Exploring relationships between joint hypermobility and neurodevelopment in children (4–13 years) with hereditary connective tissue disorders and developmental coordination disorder
Abstract
Joint hypermobility (JH) is a common, though largely ignored physical trait with increasing clinical reverberations. A few papers suggest a link between JH and selected neurodevelopmental disorders, such as developmental coordination disorder (DCD). JH is also the hallmark of various hereditary connective tissue disorders (HCTDs). Children with HCTDs may present abnormal neurodevelopment but its manifestations remain undetermined. This study examined 23 children (group 1), aged 4–13 years, with different HCTDs (i.e., 19 with hypermobile Ehlers-Danlos syndrome (EDS)/hypermobility spectrum disorder, 3 with molecularly confirmed classical EDS, and 1 with Loeys-Dietz syndrome type 1 due to TGFBR2 mutation) and 23, age- and sex-matched children with DCD (group 2). All underwent 14 different psychometric tests exploring motor, cognitive, executive-attentive, and emotional-behavior features. In group 1, 30%, 22%, and 13% patients presented DCD (with or without dysgraphia), learning disabilities, and attention deficit-hyperactivity disorder, respectively. None had cognitive delay. In group 2, 17% patients presented generalized JH and none had HCTDs. DCD children presented more motor and coordination troubles than HCTDs patients, while quality of life of children with HCTDs resulted more deteriorated due to somatic manifestations and behavioral traits. This study presents the full overview of neurodevelopmental attributes in HCTDs, and compares with standardized tools the neurodevelopmental profile of children with DCD and HCTDs. While the high rate of neurodevelopmental comorbidities in HCTDs deserves attention, the impact of a dysfunctional connective tissue in children with a primary diagnosis of DCD needs more research.
1 INTRODUCTION
Joint hypermobility (JH) is a largely ignored physical sign and defines the ability that a joint has to move beyond normal limits (Castori et al., 2017). It is common in many ethnicities and occurs in 6–57% of females and 2–35% of males (Remvig, Jensen, & Ward, 2007). JH is assessed at physical examination by an orthopedic goniometer or other objective tools. Since the 60s of the last century, JH was associated with minor musculoskeletal traits with a relatively benign course (Kirk, Ansell, & Bywaters, 1967). To date, the attribute “benign” has been removed as a wide array of musculoskeletal and extra-musculoskeletal manifestations, some of them with a disability potential, are recognized in association with JH (Castori et al., 2017).
Presentations of JH are diverse and include localized, generalized, peripheral, and historical JH (Castori et al., 2017). On a clinical perspective, their impact might diverge significantly on the basis of age at ascertainment, body distribution, and etiology. Generalized JH (GJH), commonly encountered in children, is often congenital and may indicate the existence of an underlying genetic disorder. Assessing GJH is not always an easy task due to a variety of influencing factors, such as age, sex, and ethnicity (Remvig et al., 2007). The Beighton score (BS) is the most commonly used tool for assessing GJH in a clinical setting (Juul-Kristensen, Schmedling, Rombaut, Lund, & Engelbert, 2017).
Ehlers-Danlos syndromes (EDSs) are a group of hereditary connective tissue disorders (HCTDs) sharing the triad of JH, abnormal skin texture, and fragility of internal organ and vessels with a minimum prevalence of 1/5,000 in the general population (Steinmann, Royce, & Superti-Furga, 2002). The 2017 international classification identifies 13 clinical variants due to mutations in 19 different genes (Malfait et al., 2017). Classical, vascular, and hypermobile EDS (hEDS) are the most common variants. hEDS is the unique variant lacking a confirmatory test and is, to date, included at the end of a continuous spectrum beginning with isolated, nonsyndromic JH (Castori et al., 2017; Malfait et al., 2017). All intermediate phenotypes composing this spectrum are exclusion diagnoses and correspond to the various “hypermobility spectrum disorders” (HSD). This term embraces all symptomatic individuals with JH who do not fulfill the new hEDS criteria (Castori & Hakim, 2017; Castori et al., 2017). HSD includes and, hence, substitutes the misused “joint hypermobility syndrome” (JHS) (Grahame, Bird, & Child, 2000). Before the 2017 EDS classification, hEDS and JHS were considered by some experts undistinguishable on clinical grounds, although originally defined by separate sets of criteria (Tinkle et al., 2009). Therefore, the past literature often used the term JHS/hEDS to define a broad group of patients meeting one or both set of criteria. Now, hEDS and HSDs are separate phenotypes within the same spectrum.
