Low-grade parental gonosomal mosaicism in CHD2 siblings with Smith–Magenis-like syndrome
Abstract
Loss-of-function CHD2 (chromodomain helicase DNA-binding protein 2) mutations are associated with a spectrum of neurodevelopmental disorders often including early-onset generalized seizures, photosensitivity, and epileptic encephalopathies. Patients show psychomotor delay/intellectual disability (ID), autistic features, and behavior disorders, such as aggression and impulsivity. Most reported cases are sporadic with description of germline mosaicism only in two families. We detect the first case of parental gonosomal CHD2 mosaicism disclosed by two brothers showing mild ID, born to healthy parents. The eldest brother has a history of drug-controlled generalized tonic–clonic seizures and displays sleep disorder and aggressive behavior suggestive of Smith–Magenis syndrome (SMS). Analysis of brothers’ DNAs by next-generation sequencing (NGS) custom gene panel for pediatric epilepsy and/or ID disclosed in both the same pathogenic CHD2 variant. Additional NGS experiment on genomic DNA from parents’ peripheral blood and from buccal swab raised the suspicion of low-grade gonosomal mosaicism in the unaffected mother subsequently confirmed by digital polymerase chain reaction (dPCR). This report underlines as worthwhile CHD2 screening in individuals presenting ID/developmental delay, with/without epilepsy, and behavior and sleep disorders suggestive of SMS. Detecting a CHD2 variant should prime testing probands' parents by NGS coupled to dPCR on different tissues to exclude/confirm gonosomal mosaicism and define the recurrence risk.
1 INTRODUCTION
Neurodevelopmental disorders (NDDs) include a spectrum of conditions characterized by psychomotor delay and a variable range of intellectual disability (ID) signs, with/without autistic traits and behavioral problems. Among NDDs, there are developmental and epileptic encephalopathies (DEEs) characterized by early-onset and refractory seizures. Chromodomain helicase DNA-binding protein 2 (CHD2) has been recently associated with DEEs (Lamar & Carvill, 2018), and pathogenic variants in this gene have been reported in relation to childhood-onset epilepsy in almost all the 144 surveyed cases (Zhu et al., 2022). Myoclonic, atonic, absence, or generalized tonic–clonic seizures (GTCS) are prevalent in the second year of life, associated with photosensitivity in a variable percentage of cases (Caputo et al., 2018; Chen et al., 2020). Psychomotor delay and ID are observed in most cases, while autistic traits and behavior disorders, in particular aggression (Zhu et al., 2022), may be additional features.
We describe a family with two brothers characterized by mild ID and only the elder presented a history of pharmacologically controlled childhood epilepsy and behavior problems associated with sleep disorders. Based on his clinical manifestations, the first diagnostic suspect has been Smith–Magenis syndrome (SMS; MIM #182290). The identification of a pathogenic CHD2 variant, shared with his differently symptomatic brother, led to the detection by next-generation sequencing (NGS) on parents' tissues of a low-rate gonosomal mosaicism in the mother, then confirmed by digital polymerase chain reaction (dPCR).
2 MATERIALS AND METHODS
2.1 Subjects
The study was approved by IRCCS Istituto Auxologico Italiano Ethical Committee on 2013_03_12_14-Project MOH 08C305) and was conducted according to the Declaration of Helsinki and to the Italian legislation on sensitive personal data recording. Written informed consent to the genetic test was obtained by the probands and their parents.
Patient 1 came to the attention of the Clinical Genetics Unit at the age of 24 years for undiagnosed ID and epilepsy. The clinical presentation of the patient was consistent with a mild ID, although a developmental scale was not performed. He was born to nonconsanguineous parents and is the oldest of three siblings. The second-born brother was healthy.
