Circadian Rhythms Correlated in DNA Methylation and Gene Expression Identified in Human Blood and Implicated in Psychiatric Disorders
Funding: This work was supported by National key R & D Plan of China (Grant Nos. 2022YFE0103700 to J.T.), the Science and Technology Innovation Program of Hunan Province (Grant Nos. 2021RC4018, 2021RC5027) Innovation-driven Project of Central South University (Grant No 2020CX003), the National Key R&D Project of China (Grants Nos. 2016YFC1306000), the National Natural Science Foundation of China (NSFC) (Grants Nos. 82171495, 81871057, 82022024, 31970572).
Haiyan Tang, Shanshan Chen, and Liu Yi have contributed equally to this work.
Ning Yuan, Chao Chen, and Jinsong Tang are co-corresponding authors who jointly directed this work.
ABSTRACT
Circadian rhythms modulate the biology of many human tissues and are driven by a nearly 24-h transcriptional feedback loop. Dynamic DNA methylation may play a role in driving 24-h rhythms of gene expression in the human brain. However, little is known about the degree of circadian regulation between the DNA methylation and the gene expression in the peripheral tissues, including human blood. We hypothesized that 24-h rhythms of DNA methylation play a role in driving 24-h RNA expression in human blood. To test this hypothesis, we analyzed DNA methylation levels and RNA expression in blood samples collected from eight healthy males at six-time points over 24 h. We assessed 442,703 genome-wide CpG sites in methylation and 12,364 genes in expression for 24-h rhythmicity using the cosine model. Our analysis revealed significant rhythmic patterns in 6345 CpG sites and 21 genes. Next, we investigated the relationship between methylation and expression using powerful circadian signals. We found a modest negative correlation (ρ = −0.83, p = 0.06) between the expression of gene TXNDC5 and the methylation at the nearby CpG site (cg19116172). We also observed that circadian CpGs significantly overlapped with genetic risk loci of schizophrenia and autism spectrum disorders. Notably, one gene, TXNDC5, showed a significant correlation between circadian methylation and expression and has been reported to be association with neuropsychiatric diseases.
1 Introduction
Circadian rhythms are biological processes that display an endogenous oscillation of about 24 h (Dunlap 1999). The role of these endogenous rhythms is to coordinate the body's functions with each other and with the external environment (Duffy, Zitting, and Chinoy 2015; Masri, Kinouchi, and Sassone-Corsi 2015; Salavaty 2015; Zhang et al. 2018). Disruption of circadian rhythm has been associated with or implicated in many traits, such as immunological deficiency, psychiatric disorders, obesity, metabolic disturbances, and carbohydrate intake, and so forth (Curtis et al. 2014; Lamont et al. 2010; Lemmer 2017; Levi and Schibler 2007; Ramos-Lopez et al. 2018).
Before selecting potential candidate biomarkers for future applications in medical and forensic settings, knowledge of the exogenous and endogenous regulation of gene expression rhythms is needed. DNA methylation is an epigenetic modification that regulates gene expression in response to environmental stimuli (Jaenisch and Bird 2003). One study in hamsters demonstrated that DNA methylation is vital to regulating seasonal reproduction, although seasonal variations may differ from circadian variations (Viitaniemi et al. 2019). Lim's work from a human postmortem study analyzing DNA methylation status has suggested that circadian alterations in DNA methylation drive the circadian rhythms in gene expression (Cronin et al. 2017). These findings suggest that DNA methylation may be essential in regulating circadian rhythms. However, whether circadian rhythms of DNA methylation are associated with circadian rhythms of gene expression in human blood is unknown.
Abnormalities in circadian rhythms have been reported in patients with psychiatric disorders (Li et al. 2013). The basic science and substantial clinical evidence support a link between circadian rhythm that is disrupted and major depressive disorder (Liu and Chung 2015). Decades of research have pointed out that disrupting circadian rhythms by shift work or jet lag is frequently reported in psychiatric disorders (Germain and Kupfer 2008; Harvey 2008; Vadnie and McClung 2017), can worsen or cause mood symptoms (Asaoka et al. 2013; Young 1995). Furthermore, seasonal changes in day length can affect mood (Hickie et al. 2013) (Rosenthal et al. 1984). Many circadian genes have been associated with psychiatry disorders, which implies the contribution of the circadian system to the genetic susceptibility to psychiatry disorders and suggests that different circadian genes may have specific effects (Benedetti et al. 2003; Etain et al. 2014; Mansour et al. 2006; Utge et al. 2010). Most successful treatments of psychiatric symptoms directly target the circadian system, which provides evidence that circadian disruption may underlie this disease (Duncan 1996; Germain and Kupfer 2008; Leproult et al. 2005; Robillard et al. 2018; Terman et al. 2001).