Daily practice on children with GJH and JHS/EDS (old criteria) suggests a high rate of comorbid developmental motor and coordination disorders, but evidence is still scanty (Ghibellini, Brancati, & Castori, 2015). In 2005, Adib, Davies, Grahame, Woo, and Murray reported clumsiness and symptoms of poor coordination in 125 children with JHS/EDS (old criteria). In twin studies, Kirby and colleagues suggested functional similarities between children with GJH or JHS/EDS (old criteria) and those with developmental coordination disorder (DCD) (Kirby & Davies, 2007; Kirby, Davies, & Bryant, 2005). DCD is a neurodevelopmental disorder involving motor coordination that is characterized by difficulties in learning, planning, and execution of motor tasks, and has a prevalence of 4–5% in children (Lingam, Hunt, Golding, Jongmans, & Emond, 2009). It can manifest with delay in motor milestones, poor coordination and fluency in motor actions, deficits in the execution of complex motor patterns, poor balance, and poor writing skills (Vaivre-Douret, 2014). According to the fifth edition of the diagnostic and statistical manual of mental disorders (DSM-5; APA, 2013), the diagnosis of DCD needs the exclusion of other neurologic conditions affecting movement. DSM-5 also includes EDS among the differential diagnoses and comorbidities of DCD (APA, 2013).
Although, many authors were interested in understanding the etiopathogenesis of DCD, it is still poorly understood (Visser, 2003). A link between DCD and connective tissue is also presumed by a single observation of high prevalence of GJH in children with DCD (Jelsma, Geuze, Klerks, Niemeijer, & Smits-Engelsman, 2013). In addition, speech and language disorders and poor writing skills might be frequent in subjects with GJH and JHS/hEDS (old criteria) with an impact on academic performances (Arverdson & Heintskill, 2009). Such preliminary data indicate that the link between GJH and DCD is a field of potential interest, as it might represent a model for studying mind-body connections in order to shed more light on the etiopathogenesis of a wide range of “idiopathic” neurodevelopmental disorders. Some hypothesis has been recently put forward for a possible association between JH and attention deficit-hyperactivity disorder (ADHD) (Baeza-Velasco, Sinibaldi, & Castori, 2018).
This is a pilot study comparing the neuropsychological, motor, behavioral, and psychopathological attributes of 23 children, aged from 4 to 13 years, affected by different HCTDs featuring GJH with 23 children primarily ascertained for DCD according to DSM-5 criteria. Our findings present a wider picture of neurodevelopmental disorders in children with HCTDs and contribute to the understanding of the presumed overlap between GJH and DCD.
2 PATIENTS AND METHODS
2.1 Patients’ selection
The study involved 46 patients divided into two groups of 23 individuals, aged between 4 and 13 years. The narrow age range was guided by the need of gathering data with homogeneous psychometric tools, as described below. The two groups were age- and sex-matched and included 11 males and 12 females. Group 1 consisted of 19 patients with JHS/EDS (old criteria), 3 with classical EDS (cEDS), and 1 with Loeys-Dietz syndrome type 1 (LDS1), all first assessed in a clinical genetics setting. Patients’ selection began on January 2016, before the publication of the 2017 international classification of EDS and related disorders (Malfait et al., 2017). Accordingly, individuals with JHS/hEDS (old criteria) received the diagnosis when they met the Villefranche criteria (Beighton, De Paepe, Steinmann, Tsipouras, & Wenstrup, 1998) and/or the Brighton criteria (Grahame et al., 2000), as previously detailed (Castori et al., 2014). According to the 2017 nosology, patients fitting the new criteria were affected by bona fide hEDS, while symptomatic subjects who did not meet such criteria were put under the umbrella term of HSD. Hence, the 19 patients with hEDS (old criteria) were better classified as hEDS/HSD. The diagnosis of cEDS was confirmed by the identification of a causative nucleotide change in either COL5A1 or COL5A2. In the single patient with LDS1, the diagnosis was supported by a pathogenic variation in TGFBR2. Group 2 included 23 patients primarily ascertained for DCD.