The mother had uneventful pregnancy and delivery and developmental stages of the child were reported within the normal limits (autonomous walk and first words at about 15 months). Early intellectual problems emerged at primary school, when he required support from teachers. At 9 years of age, the child presented generalized tonic–clonic epilepsy, which became completely controlled by valproic acid only by the age of 22 years. A mild ID (intelligence quotient [IQ] not available) was confirmed at the age of 15 years, associated with behavioral disorders, such as aggressiveness and severe sleep disorders (present since childhood). When he was 15 years old, a diagnosis of insulin-dependent diabetes mellitus was made. At the age of 16 years, a depressive syndrome, currently in poor pharmacological compensation, and a related psychosis were diagnosed. He sometimes presents delusional fantasies. To be noted, he does not show obvious facial syndromic anomalies. Array-comparative genomic hybridization (CGH) analysis did not evidence significant copy number variants.
Patient 2, now aged 23 years, is the third-born brother of patient 1. Pregnancy and perinatal development were normal. He acquired autonomous walking at the age of 15 months, but showed a severe speech delay, with his first words at the age of 4 years. Relationship disorders emerged at school; the clinical presentation also suggested a mild ID as for his brother (IQ not available). He has never experienced seizures, nor sleep disturbances or aggressive behavior. He has been in therapy for panic attacks for 2 years. Array-CGH analysis showed a normal profile.
2.2 NGS custom panel for diagnostic analysis
Genomic DNA was extracted from peripheral blood leukocytes using the Freedom Evo TECAN extractor (TECAN, Mannedorf, Switzerland). Epithelial buccal cells were collected in Oragene tubes, and DNA was extracted according to the manufacturer's protocol (Oragene DNA kit; DNA Genotek Inc., a subsidiary of OraSure Technologies, Bethlehem, PA, USA).
Genomic sequencing of whole coding regions and intron-exon junctions (including 20 flanking nucleotides) of the following genes: MECP2, CDKL5, FOXG1, MEF2C, STXBP1, SCN1A, PCDH19, SCN2A, GABRA1, GABRG2, SCN1B, SCN8A, CHD2, SLC25A22, PNKP, SPTAN1, PLCB1, PQBP1, SYNGAP1, KCNT1, KCNQ2, ARHGEF9, SLC2A1, KDM5C, UBE3A, SLC9A6, TCF4, ZEB2, EHMT1, ATRX, CNTNAP2, NRXN1, RAI1, HDAC4, and MBD5 was performed using the Illumina Nextera Rapid Capture Enrichment protocol (Illumina, San Diego, CA, USA) according to the manufacturer's instructions, while the uncovered genomic regions (having coverage <20×) were analyzed either by Sanger Sequencing or by Nextera-XT-Library-prep protocol (Illumina) using a MiSeq Instrument (Illumina) for sequencing.
2.3 Variant validation
The identified variant was validated in probands by Sanger sequencing using a Big-Dye® Terminator v3.1 Cycle Sequencing Kit (Thermo Fisher Scientific, Foster City, CA, USA) and analyzed on an ABI Prism 3500 Sequencer (Thermo Fisher Scientific). The primers used for Sanger sequencing are CHD2-EX7F: 5′-GGGAGTAGGGCCCAAGAATA-3′ and CHD2-EX7R: 5′-CCCGGACTTATGAGAACCAA-3′.
DNA samples from patients’ parents were analyzed by Sanger sequencing and subsequently by the Nextera-XT-Library-prep protocol.
We analyzed microsatellite parental inheritance to confirm the correct family relationship and avoid any possible sampling error. Microsatellite analysis was performed using the commercial kit ChromoQuant® QF-PCR (CyberGene AB, Solna, Sweden) which evaluates polymorphic loci on chromosomes X, Y, 13, 18, and 21, according to the manufacturer's protocol, and analyzed on ABI Prism 3500 Sequencer.
2.4 Bioinformatic analysis
The primary bioinformatic analysis was conducted following the best practices proposed by Koboldt (2020). The analysis involved the creation of fastq files from raw sequencing data using BCL2FastQ software (https://emea.support.illumina.com/sequencing/sequencing_software/bcl2fastq-conversion-software.html). Next, the Burrows-Wheeler Aligner (BWA-MEM) in conjunction with SAM Tools was used to align the DNA sequences to the hg19 genome. Indel local realignment and recalibration steps were applied to improve alignment accuracy. Quality control and coverage analysis were performed using fastqc (https://www.bioinformatics.babraham.ac.uk/projects/fastqc/) and bedtools (https://bedtools.readthedocs.io/en/latest/) software as previously described (Gentilini et al., 2019).
