP, LCA, OMA, strabismus, nystagmus, ptosis, chorioretinal coloboma, OA | + | + | + | + | ||||
| TMEM67 | Joubert syndrome | 610688 | AR | RP, LCA, OMA, strabismus, nystagmus, ptosis, chorioretinal coloboma, OA | + | + | + | + | ||||
| TRIM32 | Bardet Biedl syndrome | 615988 | AR | RCD, maculopathy, OA | + | + | + | + | + | + | ||
| TRNT1 | Sideroblastic anemia with B-cell immunodeficiency, periodic fevers, and developmental delay | 616,084 | AR | RP | + | + | + | + | ||||
| TTC8 | Bardet Biedl syndrome | 615985 | AR | RCD, maculopathy, OA | + | + | + | + | + | + | ||
| TUB | Retinal dystrophy and obesity | 616188 | AR | RCD, myopia | + | |||||||
| TUBGCP4 | Microcephaly and chorioretinopathy, autosomal recessive, 3 | 616335 | AR | Chorioretinopathy, hyperopia, microphthalmia | + | |||||||
| TUBGCP6 | Microcephaly and chorioretinopathy, autosomal recessive, 1 | 251270 | AR | Chorioretinopathy, peripheral retinal vascular abnormalities, microphthalmia | + | + | ||||||
| VPS13B | Cohen syndrome | 216550 | AR | RP, bull's eye maculopathy, myopia, retinoschisis | + | + | + | + | + | |||
| WDPCP | Bardet Biedl syndrome | 615992 | AR | RCD, maculopathy, OA | + | + | + | + | + | + | ||
| WDR19 | Senior-Löken syndrome 8 | 616307 | AR | IRD | + | + | ||||||
- Abbreviations: AD, autosomal dominant; AR, autosomal recessive; CRD, cone-rod dystrophy; IRD, inherited retinal dystrophy; LCA, Leber congenital amaurosis; OA, optic atrophy; OMA, oculomotor apraxia; RCD, rod-cone dystrophy; RP, retinitis pigmentosa; XL, X-linked.
2 EPIDEMIOLOGY AND ASSESSMENT OF ID
ID, historically termed mental retardation, is a common neurodevelopmental diagnosis affecting just over 1% of the global population (Maulik, Mascarenhas, Mathers, Dua, & Saxena, 2011). Defined by the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-5) as deficits in intellectual and adaptive functioning with onset during the developmental period (American Psychiatric Association, 2013), ID may present as a standalone diagnosis or as a feature of a broader syndrome. A typical initial presentation in young children is global developmental delay (GDD), in which significant delays occur in at least two developmental domains (Belanger & Caron, 2018; Vissers, Gilissen, & Veltman, 2016). By Age 5, many children with GDD satisfy diagnostic criteria for ID (Belanger & Caron, 2018). ID may contribute considerable financial, social, and emotional strain to affected individuals and their families (Topper, Ober, & Das, 2011). Lifetime direct and indirect costs associated with ID have been estimated at over $1 million for an individual born in the United States in the year 2000 (Centres for Disease Control and Prevention, C, 2004).
ID can vary in its severity, ranging from mild to profound. Standardized intelligence quotient (IQ) tests such as the Wechsler Adult Intelligence Scales (WAIS) or the Wechsler Intelligence Scales for Children (WISC) are commonly used to assess ID (Ropers, 2008). A formal diagnosis of ID is typically made when full-scale IQ testing reveals a score of less than 70 (Vissers et al., 2016). Traditionally, classification of the severity of ID was based on the IQ score, though recently there has been a shift to determine severity based on the individual's daily living skills and the level of support needed to complete these activities (National Academies of Sciences, Engineering, and Medicine, 2015). Obtaining reliable developmental assessments and IQ test scores becomes somewhat more challenging when applied to individuals with comorbid sensory impairments such as IRD, when most conventional assessment tools have been designed assuming the subject is able to see and hear (Tobin & Hill, 2011). Limited or absent sensory input can have a significant impact on experiential learning and consequently adaptive functioning abilities (Meacham, Kline, Stovall, & Sands, 2016). Many cases have been labeled as “special needs,” implying ID when profound co-morbid features of IRD and hearing loss are present. There remain very few validated instruments available for the neurodevelopmental assessment of individuals with sensory impairments (de Vaan et al., 2016), though some options do exist (Meacham et al., 2016; Reynell & Zinkin, 1975; Turner & Erchul, 1987).
There are manifold causes of ID including genetic and nongenetic factors. In the workup of an individual with ID, some nongenetic etiologies may include prenatal drug exposure, perinatal complications, prematurity, infections, trauma, toxins, or psychosocial factors (Belanger & Caron, 2018; Milani, Ronzoni, & Esposito, 2015). Ophthalmic and audiological testing, as well as electroencephalography if there is suspicion of seizures, are recommended first-line investigations (Belanger & Caron, 2018). When the aforementioned etiologies seem unlikely to provide a full explanation for the ID, genetic investigations should be pursued. In the absence of a suspected clinical diagnosis, a chromosomal microarray remains the recommended first-tier genetic study for an individual with GDD or ID, yielding a diagnosis in approximately 15–20% of these cases (Ellison, Rosenfeld, & Shaffer, 2013; Manning & Hudgins, 2010; Miller et al., 2010). In consanguineous families and founder populations, a single nucleotide polymorphism (SNP) array platform will also highlight regions of homozygosity, allowing the clinician to cross-reference these regions with genes of interest when determining next steps in testing. Further investigations and molecular testing should then be guided by the specific clinical presentation and associated features.
3 CLINICAL CHARACTERIZATION OF IRDS
Classifying and diagnosing IRDs may not be trivial given the inherent heterogeneity in their presentation. There are several elements of the patient history and physical examination that may lead a clinician to suspect and subsequently diagnose an IRD (Tsang & Sharma, 2018a). Symptoms of reduced vision, night-blindness, and photophobia may be elicited from history, although these may not be as readily apparent if the patient is young and the parent is unaware of problems with vision. Nevertheless, eye rubbing (oculodigital sign), nystagmus, and an inability to fix and follow a test object are all signs suggesting that a preverbal child has a significant retinopathy (Tsang & Sharma, 2018b).
In conjunction with the history and physical examination, clinicians can utilize fundoscopy and optical coherence tomography (OCT) imaging to support a diagnosis of IRD. While the phenotype can be quite variable on fundus examination, characteristic features of retinal dystrophies exist. These can include attenuation of the retinal pigment epithelium (RPE), a monolayer of pigmented cells in the outer retina, and subsequent redistribution of pigment in the inner retina (often in a “bone spicule” configuration). Furthermore, varying degrees of optic nerve pallor, retinal arteriolar attenuation, and outer retinal atrophy can be seen. An OCT image allows for the retina to be viewed in a cross-section with micrometer resolution, enabling detection of the degeneration of cells within the inner and outer retina.
A full-field electroretinogram (ffERG) performed using the International Society for Clinical Electrophysiology of Vision (www.ISCEV.org) protocol is likely the most useful test to objectively confirm dysfunction of the inner or outer retina. This test involves measuring electrical responses generated by the retina in both light- and dark-adapted conditions when the patient is exposed to flashes of light of varying intensities. The pattern of responses elicited can allow the clinician to further classify a retinal disorder as primarily involving the rod- or cone-photoreceptors or representing a more generalized retinal dystrophy (McCulloch et al., 2015; Tsang & Sharma, 2018a). The ffERG, fundus examination, and OCT images from a patient with retinitis pigmentosa are provided to illustrate typical findings of an IRD (Figure 1).
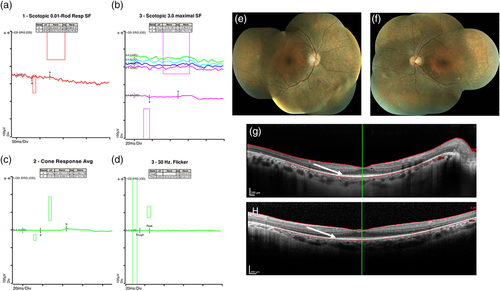
Importantly, patients with structural brain abnormalities or neurodegenerative disease may have cortical vision loss due to damaged central visual pathways. Since IRDs can present with an unremarkable fundus appearance, especially earlier in the disease course, it may be challenging to exclude an underlying retinopathy. In these cases, the ffERG becomes invaluable in facilitating this differentiation where relatively normal responses would be expected in patients with vision loss due to a structural brain abnormality in the absence of a retinopathy. Although the use of skin electrodes and newer handheld devices may allow for the ffERG to be conducted in an office setting, general anesthesia may still be required for many of these individuals.
There are several different terms used in the literature to describe specific patterns of retinal degeneration in IRDs (Nash, Wright, Grigg, Bennetts, & Jamieson, 2015). Patients with cone dystrophies, involving degeneration of cone photoreceptors, often present with impaired visual acuity, photophobia, impaired color vision, and a central visual field defect. Rod-cone dystrophies involve degeneration of the rod photoreceptors with secondary degeneration of the cone photoreceptors; retinitis pigmentosa represents the prototypical disorder in this category. Patients with rod-cone dystrophies present initially with night-blindness and a reduced peripheral visual field before manifesting signs of cone dysfunction. In contrast, patients with cone-rod dystrophies present with progressive cone degeneration followed by rod degeneration.