In both groups, JH was assessed by the BS. BS is a nine-point evaluation with attribution of one point in the presence of any of the following: (a) passive apposition of the thumb to the flexor aspect of the forearm (one point for each hand), (b) passive dorsiflexion of the V finger beyond 90° (one point for each hand), (c) hyperextension of the elbow beyond 10° (one point for each arm), (d) hyperextension of the knees beyond 10° (one point for each leg), (e) forward flexion of the trunk with the knees extended and the palms resting flat on the floor (Beighton, Solomon, & Soskolne, 1973). In noncollaborative subjects (such as, toddlers and adults in wheelchair), the upper end of the sum was reduced by one point by excluding the maneuver for forward flexion of the trunk. In this case, the highest score was 8/8. Elbow and knee extension was measured with an orthopedic goniometer. When possible, the assessment of the single components of the BS was performed according to Juul-Kristensen, Røgind, Jensen, and Remvig (2007). Although the BS is not a severity score, but a simple screening method for distinguishing between individuals with or without GJH, we assumed that the rough value of the BS is a proxy of the number of hypermobile joints (and, hence, of the extent of JH). In group 1, a minimum of 6 at the BS was fixed for inclusion and this was also the cutoff for the attribution of GJH in group 2, according to Juul-Kristensen et al. (2017). All participants underwent a full child neurology/psychiatry interview and exam for appropriate gathering of clinical and historical data. All children from group 2 (DCD group) were screened for the 2017 criteria of hEDS and for clinical signs indicative for other Mendelian HCTDs.
Parents of all patients gave their consent to the study, which is in according to the revised version of the Helsinki Declaration. This study was approved by the Local Ethic Committee (protocol no. 250/CE Lazio 1).
2.2 Psychometric tests
Children from both groups underwent rating scales to assess cognitive abilities, motor skills, and attention and the executive functions. Self-report questionnaires were administered to parents and selected patients to assess anxious and depressive symptoms, adaptive functions, behavior, and quality of life. Global cognitive abilities were assessed with the Wechsler preschool and primary scale of intelligence 3rd edition (WIPPSI-III) (Wechsler, 2002) in children from 4 to 6 years, and with the Wechsler intelligence scale for children 4th edition (WISC-IV) (Wechsler, 2003) in those with an age above 6 years.
Motor performance and handwriting skills were evaluated using the movement assessment battery for children, 2nd edition (M-ABC-2) (Henderson, Sudgen, & Barnett, 2007), the developmental test of visual-motor integration, 3rd edition (VMI) (Beery, 1997), and the Italian version of the concise assessment method for children's handwriting (BHK) (Di Brina & Rossini, 2010; Hamstra-Bletz, De Bie, & Den Brinker, 1987). M-ABC-2 is an individually administered, norm-referenced test used to identify and evaluate movement problems in children and adolescents. It can be used in patients from 3 to 16 years and consists of 24 items grouped into three categories (manual dexterity, ball skills, and static and dynamic balance) of 8 items each. VMI is a standardized test that is frequently administered to assess VMI skills. The examinee has to copy a series of geometric forms, from less to more complex, and his performance is scored depending on the accuracy of the drawings and compared with standard criteria. The VMI supplemental tests (visual perception and motor coordination) contain the same geometric forms and were designed to identify visual analysis and motor coordination difficulties. BHK is administered to assess dysgraphia. The test requires that the child copies a sample of writing within 5 min; handwriting quality is evaluated using 13 criteria and writing speed is measured. Scores of impairment higher than 21 at the scale of quality or scores below 10th percentile at the scale of speed are considered relevant. Scores are always compared to standard references.
Attention and executive functions were evaluated by the attentive-executive domain of the developmental neuropsychological assessment 2nd edition (NEPSY-II) (Korkman, Kirk & Kent, 2007). NEPSY-II is a comprehensive neuropsychological assessment battery including six domains which can be administered together or individually to children between the age of 3 and 16. The attentive-executive domain contains a series of subtests (i.e., visual attention, design fluency, auditory attention, response set, inhibition, clocks, animal sorting, and statue) that investigate selective and sustained attention, figural fluency, inhibition of automatic responses, planning and organizing a complex response, problem solving, self-regulation, and the capacity to establish, maintain, and change a response set.
Presence of anxious and depressive symptoms was evaluated with the multidimensional anxiety scale for children (MASC) (March, Parker, Sullivan, Stallings, & Conners, 1997) and the children's depression inventory (CDI) (Kovacs, 1985). MASC is a self-report, 39-item inventory, used to assess symptoms related to anxiety disorders in children and adolescents aged 8 to 19 years. It produces scores from four subscales: physical symptoms, harm avoidance, social anxiety, separation/panic, and a total score. Scores are reported on a standard scale with a mean of 50 and are considered significant if >60. CDI is a self-report, 27-item scale that measures depressive symptoms in individuals aged 7 to 17 years. Items are grouped into five areas: mood, interpersonal problems, ineffectiveness, anhedonia, and negative self-esteem. Total score ranges from 0 to 54 and is considered clinically significant for depressive symptoms if >19.
