Metabolomics in patients with psychosis: A systematic review
Abstract
The purpose of this article is to provide a comprehensive review of metabolomics studies for psychosis, as a means of biomarker discovery. Manuscripts were selected for review if they involved discovery of metabolites using high-throughput analysis in human subjects and were published in the last decade. The metabolites identified were searched in Human Metabolome Data Base (HMDB) for a link to psychosis. Metabolites associated with psychosis based on evidence in HMBD were then searched using PubMed to explore the availability of further evidence. Almost all of the studies which underwent full review involved patients with schizophrenia. Ten biomarkers were identified. Six of them were reported in two or more independent metabolomics studies: N-acetyl aspartate, lactate, tryptophan, kynurenine, glutamate, and creatine. Four additional metabolites were encountered in a single metabolomics study but had significant evidence (two supporting articles or more) for a link to psychosis based on PubMed: linoleic acid, D-serine, glutathione, and 3-hydroxybutyrate. The pathways affected are discussed as they may be relevant to the pathophysiology of psychosis, and specifically of schizophrenia, as well as, constitute new drug targets for treatment of related conditions. Based on the biomarkers identified, early diagnosis of schizophrenia and/or monitoring may be possible.
1 INTRODUCTION
Psychosis is characterized by abnormalities in an individual's perceptions, thoughts, mood, and behavior. It can be a feature of different heterogeneous psychiatric illnesses, such as schizophrenia and bipolar depression. Currently, psychotic disorders are classified and diagnosed according to the fifth edition of the Diagnostic and Statistical Manual of Mental Disorders (DSM-5) (Heckers et al., 2013). Unlike previous editions of the DSM, the DSM-5 aims to capture the underlying dimensional structure of psychosis. However, there is no laboratory diagnostic test that can be used on a clinical basis for the diagnosis and/or monitoring of psychotic disorders at the pathophysiology level. This hinders the elucidation of the different underlying pathways affected and the identification of novel candidate drugs (Casey et al., 2013).
Metabolomics, the comprehensive study of the different biochemical substances found in the body's cells, tissues, and fluids, has shown some promise with regards to biomarker discovery in psychosis. Metabolomics is able to provide a functional readout of the internal physiological state and thus constitutes a close representation of the individual's overall health status (He et al., 2012). It is based on analysis techniques capable of rapidly surveying hundreds of metabolites all at once.
Through metabolomics, biochemical signatures can be determined capturing changes in disease state, allowing subclassification of diseases, identification of biomarkers for drug response, and insight into disease mechanisms (He et al., 2012). Holmes et al. used 1H NMR to measure 152 samples of cerebrospinal fluid and found specific metabolites to be differentially regulated in patients with schizophrenia versus their healthy control counterparts (Holmes et al., 2006). The results of this pioneering study indicated that the identification of biomarkers in different body fluids could improve the understanding of schizophrenia and help diagnose its early onset. Subsequently, numerous other studies using metabolomics have been conducted to discover biomarkers in patients with psychosis. The potential of metabolomics for early diagnosis, monitoring and treatment response in patients with psychotic disorders, as well as the discovery of novel drug targets, underscores the importance to review this literature in a systematic way so as to facilitate further progress in this area of medicine.
2 RESEARCH DESIGN AND METHODS
2.1 Data sources and searches
We conducted a systematic search of published literature in 10 databases: Africa-Wide Information (Ebsco), Biosis (Ovid), Cochrane (Wiley), Embase (Ovid), Global Health (Ovid), LILACs, Medline (Ovid), PsycINFO (Ovid), PubMed (NLM), and Web of Science (Thomson Reuters). The systematic search included publications from inception until October 10, 2017 with no language restrictions. Articles identified in this search included variations of the terms metabolomics or mass spectrometry or magnetic resonance spectroscopy combined with psychosis or psychosis disorders, found as textwords in the title, abstract, or keyword fields, as well in the medical subject headings (MeSH). Animal studies were excluded as well as comments, editorials, letters, or conferences.
2.2 Study selection and eligibility criteria
There were 9,381 records identified through database searching, and 4,516 remained after duplication removal. Titles and abstracts of the 4,516 articles were independently screened by at least two independent reviewers (C.L., E.Z., X.W.) for eligibility. Studies were eligible for inclusion if they were published within the last 10 years (2007–2017), non-postmortem human studies, and if metabolites were identified using high-throughput metabolomics techniques. In cases where there was no consensus with regards to eligibility, the final decision was made after independent review of the information by the senior author of this study (Y.T.). A total of 107 articles were identified meeting our eligibility criteria.
