Diagnostic evaluation of developmental delay/mental retardation: An overview
Abstract
Mental retardation (MR) is one of the few clinically important disorders for which the etiopathogenesis is still poorly understood. It is a condition of great concern for public health and society. MR is currently defined as a significant impairment of cognitive and adaptive functions, with onset before age 18 years. It may become evident during infancy or early childhood as developmental delay (DD), but it is best diagnosed during the school years. MR is estimated to occur in 1–10% of the population, and research on its etiology has always been a challenge in medicine. The etiopathogenesis encompasses so many different entities that the attending physician can sometimes feel a “virtual panic,” starting a wide-range diagnostic evaluation. The Consensus Conference of the American College of Medical Genetics has recently established guidelines regarding the evaluation of patients with MR [Curry et al., 1997], emphasizing the high diagnostic utility of cytogenetic studies and neuroimaging in certain clinical settings. However, since then there has been substantial progress in molecular cytogenetics and neuroimaging techniques, the use of which has allowed recognition and definition of new disorders, thus increasing the diagnostic yield. This review will focus on the most appropriate investigations shown to be, at present, necessary to define the etiology of DD/MR, in the context of recommendations for the clinical evaluation of the patient with undiagnosed MR. © 2003 Wiley-Liss, Inc.
INTRODUCTION
Mental retardation (MR) represents an important chapter in medicine. It is one of the few clinically important disorders for which the etiopathogenesis is still poorly understood. It is a condition of great concern for public health and society. Public health sees it as a rather common abnormality, distributed amongst the entire population, with heavy and lifelong costs. Society sees it as a heavy burden with negative effects on productivity, associated with different degrees of limitations in self-direction and self-care, with consequent need for supervision, support, and protection. For the affected individual and his family, it represents a limitation, of variable degree, in all fields of daily living.
MR is currently defined as a significant impairment of cognitive and adaptive functions, with onset before age 18 years. According to the intelligence quotient (IQ) (obtained by assessment with one or more of the standardized, individually administered tests), MR is subgrouped in four degrees of severity: mild MR, IQ level of 50–55 to approximately 70; moderate MR, IQ level of 35–40 to 50–55; severe MR, IQ level of 20–25 to 35–40; and profound MR, IQ level of below 20–25. Usually the presenting symptoms in individuals with MR are impairments in adaptive functioning. Adaptive functioning refers to how effectively individuals cope with everyday life demands, and how well they meet the standards of personal independence expected of someone of that particular age and socioeconomic and cultural background. Adaptive functioning may be influenced by various factors such as motivation, personality style, education, social and vocational opportunities, and the general medical conditions and mental disorders that may coexist with MR. Adaptive functioning is measured by standardized scales that allow the gathering of evidence for deficits from more than one reliable source [Sparrow et al., 1984; Achenbach, 1991]. As in the assessment of cognitive functioning, consideration should be given to the suitability of the instruments to the subject's ethnic and cultural background, education, motivation, cooperation, and associated handicaps.
MR may become evident during infancy or early childhood as developmental delay (DD), but it is best diagnosed during the school years. It is estimated to occur in 1–10% of the population [McLaren and Bryson, 1987; Drillien et al., 1988; Simeonsson and Sharp, 1992; Massey and McDermott, 1995; Stevenson, 1996]; the different rates of prevalence depend on definitions used, methods of ascertainment, and population studied. MR is seen more often in males (sex ratio of 1.5:1) [Penrose, 1938; Lehrke, 1968; American Psychiatric Association, 1995]. Possible explanations may be the biological inequity between males and females conferred by the different number of sex chromosomes, and by now well-established X-linked single-gene mutations.
- 1.
Diagnosis provides prediction.
- 2.
It is often definitely sought by the family.
- 3.
Only recognition of the causes may help in establishing an accurate recurrence risk; predicting the prognosis with relative certainty; organizing appropriate laboratory testing; avoiding diagnostic evaluation of unnecessary complexity, expense, and invasiveness; establishing a health maintenance plan; starting adequate treatment, when feasible; and referring the patient and the family to a support group.