For genetic variant calling, the GATK haplotype caller algorithm (https://gatk.broadinstitute.org/hc/en-us/articles/360035894711-About-the-GATK-Best-Practices) originally developed for germline variants was used. Additionally, a specific algorithm designed for somatic variant detection, Mutect2 (https://gatk.broadinstitute.org/hc/en-us/articles/360037593851-Mutect2), operating in single sample mode, was employed. The resulting genetic variants, including both germline and somatic variants, were reported in variant call format (VCF) files. Finally, the variants were annotated using the Annovar software (https://annovar.openbioinformatics.org/en/latest/) to provide additional information about their functional impact and genomic context.
2.5 Digital PCR
dPCR assays were performed on a QuantStudio™ 3D Digital PCR System platform (all products by Thermo Fisher Scientific), using a custom-designed TaqMan SNP Genotyping Assays (primers and probes are available at assay ID ANDKGZ9; the wild-type probe is FAM-labeled, the mutant probe is VIC-labeled). Briefly, reaction mixtures were prepared by combining 50 ng of genomic DNA with the QuantStudio™ 3D Digital PCR Master Mix-v2. Each reaction mixture was loaded onto a QuantStudio™ 3D Digital PCR Chip-v2, which contains 20,000 nanowells, and cycled under standard thermal conditions for 40 cycles. End-point fluorescence data were collected and analyzed using the QuantStudio™ 3D Digital PCR Instrument and the QuantStudio™ 3D AnalysisSuite™ software. In the analysis steps, we first performed a quality check: this was based on the number of partitions that exceeded the total number of wells filled correctly. Output data were reported as copies/μL (cpm) detected on the chip, which represents the number of observed mutant alleles in the reagent mixture plus DNA. Finally, we calculated the ratio between cmp relative to the mutated allele and the cmp for the wild-type one.
3 RESULTS
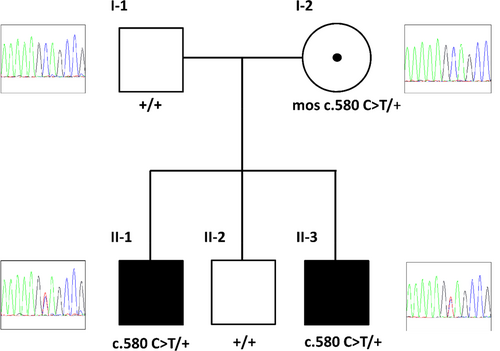
Proband 1 was addressed to the medical genetics laboratory with a clinical suspicion of SMS to investigate by NGS analysis the four main genes responsible for SMS and SMS-like syndrome: RAI1 (MIM *607642), HDAC4, MBD5, and TCF20. A custom panel of 35 genes involved in epilepsy and ID, including these four genes and, among others, the CHD2 gene, was sequenced. This analysis ruled out pathogenic variants in SMS genes, while unexpectedly a novel CHD2 heterozygous nonsense variant, NC_000015.9 g.93482836 C>T, NM_001271.3 c.580 C>T, p.(Gln194*) was identified and confirmed by Sanger sequencing. According to predictions, the variant should elicit mRNA degradation by nonsense-mediated mRNA decay (NMD). As CHD2 haploinsufficiency has been associated with a variable phenotype (Chénier et al., 2014) comprising developmental delay, ID, epilepsy (ranging from mild febrile seizures to severe epileptic encephalopathy) (Chen et al., 2020), and behavioral problems, the variant appeared compatible with the patient's and his younger brother's phenotypes. We did not identify any other variants of uncertain significance (VUS) consistent with the clinical picture. The subsequent Sanger sequencing analysis detected the same variant in the affected patient 2 and, conversely, its absence in the DNA from the peripheral blood of the healthy brother and parents, supporting its pathogenicity (Figure 1). To distinguish between a parental condition of germline mosaicism from low-grade gonosomal mosaicism, which escapes detection by Sanger sequencing, NGS was performed on the DNA from peripheral blood and buccal swabs of the parents and the healthy brother. This analysis highlighted only in the mother's buccal swab the presence of the CHD2 variant, with a variant allele frequency (VAF) of ~1% (Table 1, left). To confirm or exclude this finding, dPCR was carried out on parents' DNA samples obtained from peripheral blood and buccal swabs. The presence of the pathogenic variant was detected only in the cells of the mother's buccal epithelium, with a similar percentage to that calculated by the Mutect2 algorithm used for NGS data analysis (Table 1, right). These data highlight the presence of somatic gonosomal mosaicism in the patients’ mother.