2.3 Data extraction
From each study, we extracted the following information: author(s), year of publication and journal, study name, sample size of patients and healthy controls, patient drug therapies, analytical platform, sample type, psychosis disorder type, and significant biomarker findings.
2.4 Data analysis and organization
As part of data extraction, every significant reported metabolite was recorded from each article. These metabolites were subsequently searched on Human Metabolome Data Base (HMDB, www.hmdb.ca) by inputting the metabolite name in the search bar and selecting “metabolites” on the drop-down menu. Metabocards for each metabolite were screened for information regarding their link to psychosis; no further investigations were conducted for metabolites with no indicated link to psychosis. Metabolites with a known link to psychosis in HMDB were subsequently searched and screened by three reviewers (A.W., X.W., J.R.) on PubMed (www.ncbi.nlm.nih.gov/pubmed/) using the Advanced Search tool. In the Advanced Search builder, the following two searches were conducted: metabolite name AND “psychosis” and metabolite name AND “schizophrenia.” Additional search filter criteria included publication dates from the past 10 years and studies involving humans only. Article titles and abstracts were screened for links between the metabolite and psychosis. The complete screening methodology can be found in the included PRISMA flowchart and database search methodology (Supporting Information section).
3 RESULTS
All results from the literature search were tabulated. Table 1 summarizes the metabolites identified in at least two metabolomics studies from the articles meeting our eligibility criteria. Across the 107 initially screened articles, six metabolites were reported more than once. These metabolites were: N-acetylaspartate (NAA), glutamate, kynurenine, lactate, tryptophan, and creatine.
| Author | Metabolite | Disorder | Sample type | Analyticalplatform | NC | NP | Upregulated ordownregulated inpatients |
|---|---|---|---|---|---|---|---|
| Larabi, DI, 2017 | NAA | SCZ | Prefrontal white matter | 1H MRS | 35a | 88a | Downregulated |
| Kirtas, D, 2016 | NAA | SCZ | Thalamus, Hippocampus | 1H MRS | 30a | 49a | Downregulated |
| Jessen, F, 2013 | NAA | SCZ | Left frontal lobe, ACC | 1H MRS | 20a | 20a | Downregulated |
| Ohrmann, P, 2007 | NAA | SCZ | N/A | 1H MRS | 20b | 35b | Downregulated |
| Szulc, A, 2007 | NAA | SCZ | N/A | 1H MRS | 21a | 58a | Downregulated |
| Wood, SJ, 2008 | NAA | SCZ | N/A | 1H MRS | 19c | 34c | Downregulated |
| Mondino, M, 2013 | NAA | SCZ | Prefrontal cortex | 1H MRS | 234c | 208c | Downregulated |
| Seese, RR, 2011 | NAA | SCZ | CSF | 1H MRS | 34a | 28a | Upregulated |
| Goto, N, 2011 | NAA | SCZ | Serum | 1H MRS,HPLC-ECD | 18c | 18c | Downregulated |
| Molina, V, 2007 | NAA | SCZ, BPD | CSF | 1H MRS | 24a | 10a | Downregulated |
| Fukushima, T, 2014 | Glutamate | SCZ | Serum | HPLC | 27a | 25a | Upregulated |
| Kraguljac, NV, 2013 | Glutamate | SCZ | Hippocampus | 1H MRS | 27a | 27a | Upregulated |
| Oresic, M, 2011 | Glutamate | SCZ | Serum | UPLC-MS,GC-TOFMS | 139a | 139a | Upregulated |
| Plitman, E, 2016 | Glutamate | SCZ | N/A | 1H MRS | 63b | 64b | Upregulated |
| Friedman, JI, 2008 | Glutamate | SCZ | Brain white matter | 1H MRS | 17a | 23a | Upregulated |