Being a disorder of brain formation and function, MR may result from genetic influences, environmental insults, or a combination of the two [Moser and Wolf, 1971; Opitz et al., 1978; McLaren and Bryson, 1987]. The high frequency of the involvement of genes in the etiology of MR is reflected by the finding in Online Mendelian Inheritance in Man (OMIM) of 1,027 entries upon a search for “mental retardation.” The etiopathogenesis encompasses so many different entities that the attending physician can sometimes feel a “virtual panic,” starting a wide-range diagnostic evaluation. This feeling is magnified by the low diagnostic yield usually reported in the assessment of such patients [Moser and Wolf, 1971; Gustavson et al., 1977a,b; Laxova et al., 1977; Opitz et al., 1978; Moser et al., 1990]. However, these works mostly included patients with severe to profound MR; moreover, significant advances in laboratory testings over the last two decades have led to a substantial improvement in the diagnostic yield. The Consensus Conference of the American College of Medical Genetics has recently established guidelines regarding the evaluation of patients with MR [Curry et al., 1997]. These investigators emphasized the high diagnostic utility of cytogenetic studies and neuroimaging in certain clinical settings. A more recent study [Battaglia et al., 1999] on the diagnostic yield of the comprehensive assessment of DD/MR, while confirming the diagnostic utility of cytogenetic/molecular genetic and neuroimaging studies, suggested the usefulness of accurate electroencephalogram (EEG) recordings and stressed the importance of a thorough physical examination. Most of these points were also supported by Hunter [2000] in his review of hospital and genetics clinic records of 411 patients evaluated for MR. Contrary to the most often expressed opinion that the diagnostic yield is greater in individuals with more severe MR [Bodensteiner and Schaefer, 1995], it is now obvious to us that the likelihood of making a diagnosis is independent of the category and degree of DD/MR [Battaglia et al., 1999; Hunter, 2000]. This may in part reflect both an improved ability in studying and diagnosing more subtle patterns of malformation (e.g., mild Wolf-Hirschhorn, mild Brachmannde-Lange) and the availability of newer diagnostic techniques (e.g., fluorescence in situ hybridization (FISH), subtelomeric screening, comparative genomic hybridization, chromosome microdissection, interferometer spectral imaging or karyotyping (SKY), primed in situ labeling (PRINS), in vivo proton magnetic resonance spectroscopy (MRS) of the brain). Indeed, recent years have produced many exciting advances in dysmorphology, cytogenetics and molecular genetics, neuroimaging, and clinical neurophysiology that allow (or contribute to) the identification of the underlying cause of many previously undiagnosable cases of DD/MR.
Recent years have produced many exciting advances in dysmorphology, cytogenetics and molecular genetics, neuroimaging, and clinical neurophysiology that allow (or contribute to) the identification of the underlying cause of many previously undiagnosable cases of DD/MR.
This review will focus on the most appropriate investigations shown to be, at present, necessary to define the etiology of DD/MR, in the context of recommendations for the clinical evaluation of the patient with undiagnosed MR.
HISTORY/PHYSICAL EXAMINATION
An accurate prenatal/birth history and hereditary/familial history, together with a three-generation pedigree, should be an essential step in the evaluation of the patient with DD/MR [Curry et al., 1997].
The importance of a thorough physical examination (including a meticulous search for skin changes and a neuromotor assessment, with the documentation of minor anomalies and/or abnormal findings by detailed description and measurements) emerges from several reports in the literature [Curry et al., 1997; Root and Carey, 1997; Battaglia et al., 1999; Hunter, 2000; Nanni et al., 2001]. It is common experience that it can help either in making a diagnosis or in directing laboratory testing. Photographs and videos should be complementary tools, videotaping being invaluable in documenting posture, gait, any movement disorders, and behavior characteristics.
As emerges from literature reports, it seems that the best use of subtelomeric analysis is in patients with moderate to severe MR associated with physical anomalies.