Generalized retinal dystrophies are characterized by abnormal function and degeneration of structures in the outer retina including the photoreceptors and the RPE (Nash et al., 2015). Chorioretinal dystrophy is a less specific term describing degeneration of the outer retina coupled with thinning and atrophy of the choriocapillaris and larger choroidal vessels underlying the RPE. These various types of retinal dystrophies can be distinguished primarily based on ffERG and OCT testing and each can belong to syndromic disorders that include ID.
4 METHODS
We performed a literature search using MedLine (PubMed) and Scopus databases. Relevant MeSH terms used included retinal dystrophy, retinitis pigmentosa, retinal degeneration, intellectual disability, and cognitive impairment. The search was limited to studies published in the English language, and no restrictions were applied to the year of publication. Additional studies were retrieved using the PubMed “similar articles” option, as well as by reviewing reference lists of select articles. We supplemented our literature search by reviewing RetNet™ (https://sph.uth.edu/retnet), the Retinal Information Network (RetNet, n.d.; accessed February 24, 2020), and their catalog of genes associated with inherited retinal diseases.
4.1 IRD-ID diagnostic algorithm
We present an algorithm to aid clinicians in evaluating and classifying individuals presenting with both an IRD and ID (Figure 2). When assessing a patient with IRD-ID, the presence or absence of additional systemic features such as microcephaly, neurologic abnormalities, renal disease, or skeletal differences will help the clinician narrow the differential to a group of phenotypically related disorders. In doing so, the clinician may focus on elements of the history, physical examination, and investigations that may be most relevant in confirming or excluding a diagnosis. Furthermore, adopting a systematic approach to a patient with IRD-ID may allow for more targeted, gene-specific, or panel testing. This strategy reduces the likelihood of incidental findings, is less expensive, and provides a higher coverage (number of sequence reads) compared to broader, hypothesis-independent testing such as exome or genome sequencing (Sun et al., 2015). On the other hand, it is recognized that for some patients or in some jurisdictions, broader testing such as exome sequencing may be more appropriate as an early investigation, in which case this framework may also help the laboratory and clinician interpret patient variants.
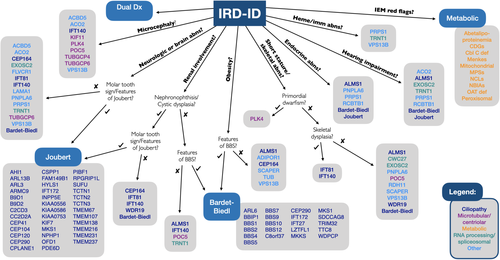
The following sections will highlight the various components of this diagnostic algorithm and present an overview of the diverse spectrum of genetic syndromes currently associated with IRD-ID. The rapid pace of gene discovery in recent years has led to several emerging candidate genes for IRD-ID disorders being described. We have included these genes for consideration as well, indicating where this is the case. Additionally, we have included a small number of syndromic IRD disorders in which ID is not a classic feature but may occasionally occur; for example, Alström syndrome.
4.2 IRD-ID syndromes with microcephaly
For an individual with IRD-ID and microcephaly, a handful of diagnoses may be considered. A classic recognizable disorder, Cohen syndrome, fits this clinical presentation. Additionally, several microtubular and centriolar disorders appear within this group. Microtubules and centrioles play important roles in cell proliferation, migration, and polarity (Kuijpers & Hoogenraad, 2011; Nigg & Raff, 2009). Impairment in their formation, structure, or function leads to abnormalities in brain development including microcephaly (Naveed et al., 2018). Microtubules and centrioles also form the building blocks of cilia (Nigg & Raff, 2009). Retinal photoreceptor cells have modified primary cilia which are important in facilitating protein trafficking between the inner and outer segments (Wheway, Parry, & Johnson, 2014).
In differentiating between the disorders in this group, consider the onset, trajectory, and severity of the microcephaly. The microcephaly seen in Cohen syndrome has its onset postnatally and is variable in severity (H. Wang et al., 1993). The same applies to ACBD5- and ACO2-related disorders, both of which are characterized by progressive, neurodegenerative clinical courses (Ferdinandusse et al., 2017; Metodiev et al., 2014). On the other hand, the microtubular and centriolar disorders within this category tend to manifest with primary or congenital microcephaly which can often be quite pronounced (Naveed et al., 2018). Finally, it is important to distinguish between absolute and relative microcephaly, an example of the latter being PLK4-related disorder in which microcephaly is present in the context of primordial dwarfism (Martin et al., 2014). Table 2 outlines the features of the IRD-ID syndromes associated with microcephaly.
| Gene (disorder), MIM | Ocular phenotype | Head size | Other systemic features | Protein function | Inheritance | Selected references |
|---|---|---|---|---|---|---|
| ACBD5 (candidate/emerging IRD-ID gene: two reports) | CRD, RCD, nystagmus, ptosis | Microcephaly (postnatal onset; progressive) | DD, white matter disease (progressive), spastic paraparesis, ataxia, proximal weakness, cleft palate | Very long-chain fatty acid CoA receptor | AR | Abu-Safieh et al. (2013), Ferdinandusse et al. (2017) |
| ACO2 (infantile cerebellar retinal degeneration) MIM: 614559 | IRD, OA, nystagmus, strabismus (Can also cause nonsyndromic OA [Metodiev et al., 2014]) | Microcephaly (postnatal onset; low-normal to −5 SD) | ID (profound), FTT, central hypotonia (severe), ataxia, athetosis, seizures, diminished DTRs (progressive), cerebral and/or cerebellar degeneration (progressive), hearing loss (sensorineural; two patients) | Tricarboxylic acid cycle enzyme | AR | Metodiev et al. (2014), Spiegel et al. (2012) |
| IFT140 (Mainzer-Saldino syndrome/short-rib thoracic dysplasia 9 with or without polydactyly) MIM: 614620 | LCA, IRD (Can also cause nonsyndromic RP or LCA; Xu et al., 2015) | Microcephaly (normal to −4 SD) | ID (normal to mild), small thorax with short ribs, phalangeal cone-shaped epiphyses, renal cysts, renal failure, hepatic disease, cerebellar ataxia | Retrograde ciliary transport | AR | Perrault et al. (2012), Schmidts et al. (2013) |
| KIF11 (microcephaly with or without chorioretinopathy, lymphedema, or mental retardation) MIM: 152950 | Chorioretinopathy, retinal detachment, myopia (Can also cause nonsyndromic FEVR; Hu et al., 2016) | Microcephaly (−1 to −7 SD) | ID (normal to moderate–severe), simplified gyration, lymphedema (congenital, bilateral, lower limbs, may resolve) | Microtubule motor | AD | Flitcroft et al. (2018), Mirzaa et al. (2014), Ostergaard et al. (2012) |
| PLK4 (microcephaly and chorioretinopathy, autosomal recessive, 2) MIM: 616171 | Chorioretinopathy, microphthalmia | Microcephaly (congenital; severe; −7 to −15 SD) | ID, primordial dwarfism (prenatal onset; height −4 to −8 SD) | Centriole biogenesis | AR | Martin et al. (2014) |
| POC5 (candidate/emerging IRD-ID gene: 1 report) | RP | Microcephaly (−3.8 SD) | SGA, short stature (−3 SD), precocious puberty, recurrent glomerulonephritis, recurrent muscle cramping (with elevated CK). Single case, the presence or degree of ID unclear (presence/degree of ID unclear) | Centriole maturation and elongation | AR | Weisz Hubshman et al. (2018) |
| TUBGCP4 (microcephaly and chorioretinopathy, autosomal recessive, 3) MIM: 616335 | Chorioretinopathy, hyperopia, microphthalmia | Microcephaly (congenital; −3 to −4 SD) | ID (normal to mild) | Microtubule nucleation | AR | Scheidecker et al. (2015) |
| TUBGCP6 (microcephaly and chorioretinopathy, autosomal recessive, 1) MIM: 251270 | Chorioretinopathy, peripheral retinal vascular abnormalities, microphthalmia | Microcephaly (congenital; severe; −7 to −11 SD) | ID, seizures, pachygyria | Microtubule nucleation | AR | Hull et al. (2019), Puffenberger et al. (2012) |
| VPS13B (Cohen syndrome) MIM: 216550 | RP, bull's eye maculopathy, myopia, retinoschisis | Microcephaly (postnatal onset; variable severity) | ID, short stature, small/narrow hands and feet, joint laxity, hypotonia, FTT followed by obesity (truncal), neutropenia, characteristic facial features, friendly disposition | Post-Golgi protein sorting, transport, modification; regulation of adipogenesis | AR | Nasser et al. (2019), H. Wang, Falk, Wensel, and Traboulsi (1993) |
- Abbreviations: AD, autosomal dominant; AR, autosomal recessive; CK, creatine kinase; CRD, cone-rod dystrophy; DD, developmental delay; DTRs, deep tendon reflexes; FTT, failure to thrive; ID, intellectual disability; IRD, inherited retinal dystrophy; LCA, Leber congenital amaurosis; OA, optic atrophy; RCD, rod-cone dystrophy; RP, retinitis pigmentosa; SGA, small for gestational age.