Three questionnaires were administered to parents to evaluate children's behavior and adaptive functions: child behavior checklist (CBCL) (Achenbach & Rescorla, 2000, 2001), Conner's (1997) parent rating scale revised: short form (CPRS-R:S), and the adaptive behavior assessment system, 2nd edition (ABAS-II) (Harrison & Oakland, 2003). CBCL is a parent respondent questionnaire widely used in research and clinical practice to detect emotional and behavioral problems in children and adolescents. In our study, we used the preschool version (CBCL1 ½ to 5) and the school age version (CBCL 6–18) which contain 100 and 113 items, respectively. Items are grouped into eight syndrome scales: anxious/depressed, withdrawn/depressed, attention problems, somatic complaints, social problems, thought problems, rule-breaking behavior, and aggressive behavior. Two higher order scales (internalizing problems and externalizing problems) combines several syndrome scales and all contribute to the total problem score. CBCL also include six “DSM-oriented” scales according to the DSM-IV diagnostic categories of affective problems, anxiety problems, somatic problems, ADHD, oppositional defiant problems, and conduct problems (APA, 1994). Scores are converted on a standard scale with a mean of 50 (standard deviation [SD] = 10); scores above 70 fall within the clinical range. CPRS-R:S is a tool used to evaluate behavioral problems and make diagnosis of ADHD in subjects aged 3 to 17 years. It contains 27 items grouped into oppositional problems, cognitive problems/inattention, and hyperactivity dimensions and provides an ADHD index. Each subscale score is based on a standard scale, with a mean of 50 (SD = 10). Scores above 60 are considered clinically significant. ABAS-II is a norm-referenced questionnaire designed to assess adaptive behavior, personal, and social skills in subjects of all ages. The tool is structured in 5 different rating forms and investigates 10 adaptive areas organized in 3 domains (conceptual, social, and practical). A general score is also derived (general adaptive composite). Scores are processed on a standard scale with a mean of 100 (SD = 15). Scores below 70 are considered clinically significant.
Quality of life was assessed with the self- and parents-reported version of the pediatric quality of life inventory (PedsQL) (Trapanotto, Giorgino, Zulian, Benini, & Varni, 2009; Varni, Seid, & Rode, 1999), which is applicable in subjects aged 2 to 18 years. The PedsQL Generic Core Scales consist of 23 items grouped in four multidimensional scales (physical functioning, emotional functioning, social functioning, and school functioning) and in three summary scores (total scale score, physical health summary score, and psychosocial health summary score). Scale and summary scores are computed by deriving the mean of each scale and converting the raw score on a 0-to-100 continuum. Learning disabilities (LDs) were evaluated with the memory-training test, 2nd edition (MT-2) (Cornoldi & Colpo, 1998) and the battery for the evaluation of developmental dyslexia and dysorthographia, 2nd edition (DDE-2) (Sartori, Job, & Tressoldi, 2007). Those are two standardized tests to assess reading, comprehension, and writing skills, designed for Italian school age children.
2.3 Neurodevelopmental profiles
All diagnoses were in according to the DSM-5 and carried out by an expert child neurologist/psychiatrist. In particular, DCD was established in subjects with a IQ >70 at WIPPSI-III or WISC-IV, according to age, and one or more of the following: (a) a total score <15th percentile at the MACB-2, or (b) a score <5th percentile in at least one of the three subscores of the MACB-2, or (c) a score <15th percentile in one or both the VMI supplemental tests, plus an impairment score >21 at the scale of quality or a score <10th percentile at the scale of speed of the BHK. The last categorical diagnosis identified a subset of patients with DCD and dysgraphia. The diagnosis of ADHD was established in children with a IQ >70 at WIPPSI-III or WISC-IV, according to age, a pattern compatible with ADHD at the CRS-R:S, and two or more scores below the mean at the NEPSY-II attentive-executive domain. LDs were diagnosed in presence of a fall <–2 SD at the MT-2 (reading LD) and/or BEDDD-2 (writing LD).
2.4 Statistical tools
A series of descriptive statistics were used to summarize pertinent study information. Chi-square or Fisher's exact test was performed for the comparison of categorical variables. Mann–Whitney nonparametric test was used for all analyses; in comparisons involving a number of subjects >30, the Student's t test was also applied. In group 2, correlations between the BS and the results of WISC-IV, WIPPSI-III, MABC-2, VMI, and NEPSY-II were investigated with the Spearman test. All p-values were reported as two-sided and p-values less than 0.01 denotes statistically significant association. All analyses were performed by using the SPSS software (SPSS version 21.0; SPSS, Inc., Chicago, IL).