| Nextera-XT-Library-prep NGS protocol | Digital PCR | ||||||
|---|---|---|---|---|---|---|---|
| Sample | Reference allele mean counts | Alternative allele mean counts | Alt/ref ratio | Reference allele, no. copies/μL (% precision) | Alternative allele, no. copies/μL (% precision) | Alt/ref ratio | |
| Peripheral blood | Father | 8893.7 | 7.3 | 0.001 | 1554.4 (1.96) | 0 (NA) | 0 |
| Mother | 6207.0 | 7.3 | 0.001 | 1123.2 (1.91) | 0.65 (92.21) | 0.001 | |
| Buccal swab | Father | 8535.5 | 8.5 | 0.001 | 701.5 (2.52) | 0 (NA) | 0.000 |
| Mother | 9459.7 | 102.0 | 0.011 | 573.9 (2.66) | 6.3 (25.03) | 0.011 | |
- Note: Left: mean base counts of the reference and alternative alleles (chr15:93482836) and the calculated alt/ref ratio obtained from NGS. Results were obtained from three different amplifications in two independent experiments. Right: the normalized number of copies with the associated precision values (Laig et al., 2020) and the calculated alt/ref ratio for the digital PCR experiment.
4 DISCUSSION
SMS is typically due to haploinsufficiency of RAI1, coding for a transcriptional factor involved in multiple functions, including embryonic neurodevelopment and neuronal differentiation, behavioral functions, and circadian activity. The SMS phenotype is characterized by distinctive facial features, developmental delay, mild-to-moderate ID, behavioral abnormalities (including severe sleep disturbance, with disruption of the circadian rhythm, stereotypies, temper tantrum, impulsivity, aggression, primarily self-inflicted or directed toward others, injurious behaviors, with particularly early onset and childhood onset) and abdominal obesity (Falco et al., 2017; Rinaldi et al., 2022). Since childhood, proband 1 displayed a severe alteration of the sleep–wake cycle, as well as aggressive behavior, mainly directed against others, clinical features that summed up to psychomotor retardation and mild ID, oriented clinicians toward the diagnostic suspicion of SMS. GTCS epilepsy, which occurred in childhood, completed the clinical picture, although epilepsy is not so frequent in RAI1-deficient patients (Carmona-Mora & Walz, 2010; Rinaldi et al., 2022). Conversely, the younger brother, later evaluated by clinicians, never displayed epilepsy, or other peculiar characteristics of SMS while suffering from relational disorders and panic attacks (anxiety). CHD2-related syndrome is typically a DEE: although CHD2 pathogenic variants have been reported in patients with NDD without epilepsy, 92% of patients with CHD2 manifest, during their lifetime, different seizure types (De Maria et al., 2022) such as GTCS, myoclonic, absences, focal-onset, myoclonic jerks (eyelid myclonia), atonic, myoclonic–atonic, tonic, and epileptic spasms. Furthermore, seizures have a wide onset time, ranging from 3 months to as long as 22 years (median age 2.5 years), and are drug-resistant in about three-fourths of the patients (Zhu et al., 2022); the nonresistant ones are generally responsive to sodium valproate (Chen et al., 2020). Photosensitivity, generally rare in other forms of epilepsy, is reported in the literature with variable percentages, ranging from 45% (De Maria et al., 2022) to 70% (Thomas et al., 2015). The epileptic picture of proband 1 overlaps with the phenotype typical of the CHD2-related syndromes. In the majority of the reported cases, a developmental delay mostly affecting speech (as in proband 2) is noticed before seizure onset, as well as aggressive and impulsive behavior, repetitive behaviors, more rarely ADHD and autism spectrum disorder (ASD) and severe psychiatric profile (Bernardo et al., 2017). These behavioral characteristics are compatible with the clinical picture of proband 1 and partially overlap those of RAI1-deficient patients. Unlike RAI1-deficient patients, to the best of our knowledge, alteration of the sleep pattern is reported in the literature only in two CHD2-mutated cases: a patient who carries a CHD2 stop mutation and, like proband 1, presents psychotic traits (Poisson et al., 2020) and two monozygotic twins both showing alteration of the circadian rhythm (A. M. Pinto et al., 2016). The overall findings suggest the importance of considering CHD2 involvement in children with developmental delay, ID with or in the absence of epilepsy, even at late onset, behavioral disturbances, in particular aggressiveness and sleep disorder reminiscent of RAI1 defects.