4.3 IRD-ID syndromes with neurologic or structural brain abnormalities
For an individual with IRD-ID and associated neurologic or structural brain anomalies, one neuroradiologic finding is particularly important. The molar tooth sign, formed by the combination of a deep interpeduncular fossa, thickened and horizontal superior cerebellar peduncles, and cerebellar vermis hypoplasia, is characteristic of Joubert syndrome, a prototypic ciliopathy (Maria, 1999). Over 35 genes have now been associated with Joubert syndrome, though some cases are more likely to present with IRD-ID than others (Bachmann-Gagescu et al., 2020). In the absence of a molar tooth sign, there are many additional disorders on the differential for a patient presenting with IRD-ID and associated neurologic or structural brain abnormalities (Table 3).
| Gene (disorder), MIM | Ocular phenotype | Neurologic or structural brain phenotype | Other systemic features | Protein function | Inheritance | Selected references |
|---|---|---|---|---|---|---|
| ACBD5 (candidate/emerging IRD-ID gene: 2 reports) | CRD, RCD, nystagmus, ptosis | White matter disease (progressive), spastic paraparesis, ataxia, proximal weakness | DD, microcephaly (postnatal onset; progressive) | Very long-chain fatty acid CoA receptor | AR | Abu-Safieh et al. (2013), Ferdinandusse et al. (2017) |
| ACO2 (infantile cerebellar retinal degeneration) MIM: 614559 | IRD, OA, nystagmus, strabismus (Can also cause nonsyndromic OA; Metodiev et al., 2014) | Central hypotonia (severe), ataxia, athetosis, seizures, diminished DTRs (progressive), cerebral and/or cerebellar degeneration (progressive) | ID (profound), FTT, microcephaly (postnatal onset; low-normal to −5 SD) hearing loss (sensorineural; two patients) | Tricarboxylic acid cycle enzyme | AR | Metodiev et al. (2014), Spiegel et al. (2012) |
| CEP164 (Nephronophthisis 15) (candidate/emerging IRD-ID gene: 1 report with ID) MIM: 614845 | IRD, LCA (Can also cause nonsyndromic LCA; Chaki et al., 2012) | Seizures, cerebellar vermis hypoplasia | ID (1 report), obesity, nephronophthisis, hepatic dysfunction, polydactyly, bronchiectasis | Primary cilia formation | AR | Chaki et al. (2012) |
| EXOSC2 (short stature, hearing loss, RP, and distinctive facies) MIM: 617763 | RP, myopia, corneal dystrophy, glaucoma | Cerebellar atrophy (mild) | ID (mild), distinctive facial features, hearing loss (sensorineural; progressive), sparse hair, premature aging, short stature, brachydactyly, broad thumbs | RNA processing and degradation | AR | Di Donato et al. (2016) |
| FLVCR1 (posterior column ataxia with RP) (candidate/emerging IRD-ID gene: 1 report with ID) MIM: 609033 | RP, bull's eye maculopathy (Can also cause nonsyndromic RP; Yusuf, Shanks, Clouston, & MacLaren, 2018) | Sensory ataxia (progressive, secondary to posterior column degeneration), diminished/absent DTRs, peripheral neuropathy | ID (1 report), scoliosis, achalasia, gastrointestinal dysmotility | Heme transportation | AR | Ishiura et al. (2011), Kuehlewein et al. (2019), Lee et al. (2018), Rajadhyaksha et al. (2010) |
| IFT81 (candidate/emerging IRD-ID gene: 1 report with IRD-ID) | RCD (Can also cause nonsyndromic CRD; Dharmat et al., 2017) | Cerebellar atrophy (1 report; however, patient also had biallelic PPT1 variants), poor balance, extrapyramidal, and pyramidal syndrome, stereotypies | ID, polydactyly (postaxial), nephronophthisis (wide spectrum of presentations; can also cause short-rib thoracic dysplasia). Further cases necessary to replicate findings of ID-RD. | Tubulin binding, anterograde ciliary transport | AR | Duran et al. (2016), Perrault et al. (2015) |
| IFT140 (Mainzer-Saldino syndrome/short-rib thoracic dysplasia 9 with or without polydactyly) MIM: 614620 | LCA, IRD (Can also cause nonsyndromic RP or LCA; Xu et al., 2015) | Cerebellar ataxia | ID (normal to mild), microcephaly (normal to −4 SD), small thorax with short ribs, phalangeal cone-shaped epiphyses, renal cysts, renal failure, hepatic disease | Retrograde ciliary transport | AR | Perrault et al. (2012), Schmidts et al. (2013) |
| LAMA1 (Poretti-Boltshauser syndrome) MIM: 615960 | IRD, high myopia, eye movement abnormalities | Cerebellar dysplasia with cysts, cerebellar vermis hypoplasia, other minor structural brain anomalies, ataxia | ID (variable), DD | Basement membrane component | AR | Aldinger et al. (2014), Poretti et al. (2014) |
| PNPLA6 (Boucher–Neuhauser syndrome/Oliver–McFarlane syndrome/Laurence–Moon syndrome) MIM: 215470/275400/245800 | Chorioretinopathy | Cerebellar atrophy, spinocerebellar ataxia (progressive), distal muscle wasting, spasticity (progressive), peripheral neuropathy (Can also cause isolated ataxia or spastic paraplegia; Synofzik et al., 2014) | ID (normal to mild; in some cases secondary to untreated endocrine deficits), hypogonadotropic hypogonadism, hypopituitarism (congenital), trichomegaly, short stature (wide spectrum of presentation) | De-esterification of phosphatidylcholine, membrane phospholipid trafficking, axonal integrity | AR | Hufnagel et al. (2015), Synofzik et al. (2014) |
| PRPS1 (Arts syndrome) MIM: 301835 | IRD (manifesting females), LCA (one male sibship), OA | Hypotonia, neuropathy (predominantly peripheral), ataxia, hyperreflexia, spastic quadriparesis | ID, hearing loss (sensorineural, profound), immune impairment (respiratory infections), diabetes insipidus (Wide spectrum of presentation; can also cause PRS-1 superactivity syndrome, hyperuricemia, nonsyndromic hearing loss, Charcot Marie Tooth disease 5; de Brouwer et al., 2010) | Purine and pyrimidine biosynthesis | XL | Al-Maawali et al. (2015), Almoguera et al. (2014), de Brouwer et al. (2010), Fiorentino et al. (2018) |
| TRNT1 (Sideroblastic anemia with B-cell immunodeficiency, periodic fevers, and DD) MIM: 616084 | RP, posterior subcapsular cataracts (Can also cause nonsyndromic RP; DeLuca et al., 2016) | Hypotonia, cerebral atrophy, epilepsy, ataxia | ID (variable), DD, B-cell immunodeficiency, periodic fevers, sideroblastic anemia (congenital), hearing loss (sensorineural), nephrocalcinosis | tRNA processing and maturation | AR | Chakraborty et al. (2014), S. Hull et al. (2016), Wiseman et al. (2013) |
| TUBGCP6 (microcephaly and chorioretinopathy, autosomal recessive, 1) MIM: 251270 | Chorioretinopathy, peripheral retinal vascular abnormalities, microphthalmia | Seizures, pachygyria | ID, microcephaly (congenital; severe; −7 to −11 SD) | Microtubule nucleation | AR | Hull et al. (2019), Puffenberger et al. (2012) |
| VPS13B (Cohen syndrome) MIM: 216550 | RP, bull's eye maculopathy, myopia, retinoschisis | Hypotonia | ID, short stature, microcephaly (postnatal onset; variable severity), small/narrow hands and feet, joint laxity, FTT followed by obesity (truncal), neutropenia, characteristic facial features, friendly disposition | Post-Golgi protein sorting, transport, and modification; regulation of adipogenesis | AR | Nasser et al. (2019), H. Wang et al. (1993) |
| Many genes (Bardet–Biedl syndrome; Figure 2) | RCD (93%), maculopathy, OA (several of the genes can also cause nonsyndromic retinal dystrophy) | Ataxia/poor coordination (40–86%) | ID (normal to severe), DD, obesity (infant onset; truncal and rhizomelic), polydactyly (postaxial), hypogonadism, genital anomalies, various renal anomalies, type 2 diabetes, dental anomalies, congenital heart anomalies, brachydactyly, syndactyly (partial), anosmia/hyposmia, hearing loss (conductive or sensorineural; several of the genes can also cause other ciliopathies) | Ciliary | AR | Beales, Elcioglu, Woolf, Parker, and Flinter (1999), Forsythe, Kenny, Bacchelli, and Beales (2018), Weihbrecht et al. (2017) |
| Many genes (Joubert syndrome; Figure 2) | RP or LCA (38%), OMA, strabismus, nystagmus, ptosis, chorioretinal coloboma, OA (several of the genes can also cause nonsyndromic IRD) | Molar tooth sign, other structural brain anomalies, encephalocele, hypotonia, OMA, seizures, ataxia | ID (normal to severe), DD (expressive speech disproportionately affected), respiratory dysregulation, nephronophthisis, cystic renal dysplasia, hepatic fibrosis, polydactyly (pre-, meso-, or post-axial), congenital heart anomalies, midline oro-facial defects, various endocrine abnormalities, hearing loss (sensorineural or conductive; several of the genes can also cause other ciliopathies) | Ciliary | AR; XL | Bachmann-Gagescu et al. (2020), Romani, Micalizzi, and Valente (2013), S. F. Wang et al. (2018) |
- Abbreviations: AR, autosomal recessive; CRD, cone-rod dystrophy; DD, developmental delay; DTRs, deep tendon reflexes; FTT, failure to thrive; ID, intellectual disability; IRD, inherited retinal dystrophy; LCA, Leber congenital amaurosis; OA, optic atrophy; OMA, oculomotor apraxia; RCD, rod-cone dystrophy; RP, retinitis pigmentosa; XL, X-linked.