Here, we sought to determine the degree of correlation in circadian rhythms between DNA methylation and gene expression in healthy human blood. We performed that the genome-wide DNA methylation rhythmic analyses on DNA from blood to find the circadian CpG sites. We also conducted a genome-wide microarray analysis on mRNA isolated from PBMCs to examine the transcriptional circadian rhythms. We tested the correlation between DNA methylation and gene expression at loci that showed circadian rhythms in both. We also explored whether rhythmic signals are enriched with genes implicated in multiple psychiatric disorders.
2 Methods
2.1 Participants and Sample Collection
Participants included eight Chinese Han males (Table 1). Written informed consent was obtained from all participants. None had extraordinary stress or disease history. Neither were they smokers nor excessive caffeine drinkers. No volunteer had traveled nor had a fever within 2 months before testing. All eight participants had average body (armpit) temperatures and blood pressure. All had regular sleep-wake cycles according to the Pittsburgh sleep quality index (PSQI) evaluation, recorded for 1 week before blood sampling (Carpenter and Andrykowski 1998).
| BM (n = 8) | BE (n = 4) | |
|---|---|---|
| Samples count | 47 | 24 |
| Sex (male/female) | 47/0 | 24/0 |
| Age (mean ± SD) | 26.25 ± 2.12 | 25.50 ± 2.84 |
| BMI (mean ± SD) | 23.65 ± 2.60 | 23.10 ± 2.84 |
- Abbreviations: BE, blood expression; BM, blood methylation; BMI, body mass index; SD, standard deviation.
The overview of the study design is shown in Figure 1. Peripheral venous blood was drawn approximately every 4 h in 1 day: 08:00, 12:00, 16:00, 20:00, and 23:00 h, ending at 04:00 h on the second day. To avoid interrupting the regular sleep–wake cycle, the fifth time point was moved from 24:00 to 23:00 h. A volume of 6 mL peripheral venous blood was drawn each time using a collector with an EDTA anticoagulant. An aliquot of 200 μL of each whole blood sample was used for DNA extraction. An aliquot of 4 mL was centrifuged at 1700 rpm for 20 min to separate plasma and peripheral blood mononuclear cells (PBMCs). The PBMCs were used for RNA extraction.
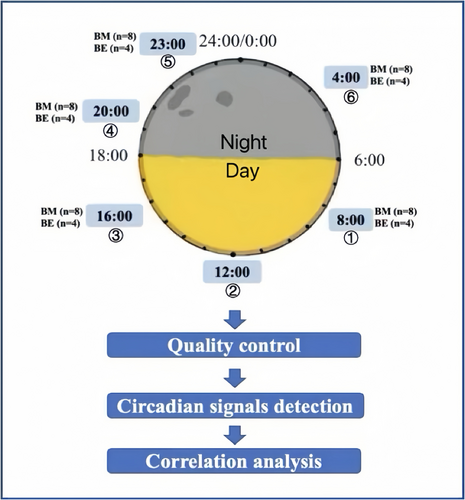
Blood DNA from all participants underwent methylation screening using Illumina Infinium HumanMethylation 450K BeadChip (Dedeurwaerder et al. 2011). Isolated total RNA was analyzed on an Agilent Bioanalyzer 2100 for RNI integrity number (RIN) quality check. Twenty-four RNA samples from four individuals with RIN >7 underwent Illumina Human-HT-12 V4 Array screening.
2.2 Microarray of DNA Methylation Analysis
We extracted DNA from whole blood using the QIAamp DNA Blood Mini Kit (QIAGEN, Netherlands). We measured DNA concentration and A260/A280 ratio using an ND-1000 spectrophotometer (Wilmington, DE). Bisulfite conversion was performed with the EZ DNA Methylation Kit (Zymo, Irvine, CA). DNA methylation was measured using the Illumina Infinium Human Methylation 450K Bead Chip (Illumina, SanDiego, CA), which contains probes totaling 485,512 CpG sites. Sample loading order was randomized to control the position effects while keeping the samples from the same individual on the same chip to minimize the batch effect (Jiao et al. 2018; Lander 1999).