| Fukushima, T, 2014 | Kynurenine | SCZ | Serum | HPLC | 27a | 25a | Upregulated |
| Linderholm, KR, 2012 | Kynurenine | SCZ | CSF | RP-HPLC | 29a | 16a | Upregulated |
| Olsson, SK, 2010 | Kynurenine | BPD | CSF | HPLC | 23a | 31a | Upregulated |
| Kegel, ME, 2014 | Kynurenine | SCZ | CSF | LC-MS | 26a | 22a | Upregulated |
| Rowland, LM, 2016 | Lactate | SCZ | CSF | 1H MRS | 29a | 27a | Upregulated |
| Fukushima, T, 2014 | Lactate | SCZ | Serum | HPLC | 27a | 25a | Upregulated |
| Holmes, E, 2006 | Lactate | SCZ | CSF | 1H MRS | 70b | 82b | Downregulated |
| Chiappelli, J, 2016 | Tryptophan | SCZ | Plasma | 1H MRS, RP-HPLC | 38a | 37a | Downregulated |
| Fukushima, T, 2014 | Tryptophan | SCZ | Serum | HPLC | 27a | 25a | Upregulated |
| Barry, S, 2009 | Tryptophan | SCZ | Plasma | HPLC | 36b | 34b | Downregulated |
| Kageyama, Y, 2017 | Creatine | SCZ | Plasma | CE-TOFMS | 19a | 17a | Downregulated |
| Koike, S, 2014 | Creatine | SCZ | Plasma | CE-TOFMS | 38c | 30c | Upregulated |
| Seese, RR, 2011 | Creatine | SCZ | CSF | 1H MRS | 34a | 28a | Upregulated |
| Ongur, D, 2009 | Creatine | SCZ, BPD | CSF | 1H MRS | 22a | 30a | Downregulated |
- Note. A summary of metabolites identified in at least two metabolomics studies from the articles meeting our eligibility criteria. Six metabolites were reported more than once across the 107 initially screened articles. These metabolites were: N-acetylaspartate (NAA), glutamate, kynurenine, lactate, tryptophan, and creatine. ACC = anterior cingulate cortex; BPD = bipolar disorder; CSF = cerebrospinal fluid; CE-TOFMS = capillary electrophoresis-time of flight mass spectroscopy; GC-TOFMS = gas chromatography-time of flight mass spectroscopy; GC-MS = gas chromatography-mass spectroscopy; 1H MRS = proton magnetic resonance spectroscopy; HPLC = high pressure liquid chromatography; HPLC-ECD = high pressure liquid chromatography-electrochemical detection; LC-MS = liquid chromatography-mass spectroscopy; N/A = not applicable; NC = number of controls; NP = number of patients; RP-HPLC = reverse-phase HPLC; SCZ = schizophrenia; UPLC-MS = ultra-performance liquid chromatography-mass spectroscopy.
- a Study involving CP patients only.
- b Study involving both FEP and CP patients.
- c Study involving FEP patients only.
Table 2 summarizes metabolites which were only found in one metabolomics study, but had significant evidence in the PubMed database, hereby defined as at least two supporting articles, for a link with psychosis. In our literature screen, we encountered 48 other metabolites that were reported once by a study on our list. We investigated each of these metabolites individually using HMDB and PubMed to search for further published evidence linking the metabolite to psychosis. There were nine metabolites that had two or more articles in PubMed highlighting a link to psychosis: linoleic acid, D-serine, 3-hydroxybutyrate, glutathione, homovanillic acid (HVA), 5-hydroxyindoleaceatic acid (5-HIAA), 3-methoxy-4-hydroxyphenylglycol (MHPG), glutamate and glutamine (Glx), and myoinositol. Some details (diagnosis, sample type, analytical platform, and numbers of affected vs. unaffected individuals) of the articles reporting each metabolite are summarized in Tables 1 and 2. Supporting Information Tables 1 and 2 provide additional details (study design, numbers of medicated vs. unmedicated patients) about each study mentioned in Tables 1 and 2.