4.4 IRD-ID syndromes with obesity
Although not uncommon in the general population, obesity in an individual with IRD-ID may suggest certain syndromes on the differential such as Bardet–Biedl syndrome. Bardet–Biedl syndrome is a genetically heterogeneous ciliopathy associated with at least 21 different genes (Forsythe et al., 2018). If Bardet–Biedl syndrome is not deemed to be a fitting diagnosis, both Alström syndrome and Cohen syndrome where childhood-onset central obesity is common may be considered as alternate possibilities in the presence of obesity (Alvarez-Satta et al., 2015; H. Wang et al., 1993). However, distinguishing features early on in their presentation help clinically separate the diagnosis of Bardet–Biedl syndrome with nyctalopia (rod-cone dystrophy) from Alström syndrome with photophobia (cone-rod dystrophy). Table 4 highlights all three of these disorders, along with a few others to consider in the individual with IRD-ID and obesity.
| Gene (disorder), MIM | Ocular phenotype | Weight | Other systemic features | Protein function | Inheritance | Selected references |
|---|---|---|---|---|---|---|
| ADIPOR1 (candidate/emerging IRD-ID gene: 1 report) | RP, myopia (Can also cause nonsyndromic RP; Zhang et al., 2016) | Obesity (truncal) | ID, DD | Glucose and lipid metabolism | AR | Xu et al. (2016) |
| ALMS1 (Alström syndrome) MIM: 203800 | CRD, nystagmus | Obesity (infant/childhood onset; truncal; associated with hyperinsulinemia) | DD (in 45%; ID uncommon but reported), hearing loss (sensorineural; progressive), insulin resistance and type 2 diabetes, hypertriglyceridemia, endocrine abnormalities (hypogonadism, hypothyroidism, female hyperandrogenism), cardiomyopathy (infant-onset dilated or later-onset restrictive), pulmonary/hepatic/renal fibrosis and dysfunction (progressive), urologic dysfunction, short stature (in adulthood) | Ciliary structure and function | AR | Alvarez-Satta, Castro-Sanchez, and Valverde (2015), Marshall, Maffei, Collin, and Naggert (2011), Nasser et al. (2018) |
| CEP164 (Nephronophthisis 15) (candidate/emerging IRD-ID gene: 1 report with ID) MIM: 614845 | IRD, LCA (Can also cause nonsyndromic LCA; Chaki et al., 2012) | Obesity | ID (1 report), nephronophthisis, hepatic dysfunction, seizures, cerebellar vermis hypoplasia, polydactyly, bronchiectasis | Primary cilia formation | AR | Chaki et al. (2012) |
| SCAPER (intellectual developmental disorder and RP) MIM: 618195 | RP (Can also cause nonsyndromic RP; Jauregui et al., 2019) | Obesity (one family) | ID (mild to severe), ADHD, short stature, brachydactyly, tapered fingers, proximally placed thumbs | Zinc finger | AR | Fasham et al. (2019), Tatour et al. (2017) |
| TUB (retinal dystrophy and obesity) MIM: 616188 (candidate/emerging IRD-ID gene: 1 report) | RCD, myopia | Obesity (early onset) | Learning difficulties (normal to mild) | G-protein coupled receptor trafficking | AR | Borman et al. (2014) |
| VPS13B (Cohen syndrome) MIM: 216550 | RP, bull's eye maculopathy, myopia, retinoschisis | Obesity (FTT evolving to obesity in teens; truncal) | ID, short stature, microcephaly (postnatal onset; variable severity), hypotonia, small/narrow hands and feet, joint laxity, neutropenia, characteristic facial features, friendly disposition | Post-Golgi protein sorting, transport, and modification; regulation of adipogenesis | AR | Nasser et al. (2019), H. Wang et al. (1993) |
| Many genes (Bardet–Biedl syndrome; Figure 2) | RCD (93%), maculopathy, OA (several of the genes can also cause nonsyndromic IRD) | Obesity (72–92%; infant onset; truncal and rhizomelic distribution) | ID (normal to severe), DD, polydactyly (postaxial), hypogonadism, genital anomalies, various renal anomalies, type 2 diabetes, dental anomalies, congenital heart anomalies, brachydactyly, syndactyly (partial), ataxia/poor coordination, anosmia/hyposmia, hearing loss (conductive or sensorineural; several of the genes can also cause other ciliopathies) | Ciliary | AR | Beales et al. (1999), Forsythe et al. (2018), Weihbrecht et al. (2017) |
- Abbreviations: ADHD, attention deficit hyperactivity disorder; AR, autosomal recessive; CRD, cone-rod dystrophy; DD, developmental delay; DTRs, deep tendon reflexes; FTT, failure to thrive; ID, intellectual disability; IRD, inherited retinal dystrophy; LCA, Leber congenital amaurosis; OA, optic atrophy; RCD, rod-cone dystrophy; RP, retinitis pigmentosa.
4.5 IRD-ID syndromes with renal involvement
Renal involvement is common in several IRD-ID syndromes. Nephronophthisis and cystic renal disease, in particular, are characteristic features shared by many ciliopathies (Gascue, Katsanis, & Badano, 2011). Symptoms of renal disease in this setting can include polyuria and polydipsia. Individuals may also develop failure to thrive, anemia, and hypertension as signs of renal dysfunction, with renal hyperechogenicity or cystic disease evident on ultrasound (Tory et al., 2009). When evaluating an individual with IRD-ID and associated nephronophthisis, Joubert syndrome should be considered as a leading possibility (Bachmann-Gagescu et al., 2020). Bardet–Biedl syndrome can present with nephronophthisis as well, though unlike Joubert syndrome, it can also be associated with numerous other structural or functional renal anomalies (Forsythe et al., 2018). Finally, both retinal and renal involvement may not necessarily be simultaneously apparent on initial presentation. For example, among the diseases associated with nephronophthisis, individuals with Senior–Löken syndrome often develop a retinopathy in childhood before manifesting overt signs of renal disease several years later (Hemachandar, 2014). Early recognition of syndromic diseases may lead to earlier medical intervention in these patients; this supports a broad, multigene approach to genetic testing to identify these cases. The features of the IRD-ID syndromes associated with renal involvement are shown in Table 5.