2.3 Microarray of Gene Expression Analysis
RNA from PBMCs was extracted with miRNeasy Mini Kit (QIAGEN, 217004). RNA concentration and A260/A280 ratio were measured using a NanoDrop spectrophotometer (ND-1000, Gene). RNA integrity number (RIN) was measured using an Agilent 2100 Bioanalyzer (Agilent Technologies). RNA samples with RIN >7 were used for the expression experiment. To achieve genome-wide expression profiling, we used the Illumina Human HT-12V4 Expression BeadChip Kit (Illumina, San Diego, CA). Sample loading order was randomized similarly to that used in our expression array to avoid position and batch effects (Chen et al. 2011; Johnson, Li, and Rabinovic 2007; Rahmani et al. 2016).
2.4 Pre-Processing
We loaded the raw intensity files of the DNA methylation array into the R (R-3.3.2) package ChAMP, calculated the proportions of methylation level (“Beta” value), and performed probe filtration. Probes were excluded from analysis with a detection p-value above 0.01 in any samples, as were bead counts less than three in at least 5% of samples, SNPs in southern Chinese populations, and probes mapped at multiple locations. From the selections, 44,2703 probes remained. We removed samples containing detection p-value above 0.1 or outlined from PCA and cluster analysis. Only one blood methylation (BM) sample was removed. Then, we used beta-mixture quantile dilation (BMIQ) to adjust the Type II probes into a statistical distribution with Type I probes—the most reliable method to reduce variability between replicates (Dedeurwaerder et al. 2011).
To detect p-values and remove background effects, we processed raw data generated from the Illumina human-HT 12-V4 platform using the Genome Studio software expression Module (Illumina, SanDiego, CA). We filtered samples with a p-value above 0.06 in more than 20% of probes according to the following standards: (1) p-values >0.06 in more than 1% of samples; (2) control probes; (3) multiple probes; and (4) replicated genes. After these exclusions, 12,364 probes remained in all samples.
To adjust for batch and positional effects, we used ComBat (Chen et al. 2011; Jiao et al. 2018; Johnson, Li, and Rabinovic 2007). To calculate cell counts for methylation data, we used ReFACTor (Rahmani et al. 2016), and to calculate cell counts for expression data, we used CIBERSORT (Newman et al. 2015). The cell-type proportion was corrected by linear regression using the R function lm().
2.5 Rhythmic Detection
Based on visual inspection of these data, we modeled these data using cosine curves. We fit the DNA methylation measures of each probe to the cosine model: M = m + βc*cos(t) + βs*sin(t) (Formula 1). The cosine model is the linearized version of the classic cosine model M = m + (β 1)*cos (t—β A) (Formula 2), for which m is the mean value at each site of all samples, β 1 is the amplitude curve, β A is the acrophase, and t is the time of sample collection. The changeable between these two algorithms according to the formulae β 1 = (βc2 + βs2)0.5 and β A = arctan2 (βs/βc). To fit the linearized version model (Formula 1), time points needed to be changed to radians (2π radians = 24 h). We defined a site or gene with a significant circadian pattern with an amplitude higher than 10% of the mean value and a q-value less than 0.1 after multiple tests (False Discovery Rate, FDR) (Storey and Tibshirani 2003; Zhang et al. 2014).
2.6 Correlation Analysis
We investigated whether a gene associated with circadian rhythms has an expression level correlated with DNA methylation. We used both methylation and expression data to perform the correlation analysis. First, we considered all possible CpG gene pairs (CGPs) for the CpG sites associated with circadian rhythms, that is, those located in the region between 5 kb upstream of the transcription start-site and 5 kb downstream of the transcription end site (Chen et al. 2014). We next performed correlation analysis using mean value of four samples' at six point. This statistic is based on Spearman's correlation coefficient between DNA methylation beta values and the gene's expression level. Multiple corrections were adjusted using FDR.
2.7 Enrichment Analysis
To explore whether genes or CpG sites associated with the psychiatric disorder show rhythmic methylation, we tested for enrichment between circadian DNA CpGs sites and EWAS results of psychiatric disorders, as well as enrichment between circadian genes and SCZ-, ASD-, and MDD- risk genes. EWAS and CpGs were collected from Hannon et al. (2016); and Jaffe et al. (2016). The risk genes of psychiatric disorders came from genome-wide association studies, differential expression, and co-expression studies. We applied gene level enrichment analysis on SCZ, ASD, and MDD separately but only used methylation level enrichment analysis on SCZ because of the limited data. All the standards for data collection of these gene sets or CpG sites are described previously (Xia et al. 2021). Fisher's exact test was used in the enrichment test. We defined significant enrichment as FDR < 0.05.