| Author | Metabolite | Disorder | Sampletype | Analyticalplatform | NC | NP | Upregulated ordownregulatedin patients |
|---|---|---|---|---|---|---|---|
| Fukushima, T, 2014 | Linoleic acid | SCZ | Serum | HPLC | 27a | 25a | Downregulated |
| Xuan, J, 2011 | Linoleic acid | SCZ | Serum | GC-MS | N/Aa | N/Aa | Downregulated |
| Hashimoto, K, 2003 | D-Serine | SCZ | Serum | HPLC | 42a | 42a | Downregulated |
| Fukushima, T, 2014 | D-Serine | SCZ | Serum | GC-MS | 27a | 25a | Downregulated |
| Fukushima, T, 2014 | 3-Hydroxybutyric acid | SCZ | Serum | HPLC | 27a | 25a | Downregulated |
| Yang, J, 2013 | 3-Hydroxybutyric acid | SCZ | Urine | GC-TOFMS | 110a | 112a | Upregulated |
| Cai, HL, 2012 | 3-Hydroxybutyric acid | SCZ | Urine | UPLC-MS, 1H MRS | 30b | 32b | Upregulated |
| Ballesteros, A, 2013 | Glutathione | SCZ | Blood | N/A | 25a | 29a | Downregulated |
| Fukushima, T, 2014 | Glutathione | SCZ | Serum | GC-MS | 27a | 25a | Downregulated |
| Palsson, E | Homovanillic acid | BPD | CSF | HPLC | 113a | 175a | Upregulated |
| Palsson, E, 2017 | MHPG | BPD | CSF | HPLC | 113a | 175a | Downregulated |
| Curcic-Blake, B, 2017 | Glx | SCZ | N/A | MRS | 30a | 67a | Downregulated |
| Chiu, PW, 2017 | Glx | SCZ | N/A | 1H-MRS | 14b | 19b | Upregulated |
| Chiu, PW, 2017 | Myoinositol | SCZ | N/A | 1H-MRS | 14b | 19b | Upregulated |
- Note. A summary of metabolites only identified in only one metabolomics study, but had evidence a link to psychosis in two or more articles in PubMed. ACC = anterior cingulate cortex; BPD = bipolar disorder; CE-TOFMS = capillary electrophoresis-time of flight mass spectroscopy; CSF = cerebrospinal fluid; GC-MS = gas chromatography-mass spectroscopy; GC-TOFMS = gas chromatography-time of flight mass spectroscopy; Glx = glutamine and glutamate; 1H MRS = proton magnetic resonance spectroscopy; HPLC = high pressure liquid chromatography; HPLC-ECD = high pressure liquid chromatography-electrochemical detection; LC-MS = liquid chromatography-mass spectroscopy; MHPG = 3-methoxy-4-hydroxyphenylglycol; N/A = not applicable; NC = number of controls; NP = number of patients; SCZ = schizophrenia; UPLC-MS = ultra-performance liquid chromatography-mass spectroscopy
- a Study involving CP patients only.
- b Study involving FEP patients only.
Figure 1 diagrammatically compares the biomarker trend among medicated and unmedicated patients with psychosis. Overall, there are insufficient studies to conclude about differences in trends between these two groups. Downregulation of MHPG and upregulation of myoinositol, 5-HIAA, and HVA are seen in medicated patients only; however, each of these metabolites was mentioned in one study only. Kynurenine is a potential biomarker for the medicated state psychosis, as it is upregulated in four studies and all of them were focused exclusively on medicated individuals. NAA is a potential biomarker for psychosis in both medicated and unmedicated states. It is downregulated in six studies with medicated and two with unmedicated patients.
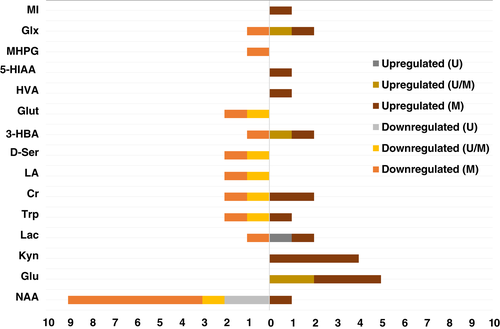
Furthermore, Supporting Information Table 3 displays the trends of the five biomarkers identified in first-episode psychosis (FEP) patients, while Supporting Information Table 4 the trends of the 13 biomarkers identified in chronic psychosis (CP) patients. More specifically, Supporting Information Table 3 summarizes the metabolites identified in at least two metabolomics studies (NAA, Glx, creatine), or in one metabolomics study with evidence linked to psychosis in two or more articles in PubMed (myoinositol and 3-hydroxybutyrate), in FEP patients. Three independent metabolomics studies showed downregulation of NAA. There were no clear trends for Glx, creatine, myoinositol, and 3-hydroxybutyrate. In contrast, Supporting Information Table 4 displays the trends for biomarkers in CP patients. These metabolites were identified in at least two metabolomics studies (NAA, glutamate, kynurenine, tryptophan, creatine, lactate, 3-hydroxybutyrate, MHPG, D-serine, glutathione, and linoleic acid) or in one metabolomics study with evidence linked to psychosis in two or more articles in PubMed (HVA and Glx). NAA, tryptophan, MHPG, D-serine, glutathione, and linoleic acid were found to be downregulated in CP, whereas glutamate, kynurenine, and lactate were found to be upregulated. No trend was identified for creatine, 3-hydroxybutyrate, HVA, and Glx, due to conflicting results or insufficient studies.