| Gene (disorder), MIM | Ocular phenotype | Renal phenotype | Other systemic features | Protein function | Inheritance | Selected references |
|---|---|---|---|---|---|---|
| ALMS1 (Alström syndrome) MIM: 203800 | CRD, nystagmus | Renal fibrosis and dysfunction (progressive) | DD (in 45%; ID uncommon but reported), obesity (infant/childhood onset; truncal; associated with hyperinsulinemia), hearing loss (sensorineural; progressive), insulin resistance and type 2 diabetes, hypertriglyceridemia, endocrine abnormalities (hypogonadism, hypothyroidism, female hyperandrogenism), cardiomyopathy (infant-onset dilated or later-onset restrictive), pulmonary/hepatic fibrosis and dysfunction (progressive), urologic dysfunction, short stature (in adulthood) | Ciliary structure and function | AR | Alvarez-Satta et al. (2015), Marshall et al. (2011), Nasser et al. (2018) |
| CEP164 (Nephronophthisis 15) (candidate/emerging IRD-ID gene: 1 report with ID) MIM: 614845 | IRD, LCA (Can also cause nonsyndromic LCA; Chaki et al., 2012) | Nephronophthisis | ID (1 report), hepatic dysfunction, obesity, seizures, cerebellar vermis hypoplasia, polydactyly, bronchiectasis | Primary cilia formation | AR | Chaki et al. (2012) |
| IFT81 (candidate/emerging IRD-ID gene: 1 report with IRD-ID) | RCD (Can also cause non-syndromic CRD; Dharmat et al., 2017) | Nephronophthisis | ID (mild), polydactyly (postaxial), cerebellar atrophy (1 report; however, patient also had biallelic PPT1 variants), poor balance, extrapyramidal and pyramidal syndrome, stereotypies, short ribs, rhizomelia/mesomelia/micromelia, ambiguous genitalia (wide spectrum of presentations) | Tubulin binding, anterograde ciliary transport | AR | Duran et al. (2016), Perrault et al. (2015) |
| IFT140 (Mainzer–Saldino syndrome/short-rib thoracic dysplasia 9 with or without polydactyly) MIM: 614620 | LCA, IRD (Can also cause nonsyndromic RP or LCA; Xu et al., 2015) | Renal cysts, renal failure | ID (normal to mild), microcephaly (normal to −4 SD), small thorax with short ribs, phalangeal cone-shaped epiphyses, hepatic disease, cerebellar ataxia | Retrograde ciliary transport | AR | Perrault et al. (2012), Schmidts et al. (2013) |
| POC5 (candidate/emerging IRD-ID gene: 1 report) | RP | Recurrent glomerulonephritis | SGA, short stature (−3 SD), microcephaly (−3.8 SD), early puberty (normal hormonal profile), recurrent muscle cramping (with elevated CK) (presence/degree of ID unclear) | Centriole maturation and elongation | AR | Weisz Hubshman et al. (2018) |
| TRNT1 (Sideroblastic anemia with B-cell immunodeficiency, periodic fevers, and DD) MIM: 616084 | RP, posterior subcapsular cataracts (Can also cause nonsyndromic RP; DeLuca et al., 2016) | Nephrocalcinosis | ID (variable), DD, hypotonia, cerebral atrophy, epilepsy, ataxia, B-cell immunodeficiency, periodic fevers, sideroblastic anemia (congenital), hearing loss (sensorineural) | tRNA processing and maturation | AR | Chakraborty et al. (2014), S. Hull et al. (2016), Wiseman et al. (2013) |
| WDR19 (Senior–Löken syndrome 8) MIM: 616307 | IRD (Can also cause nonsyndromic RP; Coussa et al., 2013) | Nephronophthisis, renal cysts (Can also cause isolated nephronophthisis; Bredrup et al., 2011) | ID, cystic bile duct dilatation, hip dysplasia, scoliosis, brachydactyly, polydactyly, pancreatic and hepatic cysts (Can also cause cranioectodermal dysplasia, short rib thoracic dysplasia; Bredrup et al., 2011) | Retrograde ciliary transport | AR | Coussa et al. (2013), Halbritter et al. (2013) |
| Many genes (Bardet–Biedl syndrome; Figure 2) | RCD (93%), maculopathy, OA (several of the genes can also cause nonsyndromic IRD) | Various renal anomalies (53–82%): Cystic renal dysplasia, hydronephrosis, scarred/atrophic kidneys, loss of corticomedullary function, developmental renal anomalies, functional renal impairment, end-stage renal disease | ID (normal to severe), DD, obesity (infant onset; truncal and rhizomelic), polydactyly (postaxial), hypogonadism, genital anomalies, type 2 diabetes, dental anomalies, congenital heart anomalies, brachydactyly, syndactyly (partial), ataxia/poor coordination, anosmia/hyposmia, hearing loss (conductive or sensorineural; several of the genes can also cause other ciliopathies) | Ciliary | AR | Beales et al. (1999), Forsythe et al. (2018), Weihbrecht et al. (2017) |
| Many genes (Joubert syndrome; Figure 2) | RP or LCA (38%), OMA, strabismus, nystagmus, ptosis, chorioretinal coloboma, OA (several of the genes can also cause nonsyndromic IRD) | Nephronophthisis or cystic renal dysplasia (25%) | ID (normal to severe), DD (expressive speech disproportionately affected), molar tooth sign, other structural brain anomalies, encephalocele, hypotonia, OMA, seizures, ataxia, respiratory dysregulation, hepatic fibrosis, polydactyly (pre-, meso-, or post-axial), congenital heart anomalies, midline oro-facial defects, various endocrine abnormalities, hearing loss (sensorineural or conductive; several of the genes can also cause other ciliopathies) | Ciliary | AR; XL | Bachmann-Gagescu et al. (2020), Romani et al. (2013), S. F. Wang et al. (2018) |
- Abbreviations: AR, autosomal recessive; CK, creatine kinase; CRD, cone-rod dystrophy; DD, developmental delay; ID, intellectual disability; IRD, inherited retinal dystrophy; LCA, Leber congenital amaurosis; OA, optic atrophy; OMA, oculomotor apraxia; RCD, rod-cone dystrophy; RP, retinitis pigmentosa; XL, X-linked.
4.6 IRD-ID syndromes with short stature or skeletal abnormalities
Several IRD-ID syndromes are associated with short stature as part of their syndromic presentation, whereas others present with specific skeletal anomalies (Table 6). Determining the age of onset of short stature can be helpful in differentiating some of these conditions. For example, in an individual with IRD-ID and short stature of prenatal onset, PLK4 which is associated with primordial dwarfism is a likely candidate gene (Martin et al., 2014). Patients with Alström syndrome, on the other hand, typically demonstrate normal growth in childhood but have a reduced final adult height due to deficiencies in growth hormone and insulin-like growth factor 1 (Romani et al., 2013). In an individual presenting with IRD-ID and thoracic dysplasia, IFT81 and IFT140 should be considered (Perrault et al., 2012; Perrault et al., 2015). Polydactyly can be seen in patients with pathogenic variants in these intraflagellar transport proteins, and can be an early presenting feature of several other ciliopathies including Bardet–Biedl and Joubert syndromes. Other, more subtle skeletal differences such as brachydactyly are reported in IRD-ID syndromes as well. Finally, short stature may be seen in patients with other medical comorbidities, particularly if not completely treated, such as renal (Table 5), endocrine (Table 7), or metabolic disorders.
| Gene (disorder), MIM | Ocular phenotype | Skeletal phenotype | Other systemic features | Protein function | Inheritance | Selected references |
|---|---|---|---|---|---|---|
| ALMS1 (Alström syndrome) MIM: 203800 | CRD, nystagmus | Short stature (in adulthood) | DD (in 45%; ID uncommon but reported), obesity (infant/childhood onset; truncal; associated with hyperinsulinemia), hearing loss (sensorineural; progressive), insulin resistance and type 2 diabetes, hypertriglyceridemia, endocrine abnormalities (hypogonadism, hypothyroidism, female hyperandrogenism), cardiomyopathy (infant-onset dilated or later-onset restrictive), pulmonary/hepatic/renal fibrosis and dysfunction (progressive), urologic dysfunction | Ciliary structure and function | AR | Alvarez-Satta et al. (2015), Marshall et al. (2011), Nasser et al. (2018) |
| CWC27 (RP with or without skeletal anomalies) MIM: 250410 | RP, LCA (Can also cause nonsyndromic RP or LCA; Xu et al., 2017) | Short stature (normal to −4 SD), brachydactyly (primarily of distal phalanges; may also see hypoplastic nails) | ID, distinctive facial features | Pre-mRNA splicing | AR | Xu et al. (2017) |
| EXOSC2 (short stature, hearing loss, RP, and distinctive facies) MIM: 617763 | RP, myopia, corneal dystrophy, glaucoma | Short stature, brachydactyly, broad thumbs | ID (mild), distinctive facial features, cerebellar atrophy (mild), hearing loss (sensorineural; progressive), sparse hair, premature aging | RNA processing and degradation | AR | Di Donato et al. (2016) |
| IFT81 (candidate/emerging IRD-ID gene: 1 report with IRD-ID) | RCD (Can also cause nonsyndromic CRD; Dharmat et al., 2017) | Short ribs, rhizomelia/mesomelia/micromelia | ID (mild), polydactyly (postaxial), cerebellar atrophy (1 report; however, patient also had biallelic PPT1 variants), poor balance, extrapyramidal and pyramidal syndrome, stereotypies, nephronophthisis, ambiguous genitalia (wide spectrum of presentations) | Tubulin binding, anterograde ciliary transport | AR | Duran et al. (2016), Perrault et al. (2015) |
| IFT140 (Mainzer-Saldino syndrome/short-rib thoracic dysplasia 9 with or without polydactyly) MIM: 614620 | LCA, IRD (Can also cause nonsyndromic RP or LCA; Xu et al., 2015) | Small thorax with short ribs, phalangeal cone-shaped epiphyses | ID (normal to mild), microcephaly (normal to −4 SD), renal cysts, renal failure, hepatic disease, cerebellar ataxia | Retrograde ciliary transport | AR | Perrault et al. (2012), Schmidts et al. (2013) |