As already suggested by the Consensus Conference of the American College of Medical Genetics [Curry et al., 1997], serial evaluations of the patient, at times over several years, are often very useful for diagnosis. Both clinical and behavior phenotypes tend to modify over time in many congenital patterns of human malformation, allowing for the eventual recognition of many conditions. Moreover, systematic follow-up is also useful for a stepwise and cost-effective approach to diagnostic testing. The frequency of evaluations should vary in relation to the age of the patient, the severity and complexity of the clinical picture, and the urgency of reproductive concerns.
CYTOGENETICS/MOLECULAR CYTOGENETICS
Contribution of chromosome aberrations to DD/MR is generally said to be elevated. Chromosome abnormalities are reported in 4–34.1% of individuals with DD/MR [Bourgeois and Benezech, 1977; Kodama, 1982; Opitz et al., 1982; Rasmussen et al., 1982; Wuu et al., 1984; Gustavson, 1977b; Srsen et al., 1989; Wuu et al., 1991; Phelan et al., 1996; Felix et al., 1998; Hou et al., 1998; Battaglia et al., 1999; Hong et al., 1999; Cora et al., 2000], and cytogenetic analysis is regarded as a mainstay in the diagnostic process. However, guidelines regarding the type and resolution of the analysis to be performed and definite clinical indications for such studies are still debated. The Consensus Conference [Curry et al., 1997] endorsed the concept that any individual with DD/MR without a definite diagnosis requires a standard cytogenetic analysis at the 500-band level. They also suggested that whenever there is a provisional diagnosis of microdeletion syndrome, a focused FISH analysis may be the first step; and in those patients whose phenotype may be shared between known nonchromosomal syndromes and chromosome aberrations (i.e., Brachmann-de Lange syndrome and dup3q26-27), high-resolution chromosome analysis should be ordered.
However, the demonstration that both false negative and false positive results occur with high-resolution banding [Kuwano et al., 1992; Delach et al., 1994; Butler, 1995] led to the conclusion that this technique is insufficient for detection of deletions [ASHG/ACMG Report, 1996]. The availability, in few research centers, of new technical developments [Knight et al., 1997] has recently allowed molecular cytogenetic approaches in the study of unexplained MR. Since the discovery that subtelomere regions are gene rich [Saccone et al., 1992], with the possible consequence that rearrangements in these sites are likely to produce clinical patterns, subtelomeric analysis, employing different methods [Xu and Chen, 2003], has been performed in cohorts of individuals with undiagnosed MR.
As emerges from literature reports [Knight and Flint, 2000; Rossi et al., 2001; Biesecker, 2002], it seems that the best use of subtelomeric analysis is in patients with moderate to severe MR associated with physical anomalies. In fact, submicroscopic subtelomeric chromosome defects have been found in 6.5–7.4% of children with moderate to severe MR [Knight and Flint, 2000; Rossi et al., 2001] vs. only 0.5% [Knight et al., 1999] or even 10.3% [Anderlid et al., 2002] of children with mild retardation. This latter discrepancy could be explained by the different size and selection of the study groups. Overall, due to the technical complexities, cost of screening, and the lack of testing facilities in every genetic center, an effective clinical preselection is recommended. For this purpose, de Vries et al. [2001] studied 29 patients with a known subtelomeric defect and assessed clinical variables such as family and birth history, facial dysmorphism, and congenital malformations. The data were compared with 110 control children with MR of unknown etiology, with normal standard cytogenetics and no detectable submicroscopic subtelomeric abnormalities. The authors concluded that good indicators for subtelomeric defects are 1) family history of MR, 2) prenatal onset growth retardation, 3) postnatal poor growth/overgrowth, 4) two or more facial dysmorphic features, and 5) one or more nonfacial dysmorphic features and/or congenital abnormalities.
Good indicators for subtelomeric defects are 1) family history of MR, 2) prenatal onset growth retardation, 3) postnatal poor growth/overgrowth, 4) two or more facial dysmorphic features, and 5) one or more nonfacial dysmorphic features and/or congenital abnormalities.
In spite of few criticisms [Baker et al., 2002; van Karnebeek et al., 2002], we believe that this five-item checklist might improve the diagnostic yield of subtelomeric analysis in the evaluation of DD/MR subjects, at least until more detailed clinical parameters and new and more efficient tests, such as genomic microarray [Xu and Chen, 2003], will be widely available.