4 DISCUSSION
Across the 107 initially screened articles, six metabolites were reported more than once: glutamate, kynurenine, tryptophan, NAA, lactate, and creatine. Five of the six metabolites followed a clear regulation trend across all related studies (Table 1). Supporting Information Figure 1 shows a visualization of the pathways that these six metabolites are implicated in.
The majority of the studies reviewed hereby were conducted in schizophrenia patients. Only two studies examined the dysregulation of metabolites in patients with bipolar disorder. Olsson and coworkers identified an upregulation of kynurenine (Olsson et al., 2010) and Palsson and coworkers identified an upregulation of HVA and a downregulation of MHPG (Palsson et al., 2017). Removal of these two studies would result in the removal of HVA and MHPG from Table 2 but would otherwise not affect our analysis. Hence, although our article was focused on psychosis, most of the findings below can also be extrapolated to patients specifically diagnosed with schizophrenia. Supporting Information Figure 2 combines different key metabolites identified in our study (Tables 1 and 2), which are found in interrelated pathways.
As summarized in Table 1, tryptophan was consistently reported to be downregulated, while kynurenine was upregulated in our study. These findings could be explained by an increase in tryptophan degradation. Tryptophan is an essential amino acid which is known to be a precursor of the neurotransmitter serotonin. Tryptophan is also a precursor for kynurenine, which has a highly active metabolic pathway in psychosis (Barry, Clarke, Scully, & Dinan, 2009; Chiappelli et al., 2016). Of note, kynurenine (a product of tryptophan metabolism) can be further metabolized into a neuroprotective agent called kynurenic acid as well as a neurotoxic agent called quinolinic acid. In neurodegenerative disorders including schizophrenia, an imbalance of these kynurenine metabolites is observed (Barry et al., 2009). The kynurenine pathway is known to be disrupted in schizophrenia; the kynurenic acid hypothesis of schizophrenia postulates that kynurenic acid is highly elevated in the brain and blocks glutamate receptors (Erhardt, Schwieler, Imbeault, & Engberg, 2017).
Moreover, our results indicate that glutamate was upregulated in patients with psychosis (Table 1). Glutamate is a nonessential amino acid and the most abundant excitatory neurotransmitter in the brain. It is used by nearly all excitatory synapses in the central nervous system. Different studies have put forth evidence supporting the glutamate hypothesis. This literature suggests that glutamatergic neurotransmission dysfunction is a central contributor to the pathogenesis of schizophrenia (Egerton & Stone, 2012). Glutamate studies in schizophrenia have generally shown that the neurotransmitter is elevated during early stages of the disease onset (Bustillo et al., 2011). Furthermore, the elevation in glutamate has been noted to possibly contribute to the negative symptoms observed in patients with schizophrenia (Egerton et al., 2012). Excessive concentrations of glutamate in the extra-cellular space of the brain cause “excitotoxicity,” a neurotoxic effect resulting in over-activation of its receptors and cell death. Therefore, levels of glutamate within the cells in the nervous system are strictly regulated by mechanisms like the conversion of glutamate to glutamine via glutamine synthetase. Further, glutamine is involved in GABA synthesis (He et al., 2012). Of note, Glx, which is a measurement of glutamate and glutamine together, was found to be upregulated in patients with schizophrenia across 17 articles and reportedly downregulated in seven articles (Table 2). Table 2 also shows that D-serine was reported to be downregulated in psychosis in the studies analyzed. D-serine is a highly abundant amino acid in the brain, which, alongside glutamate, is known to act as a co-agonist of the N-methyl-D-aspartate (NMDA) receptor (Billard, 2008; Fukushima et al., 2014). Its downregulation plays a crucial role in the hypofunction of these glutamatergic receptors, therefore contributing to the symptoms associated with schizophrenia including negative symptoms and cognitive impairment (Labrie, Wong, & Roder, 2012).