| PLK4 (microcephaly and chorioretinopathy, autosomal recessive, 2) MIM: 616171 | Chorioretinopathy, microphthalmia | Primordial dwarfism (prenatal onset; height −4 to −8 SD) | ID, microcephaly (congenital; severe; −7 to −15 SD) | Centriole biogenesis | AR | Martin et al. (2014) |
| PNPLA6 (Boucher–Neuhauser syndrome/Oliver–McFarlane syndrome/Laurence–Moon syndrome) MIM: 215470/275400/245800 | Chorioretinopathy | Short stature | ID (normal to mild; in some cases secondary to untreated endocrine deficits), hypogonadotropic hypogonadism, hypopituitarism (congenital), trichomegaly, cerebellar atrophy, spinocerebellar ataxia (progressive), distal muscle wasting, spasticity (progressive), peripheral neuropathy (Wide spectrum of presentation; can also cause isolated ataxia or spastic paraplegia (Synofzik et al., 2014)) | De-esterification of phosphatidylcholine, membrane phospholipid trafficking, axonal integrity | AR | Hufnagel et al. (2015), Synofzik et al. (2014) |
| POC5 (candidate/emerging IRD-ID gene: 1 report) | RP | Short stature (−3 SD) | SGA, microcephaly (−3.8 SD), early puberty (normal hormonal profile), recurrent glomerulonephritis, recurrent muscle cramping (with elevated CK; presence/degree of ID unclear) | Centriole maturation and elongation | AR | Weisz Hubshman et al. (2018) |
| RDH11 (retinal dystrophy, juvenile cataracts, and short stature syndrome) (candidate/emerging IRD-ID gene: 1 report) MIM: 616108 | RP, juvenile cataracts | Short stature | DD, learning difficulties, distinctive facial features, wide-spaced teeth | Retinoid reduction | AR | Xie et al. (2014) |
| SCAPER (intellectual developmental disorder and RP) MIM: 618195 | RP (Can also cause nonsyndromic RP; Jauregui et al., 2019) | Short stature, brachydactyly, tapered fingers, proximally placed thumbs | ID (mild to severe), ADHD, obesity (one family) | Zinc finger | AR | Fasham et al. (2019), Tatour et al. (2017) |
| VPS13B (Cohen syndrome) MIM: 216550 | RP, bull's eye maculopathy, myopia, retinoschisis | Short stature, small/narrow hands, and feet, joint laxity | ID, microcephaly (postnatal onset; variable severity), hypotonia, FTT followed by obesity (truncal), neutropenia, characteristic facial features, friendly disposition | Post-Golgi protein sorting, transport, and modification; regulation of adipogenesis | AR | Nasser et al. (2019), H. Wang et al. (1993) |
| WDR19 (Senior–Löken syndrome 8) MIM: 616307 | IRD (Can also cause nonsyndromic RP; Coussa et al. (2013)) | Hip dysplasia, scoliosis, brachydactyly, polydactyly | ID, nephronophthisis, renal cysts, cystic bile duct dilatation, pancreatic and hepatic cysts (Can also cause cranioectodermal dysplasia, short-rib thoracic dysplasia, isolated nephronophthisis; Bredrup et al., 2011) | Retrograde ciliary transport | AR | Coussa et al. (2013), Halbritter et al. (2013) |
| Many genes (Bardet–Biedl syndrome; Figure 2) | RCD (93%), maculopathy, OA (several of the genes can also cause nonsyndromic IRD) | Brachydactyly, syndactyly (partial), polydactyly (postaxial), | ID (normal to severe), DD, obesity (infant onset; truncal and rhizomelic), hypogonadism, genital anomalies, various renal anomalies, type 2 diabetes, dental anomalies, congenital heart anomalies, ataxia/poor coordination, anosmia/hyposmia, hearing loss (conductive or sensorineural; several of the genes can also cause other ciliopathies) | Ciliary | AR | Beales et al. (1999), Forsythe et al. (2018), Weihbrecht et al. (2017) |
- Abbreviations: ADHD, attention deficit hyperactivity disorder; AR, autosomal recessive; CK, creatine kinase; CRD, cone-rod dystrophy; DD, developmental delay; FTT, failure to thrive; ID, intellectual disability; IRD, inherited retinal dystrophy; LCA, Leber congenital amaurosis; OA, optic atrophy; OMA, oculomotor apraxia; RCD, rod-cone dystrophy; RP, retinitis pigmentosa; SGA, small for gestational age.
| Gene (disorder), MIM | Ocular phenotype | Endocrinologic phenotype | Other systemic features | Protein function | Inheritance | Selected references |
|---|---|---|---|---|---|---|
| ALMS1 (Alström syndrome) MIM: 203800 | CRD, nystagmus | Insulin resistance and type 2 diabetes, obesity (infant/childhood onset; truncal; associated with hyperinsulinemia) hypertriglyceridemia, hypogonadism, hypothyroidism, female hyperandrogenism | DD (in 45%; ID uncommon but reported), hearing loss (sensorineural; progressive), cardiomyopathy (infant-onset dilated or later-onset restrictive), pulmonary/hepatic/renal fibrosis and dysfunction (progressive), urologic dysfunction, short stature (in adulthood) | Ciliary structure and function | AR | Alvarez-Satta et al. (2015), Marshall et al. (2011), Nasser et al. (2018) |
| PNPLA6 (Boucher–Neuhauser syndrome, Oliver–McFarlane syndrome, Laurence–Moon syndrome) MIM: 215470/275400/245800 | Chorioretinopathy | Hypogonadotropic hypogonadism, hypopituitarism (congenital) | ID (normal to mild; in some cases secondary to untreated endocrine deficits), trichomegaly, cerebellar atrophy, spinocerebellar ataxia (progressive), distal muscle wasting, spasticity (progressive), peripheral neuropathy, short stature (Wide spectrum of presentation; can also cause isolated ataxia or spastic paraplegia; Synofzik et al., 2014) | De-esterification of phosphatidylcholine, membrane phospholipid trafficking, axonal integrity | AR | Hufnagel et al. (2015), Synofzik et al. (2014) |
| PRPS1 (Arts syndrome) MIM: 301835 | IRD (manifesting females), LCA (one male sibship), OA | Diabetes insipidus | ID, hypotonia, neuropathy (predominantly peripheral), ataxia, hyperreflexia, spastic quadriparesis, hearing loss (sensorineural, profound), immune impairment (severe respiratory infections; Wide spectrum of presentation; can also cause PRS-1 superactivity syndrome, hyperuricemia, nonsyndromic hearing loss, Charcot Marie Tooth disease 5; de Brouwer et al., 2010) | Purine and pyrimidine biosynthesis | XL | Al-Maawali et al. (2015), Almoguera et al. (2014), de Brouwer et al. (2010), Fiorentino et al. (2018) |
| RCBTB1 (retinal dystrophy with or without extraocular anomalies) (candidate/emerging IRD-ID gene: 1 report with ID) MIM: 617175 | RP, reticular dystrophy, chorioretinopathy (Can also cause isolated IRD, Coats disease, and autosomal dominant familial exudative vitreoretinopathy; Coppieters et al., 2016; Wu et al., 2016) | Goiter, primary ovarian insufficiency, thyroid nodules | ID (mild), hearing loss (sensorineural; adult onset), lung fibrosis | Chromatin remodeling, ubiquitination | AR | Coppieters et al. (2016) |
| Many genes (Bardet–Biedl syndrome; Figure 2) | RCD (93%), maculopathy, OA (several of the genes can also cause nonsyndromic IRD) | Hypogonadism, type 2 diabetes | ID (normal to severe), DD, obesity (infant onset; truncal and rhizomelic), polydactyly (postaxial), genital anomalies, various renal anomalies, dental anomalies, congenital heart anomalies, brachydactyly, syndactyly (partial), ataxia/poor coordination, anosmia/hyposmia, hearing loss (conductive or sensorineural; several of the genes can also cause other ciliopathies) | Ciliary | AR | Beales et al. (1999), Forsythe et al. (2018), Weihbrecht et al. (2017) |
| Many genes (Joubert syndrome; Figure 2) | RP or LCA (38%), OMA, strabismus, nystagmus, ptosis, chorioretinal coloboma, OA (several of the genes can also cause nonsyndromic IRD) | Various endocrine abnormalities (4–6%): Hypothyroidism, diabetes insipidus, growth hormone deficiency, panhypopituitarism, type 1 diabetes, precocious puberty, polycystic ovarian syndrome | ID (normal to severe), DD (expressive speech disproportionately affected), molar tooth sign, other structural brain anomalies, encephalocele, hypotonia, OMA, seizures, ataxia, respiratory dysregulation, nephronophthisis, cystic renal dysplasia, hepatic fibrosis, polydactyly (pre-, meso-, or post-axial), congenital heart anomalies, midline oro-facial defects, hearing loss (sensorineural or conductive; several of the genes can also cause other ciliopathies) | Ciliary | AR; XL | Bachmann-Gagescu et al. (2020), Romani et al. (2013), S. F. Wang et al. (2018) |
- Abbreviations: AR, autosomal recessive; CRD, cone-rod dystrophy; DD, developmental delay; ID, intellectual disability; IRD, inherited retinal dystrophy; LCA, Leber congenital amaurosis; OA, optic atrophy; OMA, oculomotor apraxia; RCD, rod-cone dystrophy; RP, retinitis pigmentosa.
4.7 IRD-ID syndromes with endocrine dysfunction
There are a handful of IRD-ID syndromes in which a higher incidence of various endocrine abnormalities can be observed (Table 7). These endocrine findings may be a defining feature of certain disorders, such as Boucher–Neuhauser syndrome where hypogonadism is frequent (Synofzik et al., 2014). The allelic disorder, Oliver–McFarlane syndrome, can present as early as infancy with features of congenital hypothyroidism, which untreated leads to intellectual disability and poor growth (Hufnagel et al., 2015). Depending on the particular testing method implemented, this central hypothyroidism may or may not be detected on a newborn screen. In other circumstances, a diagnosis of other IRD-ID syndromes with associated endocrine complications should prompt screening for these associated co-morbidities. Many of these endocrine features are readily treatable and when treated may lead to considerable improvement in quality of life.