3 Results
3.1 Characteristics of DNA Methylation Patterns
To detect significant circadian methylation CpG sites, we measured methylation levels at the genome level in 48 blood samples (8 individuals at six-time points). We investigated circadian patterns by fitting a cosine model for the DNA methylation levels at each CpG site. Following two criteria: (1) an amplitude higher than 10% of the mean value (all samples at each time point) and (2) a q-value less than 0.1 after multivariate testing, we detected 6345 CpG sites with epigenome-wide significance (FDR < 0.1) showing rhythmic oscillation in human blood (BM).
3.2 Possible Regulation of Rhythmic DNA Methylation on Gene Expression
To test whether rhythmic DNA methylation regulates gene expression in blood tissue, we applied genome-wide expression profiling on 24 samples (four individuals at six-time points). We detected 21 genes (ABCB9, CAMP, DEFA1B, DEFA3, IGJ, IGLL1, IGLL3, IKZF1, LCN2, LOC647450, LOC647506, LOC652493, LOC652694, LOC653600, MGC29506, MIR1974, MYOM2, RBPMS2, RGS1, TNFRSF17, TXNDC5) with significant circadian patterns fitting a cosine model.
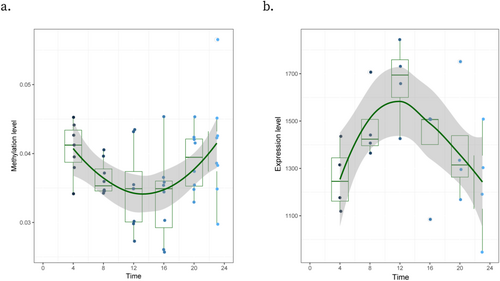
We annotated circadian CpG sites to Illumina 450K BeadChip reference and obtained the corresponding reference gene list, finding one gene (TXNDC5) with circadian oscillation for both DNA methylation and gene expression. TXNDC5 was also detected as one of our rhythmic genes best fitting cosine models. The Spearman correlation of TXNDC5's expression level and cg19116172's methylation level showed a marginally significant correlation (ρ = −0.83, p = 0.06). Besides, cg19116172 is located at 872 bp upstream of TXNDC5's transcriptome start-site. We drew the box plot to visualize this pair's pattern of methylation and expression levels, respectively. We found that the nadir of cg19116172 and the peak of TXNDC5 are located at the time point 12:00 (Figure 2). This suggests a negative regulation of DNA methylation on the gene's expression at that particular time.
3.3 Relation to Psychiatric Disorders
To investigate whether our significant circadian signals are correlated with psychiatric disorders, we compared the circadian CpG sites and their located genes with the psychiatric-related EWAS signals or genes. We found most of the circadian-related genes are enriched in schizophrenia (SCZ) and autism spectrum disorder (ASD) candidate genes, differential expression genes, or co-expression module genes (Figure 3).
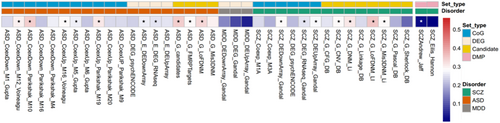
For ASD-related gene analysis, we observed significant enrichment of genes associated with circadian CpG site in the ASD- risk gene co-expression modules (max p = 7.11E−03), differentially expressed genes from psychENCODE, and other studies (max p = 1.99E−05), FMRP targets gene set (p = 4.55E−10), and candidate genes from AutDB (p = 3.01E−05).
For SCZ-related gene analysis, we observed significant enrichment of rhythmic genes with differentially expressed genes from psychENCODE and other studies (max p = 1.00E−03), missense de novo mutation genes (p = 1.52E−03). It is also enriched in loss of function de novo mutation genes (p = 4.49E−04) and copy number variance-related genes (p = 2.62E−3). Still, it was not enriched in genes identified by linkage, Sherlock, and Pascal. However, for MDD, we did not find an enrichment of circadian-related genes with differentially expressed or co-expressed genes.
At the CpG level, we used SCZ EWAS results from Hannon et al. (2016), which quantified DNA methylation from blood samples. Using an EWAS p-value less than 5.00e−05 as the cut-off, we extracted 1223 CpG loci associated with SCZ. Among these 1223 CpG sites, 5 CpGs associated with SCZ showed the circadian pattern in blood, but we did not find any enrichment. We also compared our rhythmic signals with the EWAS results of Jaffe et al. (Jaffe et al. 2016), which tested SCZ brains. Our circadian signals are enriched in these 2014 SCZ-related CpGs (p = 6.86E−03).