We believe that this five-item checklist might improve the diagnostic yield of subtelomeric analysis in the evaluation of DD/MR subjects, at least until more detailed clinical parameters and new and more efficient tests, such as genomic microarray, will be widely available.
Based on common clinical experience and literature reports, it seems relevant to underline that a growing number of DD/MR patients thought on first examination to be nonsyndromic turn out to be aneuploid or to have fragile X (FraX). In the study of Curry et al. [1996], 16/150 (11%) children with undiagnosed DD had a chromosome abnormality. Four of these 16 children were described by the clinical geneticist as nondysmorphic. In the study carried out by Battaglia et al. [1999], 10.2% of DD/MR children thought to be nonsyndromic turned out to be aneuploid, and 5.1% had FraX. Lee et al. [2001] reported a 2½-year-old Korean patient presenting with DD, speech delay, delay of gross motor milestones, and hypotonia, but no dysmorphic features, in whom cytogenetic and FISH studies showed a tandem 22/15 translocation with deletion of the 22q13.3 region and retention of the NOR of chromosome 15. A few more patients with DD, absent or severely delayed speech, hypotonia, and only minor anomalies have been reported, in whom a deletion of the 22q13 region was detected either on routine cytogenetic analysis or on FISH/molecular analysis [Precht et al., 1998; Phelan et al., 2001]. As with many terminal deletions involving pale G-band regions, the deletion can be extremely subtle (cryptic terminal rearrangements) and can go undetected on routine cytogenetic analysis (even at the 850-band level) in almost 32% of the cases [Phelan et al., 2001].
Individuals with interstitial duplication of proximal 15q may present with DD/MR and autism spectrum disorder, but without consistent dysmorphic findings [Gurrieri et al., 1999; Mohandas et al., 1999]. Based on the presence of duplicated Prader-Willi/Angelman syndrome critical region (PW/ASCR) loci and parent of origin of the duplication, the patients have been divided into three groups [Browne et al., 1997; Cook et al., 1997; Riordan and Dawson, 1998]. In the first group are patients with euchromatic variants of no clinical significance, without PW/ASCR duplication. The second group includes patients with maternally inherited PW/ASCR duplications associated with DD/MR, autism/atypical autism, learning/speech difficulties, but with no consistent dysmorphic findings. The third group includes patients with paternally inherited PW/ASCR duplications with no apparent clinical phenotype [Riordan and Dawson, 1998]. However, a patient with DD, severe speech delay, and brain anomalies, whose duplication 15q was of paternal origin and involved the PW/ASCR was reported by Mohandas et al. [1999]. The diagnosis in these patients may be missed on standard cytogenetic analysis or on FISH analysis as the sole investigation [Gurrieri et al., 1999; Thomas et al., 2002]. Complete cytogenetic and molecular characterization is recommended for the study of patients with suspected dup(15). It is, in fact, vitally important to detect these patients, since recurrence risk for these families could rise from the empiric risk of 3–7% [Jorde et al., 1990; Piven et al., 1990; Szatmari et al., 1993; Bolton et al., 1994] up to 50% in the case of maternally carried duplication, with substantial implications for other family members.
Children with DD, moderate to severe MR, severe epilepsy with seizure onset between ages 4 and 8 years, diffuse hypotonia, and autistic behavior, with no dysmorphic findings or just one to four “minor anomalies,” have been shown to have a maternally derived inv dup(15) involving the PW/ASCR [Flejter et al., 1996; Battaglia et al., 1997; Schroer et al., 1998]. There are two cytogenetic types of inv dup(15) [Maraschio et al., 1988]. One is a metacentric or submetacentric and heterochromatic chromosome, smaller or similar to a G group chromosome, dic(15)(q11). Most children with this aberration have an apparently normal phenotype [Cheng et al., 1994]. The second type of inv dup(15) is as large as, or larger than, a G group chromosome and has 15q euchromatin. It includes the PW/ASCR [Robinson et al., 1993; Blennow et al., 1995], and the cytogenetic description is dic(15)(q12 or q13). This dicentric 15 is derived from the two homologous maternal chromosomes at meiosis and is usually associated with increased maternal age and with the abnormal phenotype reported above. In all such cases, standard cytogenetics must be associated with FISH analysis.