4.8 IRD-ID syndromes with hearing loss
Hearing loss, generally bilateral, is a feature of many of the IRD-ID syndromes. Depending on how these individuals initially present, the combination of retinal dystrophy and hearing loss may lead to a presumptive diagnosis of Usher syndrome (Yan & Liu, 2010). However, ID is not typically seen in Usher syndrome and should prompt consideration of the disorders in Table 8. Sensorineural hearing loss (SNHL) tends to be the type most often associated with the IRD-ID syndromes. Profound congenital SNHL, as in type 1 Usher syndrome, can be seen in patients with PRPS1-associated Arts syndrome (de Brouwer et al., 2010). Progressive SNHL is frequent in Alström syndrome (Alvarez-Satta et al., 2015), or may represent EXOSC2- or RCBTB1-related disorders (Coppieters et al., 2016; Di Donato et al., 2016). Finally, although infrequent, progressive SNHL with onset in the first or second decade of life (Akizu et al., 2014; Singh, Shrinkhal, Pradhan, & Chakraborty, 2017) and conductive hearing loss secondary to middle ear infections (Beales et al., 1999; Kroes et al., 2010) may be observed in Bardet-Biedl and Joubert syndromes.
| Gene (disorder), MIM | Ocular phenotype | Audiologic phenotype | Other systemic features | Protein function | Inheritance | Selected references |
|---|---|---|---|---|---|---|
| ACO2 (infantile cerebellar retinal degeneration) MIM: 614559 | IRD, OA, nystagmus, strabismus (Can also cause nonsyndromic OA; Metodiev et al., 2014) | Hearing loss (sensorineural; two patients) | ID (profound), FTT, microcephaly (postnatal onset; low-normal to −5 SD), central hypotonia (severe), ataxia, athetosis, seizures, diminished DTRs (progressive), cerebral, and/or cerebellar degeneration (progressive) | Tricarboxylic acid cycle enzyme | AR | Metodiev et al. (2014), Spiegel et al. (2012) |
| ALMS1 (Alström syndrome) MIM: 203800 | CRD, nystagmus | Hearing loss (sensorineural; progressive) | DD (in 45%; ID uncommon but reported), obesity (infant/childhood onset; truncal; associated with hyperinsulinemia), insulin resistance and type 2 diabetes, hypertriglyceridemia, endocrine abnormalities (hypogonadism, hypothyroidism, female hyperandrogenism), cardiomyopathy (infant-onset dilated or later-onset restrictive), pulmonary/hepatic/renal fibrosis and dysfunction (progressive), urologic dysfunction, short stature (in adulthood) | Ciliary structure and function | AR | Alvarez-Satta et al. (2015), Marshall et al. (2011), Nasser et al. (2018) |
| EXOSC2 (short stature, hearing loss, RP, and distinctive facies) MIM: 617763 | RP, myopia, corneal dystrophy, glaucoma | Hearing loss (sensorineural; progressive) | ID (mild), distinctive facial features, cerebellar atrophy (mild), sparse hair, premature aging, short stature, brachydactyly, broad thumbs | RNA processing and degradation | AR | Di Donato et al. (2016) |
| PRPS1 (Arts syndrome) MIM: 301835 | IRD (manifesting females), LCA (one male sibship), OA | Hearing loss (sensorineural, profound) | ID, hypotonia, neuropathy (predominantly peripheral), ataxia, hyperreflexia, spastic quadriparesis, immune impairment, diabetes insipidus (Wide spectrum of presentation; can also cause PRS-1 superactivity syndrome, hyperuricemia, nonsyndromic hearing loss, Charcot Marie Tooth disease 5; de Brouwer et al., 2010) | Purine and pyrimidine biosynthesis | XL | Al-Maawali et al. (2015), Almoguera et al. (2014), de Brouwer et al. (2010), Fiorentino et al. (2018) |
| RCBTB1 (retinal dystrophy with or without extraocular anomalies) (candidate/emerging IRD-ID gene: 1 report with ID) MIM: 617175 | RP, reticular dystrophy, chorioretinopathy (Can also cause isolated IRD, Coats disease, and autosomal dominant familial exudative vitreoretinopathy; Coppieters et al., 2016; Wu et al., 2016) | Hearing loss (sensorineural; adult onset) | ID (mild), goiter, primary ovarian insufficiency, thyroid nodules, lung fibrosis | Chromatin remodeling, ubiquitination | AR | Coppieters et al. (2016) |
| TRNT1 (Sideroblastic anemia with B-cell immunodeficiency, periodic fevers, and DD) MIM: 616084 | RP, posterior subcapsular cataracts (Can also cause nonsyndromic RP; DeLuca et al., 2016]) | Hearing loss (sensorineural) | ID (variable), DD, hypotonia, cerebral atrophy, epilepsy, ataxia, B-cell immunodeficiency, periodic fevers, sideroblastic anemia (congenital), nephrocalcinosis | tRNA processing and maturation | AR | Chakraborty et al. (2014), S. Hull et al. (2016), Wiseman et al. (2013) |
| Many genes (Bardet–Biedl syndrome; Figure 2) | RCD (93%), maculopathy, OA (several of the genes can also cause nonsyndromic IRD) | Hearing loss (conductive or sensorineural) | ID (normal to severe), DD, obesity (infant onset; truncal and rhizomelic), polydactyly (postaxial), hypogonadism, genital anomalies, various renal anomalies, type 2 diabetes, dental anomalies, congenital heart anomalies, brachydactyly, syndactyly (partial), ataxia/poor coordination, anosmia/hyposmia (several of the genes can also cause other ciliopathies) | Ciliary | AR | Beales et al. (1999), Forsythe et al. (2018), Weihbrecht et al. (2017) |
| Many genes (Joubert syndrome; Figure 2) | RP or LCA (38%), OMA, strabismus, nystagmus, ptosis, chorioretinal coloboma, OA (several of the genes can also cause nonsyndromic IRD) | Hearing loss (3%; sensorineural or conductive) | ID (normal to severe), DD (expressive speech disproportionately affected), molar tooth sign, other structural brain anomalies, encephalocele, hypotonia, OMA, seizures, ataxia, respiratory dysregulation, nephronophthisis, cystic renal dysplasia, hepatic fibrosis, polydactyly (pre-, meso-, or post-axial), congenital heart anomalies, midline oro-facial defects, various endocrine abnormalities (several of the genes can also cause other ciliopathies) | Ciliary | AR; XL | Bachmann-Gagescu et al. (2020), Romani et al. (2013), S. F. Wang et al. (2018) |
- Abbreviations: AR, autosomal recessive; CRD, cone-rod dystrophy; DD, developmental delay; DTRs, deep tendon reflexes; FTT, failure to thrive; ID, intellectual disability; IRD, inherited retinal dystrophy; LCA, Leber congenital amaurosis; OA, optic atrophy; OMA, oculomotor apraxia; RCD, rod-cone dystrophy; RP, retinitis pigmentosa; XL, X-linked.
4.9 IRD-ID syndromes with hematologic or immunologic abnormalities
A few of the IRD-ID syndromes, highlighted in Table 9, present with associated hematologic or immunologic abnormalities that may have important clinical consequences. Noncyclic neutropenia is a feature of Cohen syndrome along with recurrent infections and aphthous ulcers; although the neutropenia is typically mild to moderate, granulocyte colony-stimulating factor therapy is sometimes administered (H. Wang et al., 1993). B-cell immunodeficiency in an individual with IRD-ID suggests a TRNT1-related disorder, in which periodic fevers and congenital sideroblastic anemia may also be present (Chakraborty et al., 2014). Arts syndrome may cause immune impairment as well, particularly with severe respiratory infections (de Brouwer et al., 2010). Although it should be noted that any of the IRD-ID syndromes with renal involvement may manifest with anemia secondary to renal dysfunction; these syndromes are not included within this section.
| Gene (disorder), MIM | Ocular phenotype | Hematologic or immunologic phenotype | Other systemic features | Protein function | Inheritance | Selected references |
|---|---|---|---|---|---|---|
| PRPS1 (Arts syndrome) MIM: 301835 | IRD (manifesting females), LCA (one male sibship), OA | Immune impairment (severe respiratory infections) | ID, hypotonia, neuropathy (predominantly peripheral), ataxia, hyperreflexia, spastic quadriparesis, hearing loss (sensorineural, profound), diabetes insipidus (Wide spectrum of presentation; can also cause PRS-1 superactivity syndrome, hyperuricemia, nonsyndromic hearing loss, Charcot Marie Tooth disease 5 (de Brouwer et al., 2010)) | Purine and pyrimidine biosynthesis | XL | Al-Maawali et al. (2015), Almoguera et al. (2014), de Brouwer et al. (2010), Fiorentino et al. (2018) |
| TRNT1 (Sideroblastic anemia with B-cell immunodeficiency, periodic fevers, and DD) MIM: 616084 | RP, posterior subcapsular cataracts (Can also cause nonsyndromic RP; DeLuca et al., 2016) | Sideroblastic anemia (congenital), B-cell immunodeficiency | ID (variable), DD, hypotonia, cerebral atrophy, epilepsy, ataxia, periodic fevers, hearing loss (sensorineural) | tRNA processing and maturation | AR | Chakraborty et al. (2014), S. Hull et al. (2016), Wiseman et al. (2013) |
| VPS13B (Cohen syndrome) MIM: 216550 | RP, bull's eye maculopathy, myopia, retinoschisis | Neutropenia (mild to moderate), recurrent infections, aphthous ulcers | ID, microcephaly (postnatal onset; variable severity), short stature, small/narrow hands and feet, joint laxity, hypotonia, FTT followed by obesity (truncal), characteristic facial features, friendly disposition | Post-Golgi protein sorting, transport, and modification; regulation of adipogenesis | AR | Nasser et al. (2019), H. Wang et al. (1993) |
- Abbreviations: AR, autosomal recessive; DD, developmental delay; FTT, failure to thrive; ID, intellectual disability; IRD, inherited retinal dystrophy; LCA, Leber congenital amaurosis; OA, optic atrophy; RP, retinitis pigmentosa; XL, X-linked.