Finally, the two brothers carrying the same pathogenic variant, express a different phenotype, not only as regards epilepsy but also behavioral and sleep disturbances. This discrepancy has been similarly observed in reported family cases where affected family members show differences in clinical manifestation (Feng et al., 2022; Lebrun et al., 2017; Petersen et al., 2018) as well as in unrelated patients with the same pathogenic variant, but discrepant phenotypes (De Maria et al., 2022). The overall evidence points to the difficulty of establishing a genotype–phenotype correlation for CHD2-caused disorder and to the existence of genetic and environmental modifiers.
The CHD2 gene codes for a chromatin assembly ATPase, which catalyzes the assembly of chromatin into periodic arrays of nucleosomes (Liu et al., 2015) and modulates gene expression via the ability to remodel chromatin structure (Chénier et al., 2014). To date (July 2023) more than 140 CHD2 variants associated with clinical phenotype are reported in the Human Gene Mutation Database HGMD professional (https://www.hgmd.cf.ac.uk/ac/index.php), including partial or complete deletion of the gene, as well as point mutations with a prevalence of nonsense/truncating variants, likewise, the one here described. These variants generally lead to mRNA degradation by NMD (De Maria et al., 2022). Our data confirm that the pathogenic mechanism determining the CHD2-associated phenotype is haploinsufficiency (Lamar & Carvill, 2018) as demonstrated by studies in zebrafish models (Suls et al., 2013).
The mutations are almost exclusively de novo: only five variants were inherited from asymptomatic (Chen et al., 2020; Feng et al., 2022; Galizia et al., 2015), mildly affected (Chen et al., 2020) or affected parents (Petersen et al., 2018). Six cases with more than one affected family members are described (Chen et al., 2020; Feng et al., 2022; Galizia et al., 2015; Lebrun et al., 2017; Petersen et al., 2018; A. M. Pinto et al., 2016) including two cases of germline mosaicism (Lebrun et al., 2017; D. Pinto et al., 2014) (see Table 2).
| Patient 1 | Patient 1 phenotype | Patient 2 | Patient 2 phenotype | Inheritance | Parent phenotype | Mosaicism | Pathogenetic variant | References | |
|---|---|---|---|---|---|---|---|---|---|
| 0 | Male | Mild ID-epilepsy (GTCS)-behavioral disorders (aggressiveness) and severe sleep disorder | Male | Mild ID | Maternal (gonosomal mosaicism) | Normal | Gonosomal mosaicism | p.(Gln194*) | Our case |
| 1 | Male | ASD-ID-epilepsy (AS and GTCS) responsive to antiepileptic drug-behavioral disorders (aggressiveness and repetitiveness) | Male | ASD-mild ID-behavioral disorders (aggressiveness) | De novo | Normal | Germline mosaicism | p.(Thr645Met) | Lebrun et al. (2017) |
| 2 | Male | ASD-mild ID | Male | ASD-mild ID-epilepsy | De novo | Normal | Germline mosaicism | 83Kb deletion (removes first six exons of CHD2) | D. Pinto et al. (2014) |
| 3 | Female | ID-global developmental delay-drug-resistant epilepsy-ADHD | Mother | Bipolar disorder-ADHD-language delay-epilepsy responsive to antiepileptic drug | Maternal | Affected | p.(Glu210*) | Petersen et al. (2018) | |
| 4 | Male (dyzigotic twin) | Epilepsy (GTCS-SE-FS+) | Male (dyzigotic twin) | Epilepsy (GTCS-FS+) | Paternal | Febrile seizures in infancy | p.(Met1744Ile) | Chen et al. (2020) | |
| 5 | Female (monozygotic twin) | ASD-ID-epilepsy (AS)-stereotypic movements-circadian rhythm alteration | Female (monozygotic twin) | ASD-ID-epilepsy (AS)-stereotypic movements-circadian rhythm alteration | De novo | Normal | p.(Gln1392Thrfs*17) | A. M. Pinto et al. (2016) | |