DD, usually of mild degree, and hypotonia, associated either with a normal phenotype or with a Prader-Willi-like phenotype, have been described in patients with UPD14mat [Papenhausen et al., 1995; Berends et al., 1999; Hordijk et al., 1999; Martin et al., 1999]. UPD14pat has also been shown to be associated with MR and a normal phenotype [Papenhausen et al., 1995] or with MR and different types of anomalies [Walter et al., 1996; Cotter et al., 1997]. UPD14 seems therefore another possible cause of DD/MR and should be searched for at least in definite cases by means of DNA analysis. This could further improve the diagnostic yield and counseling in these families.
Mild to severe DD/MR, associated with hypotonia and variable signs of nonspecific developmental abnormalities, has been reported in individuals with either a de novo 16p deletion [Lindor et al., 1997] or a subtelomeric 16p deletion as a consequence of an inherited balanced cryptic subtelomeric translocation, t(3;16)(q29;p13.3), segregating in the family [Holinski-Feder et al., 2000]. In the latter cases, the authors were able to uncover the etiology of DD/MR following a genome search (due to the large size of the family) and subsequent FISH analysis with subtelomeric 16p probes (extended cytogenetic analysis, including the use of high-resolution karyotyping, multiplex (M-) FISH, and DNA FraX, could not reveal the cause of DD/MR). Such cases once more point out the importance of subtelomeric chromosomal microrearrangements in idiopathic MR, not only in sporadic cases or in small families, but also in large pedigrees, irrespective of the suspected inheritance pattern.
Since the description of mutations in the methyl-CpG binding protein 2 (MECP2) gene in Rett syndrome (RTT; MIN 312750) [Amir et al., 1999; Bienvenue et al., 2000; Cheadle et al., 2000; De Bona et al., 2000; Huppke et al., 2000; Kim and Cook, 2000; Xiang et al., 2000], a few reports have shown that MECP2 mutations are not necessarily lethal in males. The male patients can show severe MR with progressive neurological symptoms [Meloni et al., 2000; Villard et al., 2000] or a nonfatal, nonprogressive encephalopathy [Imessaoudene et al., 2001] or an Angelman-like phenotype [Watson et al., 2001] or a moderate to severe nonspecific X-linked MR (MRX) [Orrico et al., 2000; Couvert et al., 2001]. Even more intriguing appears to be the possibility that males with mild nonspecific MR with no phenotypic anomalies can have an in-frame deletion in MECP2 [Yntema et al., 2002]. These authors screened the DNA of one affected male from 176 families, collected by the European XLMR consortium, in which MR occurred as a trait compatible with X-linked inheritance. The screening was performed for mutations in the entire coding region of the MECP2 gene. A mutation was detected only in one family, which included three affected males in two generations. All affected males showed mild nonspecific MR without any physical or neurological anomalies. How MECP2 mutations lead to MR is unclear as of yet, but these reports disclose new horizons concerning the diagnostic evaluation of DD/MR, particularly bearing in mind that only few years ago, it was common belief that mild DD/MR could be due to cultural and familial rather than pathological causes.
Recent reports in the literature draw attention to a complex developmental disorder characterized by MR, delayed motor development, and distinct facial features (hypertelorism, abnormal eyebrows, low nasal root, prognathism), associated in some patients with microcephaly, epilepsy, Hirschsprung disease (HSCR) or just constipation, heart defects, hypospadias, and corpus callosum agenesis. In a minority of cases, a de novo translocation involving chromosome 2q22 or an interstitial deletion of chromosome 2q22 was detected on standard cytogenetics [Lurie et al., 1994; Mowat et al., 1998; Amiel et al., 2001; Wakamatsu et al., 2001], whereas in the remainder, a variety of different mutations in (ZFHX1B) SMADIP1, encoding Smad-interacting protein1 (SIP1), were detected on molecular analysis [Yamada et al., 2001; Zweier et al., 2002]. This syndrome appears to be less rare than originally expected, and we believe that SMADIP1 mutations should be part of the diagnostic evaluation of DD/MR patients presenting with distinct facial features, +/− microcephaly, +/−HSCR/constipation, +/− corpus callosum agenesis.