4.10 IRD-ID in inborn errors of metabolism
Both IRD and ID are observed in several inborn errors of metabolism (IEMs; Poll-The & Maillette de Buy Wenniger-Prick, 2011; Rajappa, Goyal, & Kaur, 2010). Although a detailed review of metabolic disorders that can manifest with IRD-ID lies outside the scope of this review, the clinician should consider this category in the diagnostic workup of a patient.
IRD and ID can result from peroxisomal disorders, several mitochondrial disorders, lysosomal storage disorders such as the mucopolysaccharidoses and neuronal ceroid lipofuscinoses, congenital disorders of glycosylation, neurodegeneration with brain iron accumulation, abetalipoproteinemia, Menkes disease, cobalamin C deficiency, and ornithine aminotransferase deficiency (Poll-The & Maillette de Buy Wenniger-Prick, 2011; Rajappa et al., 2010; Werdich et al., 2014). These disorders tend not to present with IRD-ID alone, as the biochemical pathways involved are implicated in multiple cellular processes. In general, additional features that should raise suspicion for these IEMs include developmental regression, failure to thrive, a progressive neurodegenerative course, multisystem involvement, or clinical deterioration in times of increased metabolic demand (Saudubray, Berghe, & Walter, 2012). Some disorders manifest with suggestive signs, for example, coarse facial features and corneal clouding in some mucopolysaccharidoses, or kinky and brittle hair in Menkes disease. Others, such as mitochondrial disorders and congenital disorders of glycosylation, can present in any organ system at any age (Saudubray et al., 2012). Prompt diagnosis may in some cases lead to the early implementation of treatment options (Rajappa et al., 2010). Of note, some of these IEMs do also have milder forms which may present with IRD without neurodevelopmental features, for example, PEX1- and PEX6-related Heimler syndrome and certain variants in HGSNAT (Haer-Wigman et al., 2015; Ratbi et al., 2015).
Newborn screening, which varies by region, does not detect the majority of the aforementioned IEMs associated with IRD-ID (Therrell et al., 2015). An exception is mucopolysaccharidosis type I, which has recently been added to the Recommended Uniform Screening Panel (RUSP) in the United States, although the implementation remains variable (RUSP, 2018). When suspecting an IEM in an individual with IRD-ID, the Treatable Intellectual Disability Endeavor (TIDE) protocol is one resource which may guide biochemical testing (van Karnebeek, Shevell, Zschocke, Moeschler, & Stockler, 2014). Specifically, useful initial investigations for the metabolic IRD-ID disorders include plasma amino acids, total homocysteine, copper and ceruloplasmin, urine organic acids, and urine glycosaminoglycans. These investigations can assist in the workup of mucopolysaccaridoses, Menkes disease, cobalamin C deficiency, and ornithine aminotransferase deficiency. Additionally, the clinician can consider very long-chain fatty acids if a peroxisomal disorder is suspected, transferrin isoelectric focusing in the case of a congenital disorder of glycosylation, and a lipid profile for possible abetalipoproteinemia. Magnetic resonance imaging of the brain can be a useful adjunct as well, particularly if considering mitochondrial or peroxisomal disorders, neuronal ceroid lipofuscinosis, or neurodegeneration with brain iron accumulation. Broad molecular testing such as exome sequencing, which is increasingly being applied earlier in the diagnostic workup, can of course also be quite helpful in confirming the diagnosis of an IEM.
4.11 IRD-ID as a dual diagnosis
Finally, despite Occam's razor, at times a single unifying genetic diagnosis may not explain an individual's entire phenotype. In such cases, the clinician must keep in mind the possibility of a dual diagnosis (Balci et al., 2017; Posey et al., 2017). This is especially relevant when it comes to the IRD-ID syndromes, as both IRD and ID are independently common. Likely some older reports in the literature of single instances of ID associated with established retinal disorder genes (or vice versa) may have resulted from separate molecular genetic disorders. Finally, a significant proportion of the genes and syndromes reviewed above have a wide phenotypic spectrum and may also cause nonsyndromic ophthalmologic manifestations or systemic disease without ocular features. Thus, even in a scenario where an individual is found to have a pathogenic result in one of the genes highlighted within this review, careful consideration of the variant in question and the individual's clinical phenotype remains invaluable in establishing an accurate diagnosis.
4.12 Emerging evidence for IRD-ID disorders
Although we have provided a summary of the existing evidence for genes associated with IRD-ID phenotypes, the list of IRD-ID disorders will likely continue to grow. We conclude our review by sharing a clinical encounter that highlights evidence for the nonrandom association of IRD with ID.
4.13 KIZ (MIM# 615757)
We examined a 23-year-old male Sudanese refugee who presented with GDD, short stature, kyphoscoliosis, presumed hearing loss (awaiting formal testing), high myopia (−20D), and a retinal dystrophy. A retinal dystrophy panel consisting of 266 genes (Blueprint Genetics, Helsinki, Finland) identified two heterozygous variants in KIZ (MIM# 615757): c.1612 + 2 T > G, a consensus splice donor variant; c.709C > A, p.(Pro237Thr), a missense variant. This panel essentially ruled out a collagenopathy, including Kniest dysplasia. Neither variant in KIZ has been reported before in association with human disease. Using the American College of Medical Genetics and Genomics (ACMG) classification guidelines, the consensus splice donor variant was classified as likely pathogenic and the missense variant as a variant of uncertain significance (VUS). Unfortunately, neither parent was available for segregation analysis.
The KIZ gene encodes a centrosomal protein kizuna located in the basal body of cilia. Mutations in KIZ have been shown to cause rod-cone dystrophy; however, there have been no reports of a syndromic presentation (El Shamieh et al., 2014, 2017; Gustafson et al., 2017). Given the exceptionally rare occurrence of the above variants individually in control databases combined with the known association of KIZ with retinopathy, we believe that these changes explain our patient's ocular phenotype. An alternate genetic cause, however, cannot be entirely excluded. As this disorder is a ciliopathy, it is plausible that mutations in KIZ can also affect other commonly involved systems, providing an explanation for our patient's developmental delay and vertebral anomalies. However, the possibility of a dual diagnosis remains. Examining parents and analyzing the pedigree may help clinicians clarify both single and dual genetic diagnoses and nongenetic contributions to disease. Future functional studies that examine these variants in a model organism, as well as further replication in other clinic patients, may also help to clarify this observation.
5 CONCLUSION
The molecular etiologies underlying the clinical presentation of IRD-ID are diverse, encompassing a variety of mechanistic pathways and classes of disorders. These disorders themselves are complex as well, with multi-system involvement. For many of these patients, timely diagnosis can lead to specific screening for associated features or complications, with the potential to lead to improved outcomes.
When a first-line chromosomal microarray does not yield a diagnosis in the individual with IRD-ID, there are many single-gene disorders to consider in the differential, as highlighted in this review. Although broad, nonhypothesis-driven testing such as exome or genome sequencing are now available as diagnostic tools to many clinicians, in reality, these tests are still not readily accessible to all. Multigene panel-based testing remains a reasonable option for individuals with IRD-ID and may be a more efficient alternative in some cases. If the clinician chooses to pursue panel-based testing, whether based primarily on the ocular phenotype, the cognitive phenotype, or other systemic features, this review can help the clinician ensure that the selected panel is comprehensive in its coverage of relevant genes on the differential diagnosis. Regardless of the testing approach used, the diagnostic framework presented here also provides a useful reference both in the initial assessment and test result in interpretation of an individual with IRD-ID, particularly if the phenotype does not seem to have been explained entirely by the results.
With the significant advances within the field in recent years, the list of IRD-ID disorders has expanded rapidly and continues to grow. Despite all that we currently understand about these disorders and their underlying molecular basis, there continue to be patients with IRD-ID that remain unsolved. Candidate genes and new disorders continue to emerge over time. Continued research, collaborative efforts, and patient engagement will be essential for furthering gene discovery with the goal of eventually being able to end the diagnostic odyssey and provide a timely answer to all of our patients with IRD-ID.
ACKNOWLEDGEMENTS
This research was supported by the Clinician–Scientist Emerging Leader Award from Fighting Blindness Canada (MDB). The authors gratefully acknowledge the financial support of Michelle and Cam Robinson for research on heritable ocular disorders.
Open Research
DATA AVAILABILITY STATEMENT
Data sharing not applicable—no new data generated