| 6 | Male | Epilepsy (EMA) | - | - | Maternal | Normal | Incomplete penetrance? | p.(Pro218Leu) | Galizia et al. (2015) |
| 7 | Male | Developmental delay-epilepsy (GTCS-MS) responsive to antiepileptic drug | - | - | Paternal | Normal | Incomplete penetrance? | c.5153+2 T>C | Chen et al. (2020) |
| 8 | Male | Developmental delay-epilepsy (GTCS-AS-AtS) | Female | Normal intelligence-epilepsy (GTCS) responsive to antiepileptic drug | Paternal | Normal | Incomplete penetrance? | p.(Val1246Leu)/VUS? | Feng et al. (2022) |
- Abbreviations: ADHD, attention-deficit hyperactivity disorder; AS, absence seizures; ASD, autism spectrum disorder; AtS, atonic seizures; EMA, eyelid myoclonia with absences; FS+, febrile seizure plus; GTCS, generalized tonic–clonic seizures; ID, intellectual disability; MS, myoclonic seizures; SE, status epilepticus.
Our siblings inherited a pathogenic CHD2 variant from their unaffected mother, and although the presence of the pathogenic variant has not been directly assessed in gonadal tissue, our family represents the third case of germline mosaicism and the first case of gonosomal mosaicism involving CHD2. The NGS analysis for somatic variants on the DNA from the mother's buccal swab allowed us to suspect a condition of gonosomal mosaicism. Parental gonosomal mosaicism with a higher number of cells has been described for other genes responsible for epileptic encephalopathies (Stosser et al., 2018). Our data allow to hypothesize that gonosomal mosaicism may underlie germline mosaicism, relatively frequent in the CHD2 gene, although its real frequency is yet unestimated. Dedicated NGS somatic analyses and dPCR may represent a powerful tool to explore the real occurrence of this phenomenon, which may have a relevant impact on genetic counseling, considering that in the presence of gonosomal mosaicism the recurrence risk is significantly higher (Bai et al., 2023). Additional molecular investigation by high-coverage NGS/dPCR on at least two parental tissues should be routinely performed to detect parental mosaicism and define a proper recurrence risk, considering also that low levels of mosaicism in the blood could be predictive of higher levels in other tissues (Rahbari et al., 2016). Even if it is challenging, it could be proposed also to couples with a single child carrying a de novo pathogenic variant in the CHD2 gene.
AUTHOR CONTRIBUTIONS
Francesca Cogliati and Silvia Russo conceived the study. Francesca Cogliati, Sara Perego, and Maura Masciadri carried out the experiments and analyzed the NGS genetic data. Berardo Rinaldi, Donatella Milani, and Maria Francesca Bedeschi clinically evaluated the patients and hypothesized the clinical suspicion. Letizia Straniero, Valeria Rimoldi, and Rosanna Asselta performed and interpreted the dPCR experiment. Davide Gentilini handled bioinformatics data analysis. Francesca Cogliati drafted the manuscript. Silvia Russo and Lidia Larizza read and revised the manuscript with the help of Maria Francesca Bedeschi and Rosanna Asselta. Silvia Russo recruited the funding for the study. All authors read and approved the final manuscript.
ACKNOWLEDGMENTS
We acknowledge the patients' family for the collaboration.
FUNDING INFORMATION
This work was funded by BIBLIOSAN.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflicts of interest.
CONSENT STATEMENT
Written informed consent and consent to publish were obtained from the family.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are openly available in Harward dataverse at https://doi.org/10.7910/DVN/O46MPP.