From recent literature reports, it looks like 1p36.3 deletions account for 0.5–0.7% of idiopathic MR [Giraudeau et al., 1997, 2001]. This emerging chromosomal disorder is associated with DD/MR, hypotonia, growth abnormalities, and distinct craniofacial dysmorphism (prominent forehead, deep-set eyes, flat nasal bridge, midface hypoplasia, pointed chin) [Slavotinek et al., 1999]. Cardiomyopathy, seizures, and enlarged ventricles occasionally associated with a squat corpus callosum or leukodystrophy can also occur [Battaglia et al., 2001b]. Although the deletion can be detected by high resolution banding (HRB), confirmation by FISH is required in most cases, and subtelomeric FISH analysis has been necessary in others [Giraudeau et al., 1997, 2001; Riegel et al., 1999; Battaglia et al., 2001b]. Based on the relative frequency of this chromosomal aberration in DD/MR children with nonspecific anomalies, we would recommend that subtelomere analysis for 1p36 be routinely performed in patients with unclassified multiple congenital anomalies (MCA)/MR syndromes or apparent idiopathic DD/MR.
Recently, Williams et al. [2001] drew attention to the Angelman syndrome (AS) mimicking conditions and phenotypes. These were grouped into the areas of chromosome, single-gene, and symptom complex anomalies. 22q13.3 terminal deletions seem to be the most mimicking of the AS among chromosome aberration. However, other chromosome anomalies, such as duplication of 15q11-13 (on rare cases), interstitial deletion of 2q21-23, 17q23.2, 4q, should be kept in mind when evaluating a patient with an AS clinical phenotype. Rett syndrome is probably the most common AS mimicker during the infant and toddler ages [Ellaway et al., 1998]. Methylene tetrahydropholate reductase deficiency (MTHFR) can present with DD/MR, absent speech, ataxia, seizures, and happy demeanor [Arn et al., 1998]. Profound MR and protruding tongue can be seen in ATR-X syndrome [Gibbons et al., 1995]. However, patients with nonspecific MR (even of mild degree), no positive family history, and no laboratory evidence for alpha-thalassemia have also been reported to have mutations of the ATR-X gene [Villard et al., 1999; Guerrini et al., 2000].
METABOLIC WORKUP
The importance of inborn errors of metabolism as a cause of DD/MR conditions is widely recognized. However, given their generally low prevalence in children with DD/MR (0–5%) [Opitz et al., 1982; Wuu et al., 1991; Majnemer and Shevell, 1995; Allen and Taylor, 1996], the Consensus Conference [Curry et al., 1997] recommended that “metabolic testing be selective and targeted at the suspected category of disorder.” Identical suggestions derived from following studies on DD/MR patients [Battaglia et al., 1999; Hunter, 2000].
However, it's worth noting that patients presenting with nonregressive early-onset encephalopathy characterized by severe neonatal hypotonia, DD, and cerebellar signs, but with no dysmorphic features or only minor facial anomalies, have been reported to have CDG-1a [Drouin-Garraud et al., 2001]. Congenital disorder of glycosylation (CDG) is a group of metabolic disorders usually presenting with severe neurological manifestations and multisystemic involvement, first described by Jaeken et al. [1980]. The awareness that such a disorder may have a discrete clinical presentation should prompt a preliminary routine test based on isoelectric focusing of serum transferrins or on Western blot analysis of the serum glycoproteins [Seta et al., 1996].
Salomons et al. [2001] described a male child presenting at age 6 years with mild MR, severe speech delay, and hypotonia who was found to have increased levels of creatine in urine and plasma. Further investigations led to the identification of a new creatine deficiency syndrome.