In summary, thus far, we have shown that three (tryptophan, kynurenine, glutamate) of the six metabolites reported as biomarkers for psychosis in more than one metabolomics studies map to closely linked metabolic pathways (Table 1; Supporting Information Figure 1). We have also shown that two (D-serine, Glx) of the nine biomarkers having two or more published articles in PubMed for a link to psychosis are also relevant to these pathways (Table 2; Supporting Information Figure 2). If one takes in consideration, the prior literature suggesting that elevated kynurenine levels appear to be related to psychotic symptoms and cognitive impairments due to its ability to control glutamatergic and dopaminergic neurotransmission (Erhardt et al., 2017), two more metabolites from Table 2 (namely, MHPG and HVA) could be linked to this emerging pathway. Monoaminergic signaling is known to be altered in the pathophysiology of psychosis as part of the dopamine hypothesis of schizophrenia. HVA is a dopamine metabolite, and hyperactive dopaminergic signaling is a key idea in the dopamine hypothesis of schizophrenia. HVA was shown to be upregulated in Table 2 but variable changes in its levels have been reported across different papers in patients with schizophrenia (Andreou et al., 2014, 2015; Carlborg, Jokinen, Nordstrom, Jonsson, & Nordstrom, 2011). In a closely related pathway, MHPG is a modified metabolite of another catecholamine, namely normetanephrine. Similar to the trend noted for MHPG in Table 2, four recent studies discussing the role of MHPG in schizophrenia, all indicated a downregulation of this metabolite in affected individuals (Andreou et al., 2014; Comasco et al., 2016; Liu et al., 2015; Palsson et al., 2017). The last three metabolites reported in two or more metabolomics studies in patients with psychosis (lactate, creatine, NAA) are directly or indirectly related with mitochondrial metabolism.
Three metabolomics studies from Table 1 highlighted the importance of lactate in psychosis. As a biomarker for psychosis, lactate was found to be upregulated in two of the screened studies, and downregulated in another. Lactate shows potential to be a promising biomarker for psychosis due to its involvement in bioenergetics pathways, those of which are known to be altered in psychosis (Rowland et al., 2016). A possible reason for increased levels of lactate in schizophrenia is oxidative stress-related mitochondrial dysfunction, or vice versa, mitochondrial dysfunction-induced oxidative stress (Wang, Shao, Sun, & Young, 2009). Impaired brain energy metabolism and mitochondrial dysfunction have been linked with psychosis, schizophrenia, and other neuropsychiatric disorders (Bubber et al., 2004; Hazlett et al., 2004; Henneman, Altschule, & Goncz, 1954; Liu et al., 2015; Olsen et al., 2008; Wesseling et al., 2013; Yang et al., 2013).
Creatine was reported in a total of four studies, but no clear trend could be deciphered. Two studies reported a downregulation and the other two an upregulation of creatine in patients with psychosis. While creatine is a known neuromodulator and key regulator of energy metabolism, its role in psychosis is still not well defined except for the fact that altered brain creatine pathways are linked with psychosis (Allen, 2012). Further research is necessary to determine if creatine is a trait or state marker for psychosis. For instance, a 2009 study by Keshevan and coworkers found that healthy adolescents at high risk for developing schizophrenia had markedly lower creatine levels in their caudate nucleus compared to low risk adolescents (Keshavan et al., 2009). Conversely, a study involving twin pairs discordant for schizophrenia indicated that the twins at high risk for developing the disease exhibited a greater total hippocampal creatine concentration (Lutkenhoff et al., 2010).