4 Validation
First, to verify whether this model is feasible, we simulate a set of data by fitting the standard cosine model shifting the amplitude and phase by 0.5 times. We found that all the fitting parameters (phase, acrophase, and amplitude) generated from this model matched the simulation data. Second, we drew a boxplot using the methylation level of each CpG site that showed a clear circadian pattern in 24 h. We then randomly selected 12 CpG signals from our circadian methylation sites and validated their methylation levels using the pyrosequencing method. All tested sites had DNA methylation levels that were significantly correlated between the two methods (p < 0.05). Ten of the tested CpG sites had a correlation coefficient p > 0.6.
5 Discussion
We explored the genome-wide DNA methylation and gene expression related to circadian rhythm in human blood in 24 h. We recruited eight young male adult volunteers. All these volunteers are healthy, non-smoking, have similar sleep habits, and within a relatively narrow age range. They did not have long-distance travel during the last 2 months before the test. We were able to avoid biases caused by these confounding factors and environmental-specific effects due to geographic location, all of which have been shown to impact gene expression variation and DNA methylation. The Illumina Human Methylation450K BeadChip and the Illumina HumanHT-12 v4 Expression BeadChip were used to provide genome-wide coverage of DNA methylation and mRNA expression, respectively. By doing so, we detected the negative circadian regulation in TXNDC5. Thioredoxin domain-containing protein 5 (TXNDC5) is a member of the protein disulfide isomerase (PDI) family, which catalyzes protein folding and thiol-disulfide exchange reactions (Sullivan et al. 2003). TXNDC5 is involved in apoptosis, migration, and antioxidative stress (Chawsheen et al. 2018; Horna-Terron et al. 2014). As a known functional gene expressed in the central nervous system, TXNDC5 has been reported to be associated with neuropsychiatric diseases. TXNDC5 showed a marginally significant association with schizophrenia after multiple testing adjustments (Lin et al. 2009) and transcript and expression changes in rat primary cortical neurons with tau isolated from Alzheimer's disease brains (Ficulle et al. 2022).
Additionally, we detected 6345 CpG sites that have epigenome-wide significant fit to the circadian pattern of methylation variation in blood. We also detected 21 genes that have genome-wide significant fit to the rhythmic oscillation in gene expression. Our results suggested that there are detectable daily rhythms for DNA methylation and RNA expression in human blood, even using a relatively, small sample size.
Our results are consistent with previous studies. To test the replication of our circadian genes with others, we checked five databases that focused on circadian expression genes in animal and human models. Our results were well replicated, except for those genes with LOC identifiers. All 21 significant circadian genes showed rhythmic in one or more databases. ABCB9 (ATP Binding Cassette Subfamily B Member 9), the membrane-associated protein encoded by this gene, is a member of the superfamily of ATP-binding cassette (ABC) transporters; also a rhythmic gene in these five databases and replicated in various tissues. CAMP, IGLL1, LCN2, MYOM2, and TXNDC5 also existed rhythmic in one or more such databases.
Our results indicate that DNA methylation and gene expression have circadian variations in a 24-h cycle. The DNA methylation has a moderately negative regulation on gene expression. A study found that 21 loci in the circadian rhythm genes were found to be significantly methylated in night shift workers compared with day shift workers, suggesting that methylation changes of these circadian rhythm genes may lead to differential changes in the expression of these genes between night shift and day shift workers (Bhatti et al. 2015). In the brain of dementia patients, the cycle of DNA methylation contributes to the regulation of the circadian rhythm of BMAL1, one of the core components of the biological clock (Cronin et al. 2017). Moreover, another study of mouse skeletal muscle cells showed that circadian expression might be regulated by rhythmic DNA methylation, but a strong rhythmic association between transcriptome and CpG methylation has not been established (Ali Altıntaş et al. 2020).
We found the CpG site, Cg19116172, had concerted variation with the nearby gene TXNDC5. The CpG site was 872 bps upstream from gene's transcriptome start-site, suggesting that CpG may work as the regulatory element. We detected a modest correlation between this CGP using Spearman's correlation analysis, which suggests a regulatory relationship. Previous findings similarly supported a causal role for DNA methylation as a modulator of circadian rhythms (Cronin et al. 2017; Li et al. 2020).