It seems, therefore, worthwhile suggesting a careful consideration of a “general first-step” metabolic workup in all DD/MR children, in order to avoid missing some metabolic conditions with a discrete clinical presentation.
It seems, therefore, worthwhile suggesting a careful consideration of a “general first-step” metabolic workup in all DD/MR children, in order to avoid missing some metabolic conditions with a discrete clinical presentation.
NEUROIMAGING STUDIES
An individual with MR has, by definition, a functionally abnormal brain, which does not necessarily match with anatomical abnormalities. Structural brain abnormalities have been described in 34–98% of deceased severely retarded patients undergoing neuropathologic studies [Crome, 1960; Warkany, 1971; Polednak, 1974], whereas abnormalities on neuroimaging were reported in 9–60% of living patients [Moeschler et al., 1981; Lingam et al., 1982; Sugimoto et al., 1993; Majnemer and Shevell, 1995; Curry et al., 1996; Root and Carey, 1997; Hunter, 2000]. This wide range of results is probably related to the date and type of study (computed tomography (CT) vs. magnetic resonance imaging (MRI)), and to the patient selection criteria. According to the literature data, the Consensus Conference [Curry et al., 1997] stated that “neuroimaging appears to have an especially important role in patients with microcephaly or macrocephaly, seizures, loss of psychomotor skills and neurologic signs,” while its value in the normocephalic patient with no focal neurological signs is unclear.
It is worth mentioning that recently two sisters, aged 4 and 6 years, respectively, were referred to the institute of one of the authors (A.B.) because of mild MR and severe language delay. Parents were unrelated. There were no focal neurological signs, and occipitofrontal circumference (OFC) and physical examination were normal. Standard cytogenetics, routine blood and urine analysis, and metabolic workup were normal. Nonetheless, both girls underwent conventional MRI and brain proton MRS. MRI was normal, whereas MRS disclosed the total absence of creatine/phosphocreatine peak in the periventricular white matter, the cerebellum, and the parieto-occipital cortex [Bianchi et al., 2000]. Consequent investigation of the creatine biosynthetic pathway led to the identification of a new inborn error of creatine metabolism [Item et al., 2001]. Creatine monohydrate oral administration resulted in almost complete brain creatine level restoration along with improvement of the patients' disabilities.
MRS of the brain showing an almost complete absence of the creatine signal again prompted further investigations leading to diagnosis in the patient reported by Salomons et al. [2001] (see above), whose OFC and neurological examination were normal.
In this light, it seems appropriate to perform state-of-the-art neuroimaging studies also in normocephalic DD/MR patients with no focal neurologic signs, in order to avoid missing the recognition of potentially “treatable” neurologic disorders [see Battaglia, 2003].
It seems appropriate to perform state-of-the-art neuroimaging studies also in normocephalic DD/MR patients with no focal neurologic signs, in order to avoid missing the recognition of potentially “treatable” neurologic disorders.
EEG STUDIES
Until a few years ago, very little, if anything, had been reported on the value of EEG studies in MR patients. Recently, Battaglia et al. [1999] reported a relatively high diagnostic yield of EEG investigations in DD/MR patients. They showed the usefulness of waking/sleep video-EEG-polygraphic studies not only in the presence of a clinical history of seizures/epilepsy, but also in other specific clinical settings, such as significant language impairment, Angelman syndrome, inv dup(15) syndrome, and Wolf-Hirschhorn syndrome. In all these conditions, EEG can be helpful both for adequate treatment and for diagnostic purposes [Battaglia et al., 1996, 1997, 2001a, 2002; Guerrini et al., 1996]. In addition, there are a few neurometabolic disorders in which EEG/evoked potentials show a typical pattern, highly suggestive of the diagnosis [Pampiglione and Harden, 1974; Harden and Pampiglione, 1982]. Still, particular EEG patterns observed in some genetic/chromosomal disorders may contribute to the understanding of the underlying pathophysiology [Kivitie-Kallio and Norio, 2001].