N-acetylaspartate is highly abundant in neurons and considered to be an indicator of neuronal integrity and viability, as well as a marker of mitochondrial dysfunction (Larabi et al., 2017; Moffett, Ross, Arun, Madhavarao, & Namboodiri, 2007). It is synthesized in neuronal mitochondria from the amino acid aspartate and acetyl-coenzyme A (Patel & Clark, 1979). Among the articles reviewed, there was a strongly reported trend of its downregulation in patients with psychosis. Given NAA is the source of acetate for lipid and myelin synthesis in oligodendrocytes (i.e., the glial cells that myelinate neuronal axons), the downregulation noted in our study is consistent with the literature suggesting myelination abnormalities in psychosis (Flynn et al., 2003; Mighdoll, Tao, Kleinman, & Hyde, 2015). Moreover, NAA may function as a neurotransmitter in the brain. When NAA is coupled with glutamate via a peptide bond, N-Acetylaspartylglutamate (NAAG) is formed. NAAG is a neuropeptide that acts on metabotropic glutamate receptors (Coyle, 1997; Fricker et al., 2009; Lodder-Gadaczek, Becker, Gieselmann, Wang-Eckhardt, & Eckhardt, 2011; Neale, Bzdega, & Wroblewska, 2000; Yan, Ishihara, Serikawa, & Sasa, 2003) and may have a neuroprotective role (Bruno, Wroblewska, Wroblewski, Fiore, & Nicoletti, 1998). The Seese and coworkers study was the only study that reported an increase of NAA in a psychosis patient (Seese et al., 2011). Interestingly, this study focused on child-onset schizophrenia, whereas the other studies investigated adolescent and adult-onset schizophrenia, raising the possibility of differences at the pathophysiology level between child-onset and adult-onset schizophrenia. In summary, the NAA is one of the most robust biomarkers identified in our study, as it is supported by 10 different studies (Table 1) and is able to link many different pathophysiological findings identified in our study. These include abnormalities in energy metabolism, the glutamate pathway, neurotransmission, and possibly in myelination. Moreover, a decrease in NAA may render the nerve cells more vulnerable due to a secondary decrease of NAAG related neuroprotection.
In accordance with a deficiency in neuroprotection, myoinositol was found to be upregulated in two studies (Table 2), suggesting activation of glial cells and a possible explanation for the neuroinflammation that occurs within the early stages of schizophrenia (Plitman et al., 2016). Glutathione and linoleic acid were two other biomarkers downregulated in psychosis which have a potential neuroprotective effect (Table 2). Glutathione is a major cellular redox regulator and antioxidant which protects cells from damage induced by reactive oxygen species (Lavoie et al., 2017). It has been noted that glutathione levels are decreased in the brain, CSF, erythrocytes, and plasma of schizophrenia patients (Lavoie et al., 2017). Heightened levels of oxidative stress in cortical regions are characteristic of schizophrenia (Yao, Leonard, & Reddy, 2006); the decrease in available glutathione could potentially be the result of impaired synthesis or an exaggerated demand for antioxidative defense (Ballesteros et al., 2013). Linoleic acid is an essential doubly unsaturated fatty acid that widely occurs in plant glycosides. Fatty acids are known to possess neuroprotective effects through inflammation suppression, neurogenesis regulation, and oxidative stress protection (Hashimoto et al., 2003), and some evidence for a neuroprotective role of linoleic acid is available (Monaco et al., 2018). Of note, fatty acid biosynthesis pathways are disrupted in patients with psychosis which causes their downregulation in vivo (McEvoy et al., 2013). This is consistent with the abnormalities seen in 3-hydroxybutyrate in the studies reviewed (Table 2). 3-hydroxybutyrate is a ketone body found to be elevated in blood and urine in ketosis. It can be metabolized by the brain for energy during hypoglycemia (Akram, 2013). Previous studies have found that there is an insufficient supply of glucose to the brain in patients with schizophrenia, which would therefore cause an increase in fatty acid catabolism and ultimately ketogenesis. Two of the studies reviewed as part of our search noted the upregulation of this metabolite while one noted its downregulation in patients with schizophrenia (Table 2). Urine 3-hydroxybutyrate and a panel of five serum biomarkers showed promising results to potentially diagnose patients with schizophrenia (Yang et al., 2013).
In an effort to understand the timing of the different metabolic changes, the biomarkers identified in FEP patients were contrasted with those in CP patients (Supporting Information Tables 3 and 4). Multiple independent metabolomics studies in FEP showed an abnormality in NAA, supporting that its downregulation is an early pathophysiological change (Supporting Information Table 3), which persists in patients with CP (Supporting Information Table 4). Early onset abnormalities seen in FEP that persist in CP were also seen for glutamate, 3-hydroxybutyrate, and creatine, however, no clear trend of upregulation or downregulation was observed. Interestingly, myoinotisol was only seen in FEP suggesting that glial cells activation is an early step in the pathophysiology of psychosis (Chiu et al., 2018). Elevation of myoinositol in addition to elevated glutamate and choline was also noted in a different study targeting patients with FEP. This study suggested glial activation and a possible explanation for the neuroinflammation that occurs within the early stages of schizophrenia (Plitman et al., 2016).