Moreover, we observed an inverse pattern between the boxplot of this pair's methylation and expression level, which also indicates negative regulation of DNA methylation to circadian gene expression. It was reported that selected genes in specific regions of the hamster brain have seasonal rhythms (Stevenson and Prendergast 2013). The seasonal rhythm of DNA methylation may affect these rhythms (Alvarado et al. 2015). In a recent human study, Lim et al. examined levels of DNA methylation sites across the genome in the post-mortem dorsolateral prefrontal cortices of 758 donors (Lim et al. 2017). They found significant 24-h rhythms and seasonal rhythms in the human brain DNA methylome and transcriptome. Yet, they did not investigate which methylation sites might show significant 24-h rhythms. The likely explanation for this discrepancy would be the coverage of methylation sites and the difference between techniques used for measuring DNA methylation. Our study examined individual DNA methylation sites on an epigenome-wide scale, including >400,000 CpG sites, whereas other recent studies examined only DNA methylation sites within promoter regions (Satou et al. 2013). Apart from these technical reasons, the difference in discrete circadian times examined in these studies may contribute to this discrepancy, too. For example, Baehr's study only tested at two time-points (Baehr, Revelle, and Eastman 2000). Limited time points are likely to reduce the study's power to detect DNA methylation rhythms. Whereas in the current study, we tested six time-points over 24-h.
We further explored relationships between the detected circadian methylation signals and various psychiatric disorders. We performed a multiplex comparison between circadian epigenetic signals as well as their corresponding genes with psychiatric-disorder-related genes or EWAS signals. We found a relatively high proportion of circadian-related genes, with 50% in SCZ and 53% in ASD, are enriched among candidate genes, differential expression genes, or co-expression modules associated with both conditions. Future studies of mechanisms in psychiatric disorders, circadian rhythms, or regulation may be taken into consideration.
There are several limitations to our study that should be addressed. First, we only detected 21 circadian genes due to the limited sample size, which may restrict the comprehensiveness and generalizability of our findings. The lack of female participants in our study prevents us from investigating potential sex differences in circadian gene expression, as highlighted in Xia et al. (2021). Additionally, our study focused exclusively on young men, limiting the age diversity of our sample. This age homogeneity means our findings may not be applicable to broader age groups.
All our peripheral samples were derived from bulk tissues, which might obscure circadian patterns specific to individual cell types. This limitation suggests that future studies should incorporate single-cell analyses to uncover cell type-specific circadian rhythms. Moreover, the circadian patterns observed in our study based on a single 24-h period. This approach could introduce randomness and does not account for day-to-day variability in circadian rhythms. Future research should investigate multiple time points across several days to provide a more robust and accurate understanding of circadian gene expression patterns.
Future research should incorporate larger and more diverse sample populations to enhance the robustness and generalizability of our findings. Including participants of different sexes, ages, and ethnic backgrounds will provide a more comprehensive understanding of circadian variations in DNA methylation and gene expression. Furthermore, investigating the potential mechanisms by which circadian methylation influences gene expression and contributes to the risk of psychiatric disorders is crucial. Moreover, exploring tissue biology in other peripheral human tissues, such as adipose and skin, can provide valuable insights into the circadian regulation of DNA methylation and gene expression.
By addressing these constraints, future studies can enhance the generalizability and depth of understanding of circadian gene expression and its implications for health and disease.
6 Conclusion
In summary, our results provide a critical baseline for further investigation into circadian dynamics of DNA methylation and gene expression in blood. We found that DNA methylation and gene expression have coordinated variations within 24 h. Our study identified significant methylation signals and expression signals, which could be related to psychiatric disorders. Notably, our findings suggest that TXNDC5 may be an important candidate for further investigating the molecular basis of psychiatric disorders.
Author Contributions
Haiyan Tang: data analysis, validation, writing – original draft, writing – review and editing. Shanshan Chen: data analysis, writing – original draft, writing – review and editing. Liu Yi: data acquisition, analysis, writing – review and editing. Sheng Xu, Huihui Yang, Zongchang Li, Ying He, Yanhui Liao, Lin Gu, Xiaogang Chen: data analysis, writing – original draft. Chunyu Liu: design, validation, writing – review and editing. Ning Yuan: design, data analysis, writing – review and editing. Chao Chen: design, data analysis, validation, writing – review and editing, funding acquisition. Jinsong Tang: design, data acquisition, analysis, validation, writing – review and editing, funding acquisition.
Acknowledgments
We gratefully acknowledge Professor Huayun Chen's advice on statistical analysis.
Conflicts of Interest
The authors declare no conflicts of interest.
Open Research
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.