CONCLUSIONS
As already discussed [Battaglia et al., 1999], we would suggest that the practitioner follow an algorithm for the “rational evaluation” of a patient with DD/MR,
We would suggest that the practitioner follow an algorithm for the “rational evaluation” of a patient with DD/MR.
and given the latest scientific and technical advances, this should now be updated as from Figure 1. Based on historical and physical examination, the workup of a DD/MR patient can follow various pathways that guide decisions regarding the most appropriate laboratory testing and imaging studies.
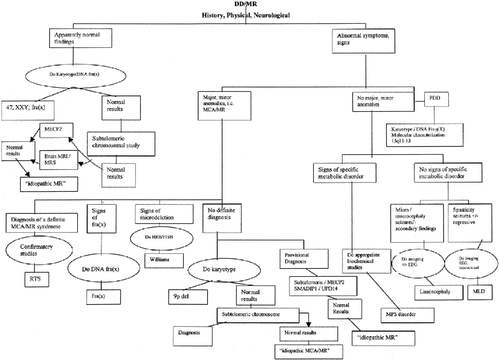
Algorithm for the “rational evaluation” of a patient with DD/MR. [Modified from Battaglia A, Bianchini E, Carey JC. 1999. Am J Med Genet 82:60–66. Published by permission of the author and Wiley-Liss, Inc.]
As mentioned above, during recent few years there has been substantial progress in molecular cytogenetics and neuroimaging techniques, the use of which have allowed recognition and definition of new disorders, thus increasing the diagnostic yield.
- 1.
Patients thought to be nonsyndromic—Do HRB + (if not microcephalic) DNA FraX analysis, and if normal, proceed to subtelomeric analysis; if normal, consider DNA analysis searching for MECP2 mutations/deletions, and brain MRS.
- 2.
Patients with physical anomalies +/− family history of MR, +/− prenatal and/or postnatal growth delay, overgrowth—Do HRB + (if not microcephalic) DNA FraX analysis, and if normal, do subtelomeric analysis.
- 3.
Patients with autism spectrum disorder but no dysmorphic findings or just one to four minor anomalies +/− epilepsy—Do standard cytogenetics and complete cytogenetic/molecular characterization of 15q11-13 region.
- 4.
Patients with hypotonia and a Prader-Willi syndrome-like phenotype—Do DNA methylation studies; if normal, do DNA FraX analysis; if normal, do DNA analysis for UPD14, and research for MECP2 mutations [Kleefstra et al., 2002].
- 5.
Patients with an Angelman syndrome-like phenotype—Do DNA methylation studies; if normal, do HRB, and if normal, proceed to subtelomeric analysis (to exclude 22q13.3 aberrations), and consider comparative genomic hybridization (to exclude 2q21-23 or 4q or 17q23.2 aberrations); or if normal, do DNA analysis searching for MECP2 mutations/deletions; and if normal, do complete cytogenetic/molecular characterization of 15q11-13 region; and/or search for methylene tetrahydropholate reductase deficiency (MTHFR); and/or do mutation testing for the ATR-X gene; and/or molecular analysis searching for mutations in the SMADIP1 gene.
- 6.
Patients with distinct facial features +/− microcephaly +/− HSCR/constipation +/− midline defects—Do HRB searching for 2q22 aberrations, and if normal, do molecular analysis searching for mutations in the SMADIP1 gene.
- 7.
Patients with either progressive or nonprogressive neurological symptoms—(Especially if males) do DNA analysis searching for MECP2 mutations/deletions, plasma/urine creatine levels, and (especially if cerebellar signs are present) isoelectric focusing of serum transferrins or Western blot analysis of the serum glycoproteins; and eventually appropriate metabolic workup (see below).
- 8.
Patients with clinical and physical findings suggestive of metabolic disorders—Do the appropriate metabolic workup [see Kahler and Fahey, 2003].
Careful waking/sleep video-EEG-polygraphic studies should be taken into consideration at least in definite cases. Systematic follow-up of the patient should be carried out routinely. On many occasions this has been helpful for diagnosis.
Although it is obvious to us that no set protocol can replace the individual clinician's freedom to decide what test to carry out, we believe that the above recommendations might help toward a more rational and comprehensive assessment of the DD/MR patient.