In contrast, Supporting Information Table 4 displays the trends of 13 biomarkers in CP patients, some of which were not reported in FEP patients (specifically, tryptophan, D-serine, HVA, MHPG, glutathione, and linoleic acid were downregulated, whereas kynurenine and lactate were upregulated, in CP). Some of the changes seen in CP are expected to be due to exposure to antipsychotic medication. Figure 1 diagrammatically compares the biomarker trends among medicated and unmedicated patients with psychosis (also see Supporting Information Tables 1 and 2). Overall, there are insufficient studies to conclude about differences in trends between these two groups. Downregulation of MHPG and upregulation of myoinositol, 5-HIAA, and HVA are only seen in medicated patients. However, in our search, each of these metabolites was mentioned in one study only. Kynurenine is a potential biomarker for the medicated state of psychosis, as it is upregulated in four studies and all of them were focused exclusively on medicated individuals. NAA is a potential biomarker for psychosis in both medicated and unmedicated states. It is downregulated in six studies with medicated and two with unmedicated patients, supporting our above-mentioned hypothesis about its early and central role in the pathophysiology of psychosis.
5 LIMITATIONS
Our study could not take into account important covariates such as, clinical severity, compliance and timing of medication intake relative to sample collection, therapeutic responsiveness, the age, sex and ethnicity of participants, as well as, the overall progression of disease. Future prospective metabolomics studies should take these elements into consideration.
6 CONCLUSION AND FUTURE DIRECTIONS
In this systematic review, we identified six biomarkers for psychosis (NAA, glutamate, kynurenine, lactate, tryptophan, creatine) that were reported to be abnormal in psychosis in two or more independent metabolomics studies meeting our search criteria. Four additional metabolites were encountered in a single metabolomics study but had significant evidence (two supporting articles or more) for a link to psychosis based on PubMed: linoleic acid, D-serine, glutathione, and 3-hydroxybutyrate. Our systematic review of the different metabolomic studies highlights several pathways that appear to be important in the pathophysiology of schizophrenia. Our findings include abnormalities in the regulation of different amino acids (e.g., glutamate, D-Serine, tryptophan, tyrosine-dopamine pathway, aspartate-NAA synthesis pathway) and neurotransmission. Moreover, abnormalities in molecules with neuroprotective function, as well as, abnormalities in pathways important for myelination, glial cell activation, redox regulation, and overall mitochondrial function are also highlighted in our manuscript. Some of these pathways may constitute new drug targets for treatment of schizophrenia and related conditions. Furthermore, the metabolites identified from this study can serve as biomarkers for early diagnosis of individuals at risk for schizophrenia, monitoring the progression of disease and response to treatment. However, further validation based on large prospective studies is required before clinical translation of the biomarkers identified. Ideally, a prospective study using these biomarkers should include a large number of untreated FEP patients who will be followed over time to better evaluate the impact of medications and explore how FEP differs from CP. Urine, blood, and CSF samples could be collected at regular intervals. The clinical status (decompensation vs. remission; severity of symptoms), the medications and compliance of the patient, as well as the time at which the patient took each medication with regards to the time of sample collection, should be documented. A short dietary history for the intake prior to sample collection might also be useful when interpreting the metabolomics results.
Finally, genomic studies exploring the genetic architecture of schizophrenia could benefit by placing particular emphasis on the pathways highlighted in our study. Our hypothesis is that there exists an overall metabolic pathway, including several of the metabolites identified in our study, which is affected in patients with schizophrenia. However, different subgroups of patients likely have different combinations of predisposing genetic and environmental factors affecting this pathway. Metabolomics could potentially help us identify more homogeneous groups of patients with schizophrenia at the pathophysiology level, ultimately opening the way to more targeted treatments.
CONFLICT OF INTEREST
The authors have no conflicts of interest to report.
ACKNOWLEDGMENTS
We thank Ankur Krishnan for the feedback he provided in analyzing the pathways corresponding to the screened metabolites. We thank Kellie MacDonald for constructing the Supporting Information figures that analyze the screened metabolite pathways. We thank the Montreal Children's Hospital Foundation for supporting Dr. Trakadis' research.
AUTHORS' CONTRIBUTIONS
YT designed and coordinated the study, as well as synthesized the results. EG and YT designed the systematic search methodology and EG performed the search. CL, AW, XW, JR, EZ screened the articles and reviewed the literature. CL put the first draft of the article together. All authors reviewed and provided feedback on the manuscript.
DECLARATION OF INTEREST
None.