3 RESULTS
3.1 Demographic and general data
Comparison of demographic and general developmental data of patients belonging to groups 1 and 2 is summarized in Table 1. All values are comparable except the BS. This is in line with patients’ selection as GJH is a major feature/diagnostic criterion for many HCTDs, especially hEDS and cEDS, while it is not currently considered in the initial assessment of children with DCD.
| Characteristics | Group 1 | Group 2 | p-value |
|---|---|---|---|
| No. of patients | 23 | 23 | – |
| Males/females | 11/12 | 11/12 | – |
| Age, range (median) [years] | 4.7–13.2 (9.1) | 4.9–13.2 (9.3) | .913 |
| Beighton score, range (median) | 6–9 (7) | 2–8 (4) | <.001 |
| Crawling, range (median) [months] | 8–11 (9) | 8–11 (9) | .854 |
| Autonomous walking, range (median) [months] | 12–20 (14) | 12–18 (13) | .309 |
| First words, range (median) [months] | 10–18 (12) | 10–18 (12) | .298 |
| First sentences, range (median) [months] | 24–36 (30) | 24–36 (30) | .485 |
- Significant p-values are in bold (Mann–Whitney test). Group 1: 19 patients ascertained for hypermobility spectrum disorder/hEDS, 3 for cEDS, and 1 for Loeys-Dietz syndrome type 1. Group 2: 23 patients ascertained for DCD. cEDS = classical EDS; DCD = developmental coordination disorder; EDS = Ehlers-Danlos syndrome; hEDS = hypermobile EDS.
3.2 Neuropsychiatric features in children with HCTDs (group 1)
Figure 1 describes the distribution of neurodevelopmental comorbidities in group 1. Neurodevelopmental comorbidities are observed in 61% of the sample, with DCD (7/23; 30%) and LDs (5/23; 22%) being the most common. Among the seven patients with DCD, three (13%) also displayed dysgraphia and one (4.3%) had DCD plus ADHD. As two additional patients showed a neurodevelopmental profile restricted to ADHD (i.e., not in combination with DCD or LDs), we had three individuals with ADHD (3/23; 13%). Within group 1, a comparison between children with neurodevelopmental comorbidities (14/23) and those without (9/23) was carried out (Mann–Whitney test). No significant differences resulted from the comparison for all tests and domains. The comparison between males and females did not find any significant difference (Mann–Whitney test).
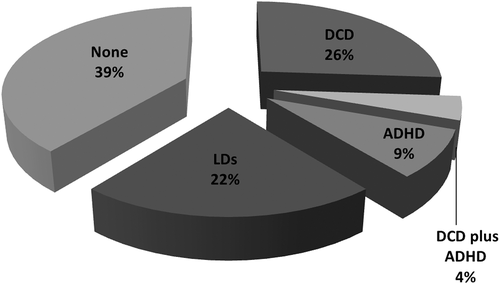
Graphical summary of the neurodevelopmental comorbidities in the 23 pediatric patients with HCTDs (Group 1). ADHD = attention deficit-hyperactivity disorder; DCD = developmental coordination disorder; HCTD = hereditary connective tissue disorder; LDs = learning disabilities
3.3 Patterns of JH and its relationship with key neuropsychiatric features in children with DCD (group 2)
Patients with a primary diagnosis of DCD (group 2) were first screened for the BS. Table 2 summarizes the distribution of BS values in the sample. Four (17.4%) patients had a BS ≥6 and hence presented GJH according to age (Juul-Kristensen et al., 2017). All others presented more restricted variants of JH, including localized and peripheral JH. No patient had a BS below 2. Spearman test was used to explore possible relationships between the value of the BS and the results of WISC-IV/WIPPSI-III, MABC-2, VMI, and NEPSY-II. Positive correlation was found for the Inhibition subtest part A the NEPSY-II executive-attentive domain (r = .709; p = .001). A comparison between DCD patients with (# 4) and without (# 19) GJH, and between males and females gave negative results for all items (Mann–Whitney test). None of the DCD patients met the 2017 criteria for a diagnosis of hEDS or presented features prompting molecular testing for rarer HCTDs. Patients were selected from those in whom all neurological disorders put in the differential diagnosis of DCD (DSM-5) had been already excluded by the child neurologist.
| Beighton score | No. of patients | Sex | % | Cumulative % |
|---|---|---|---|---|
| 8 | 1 | 1F | 4.3 | 4.3 |
| 7 | 0 | – | – | 4.3 |
| 6 | 3 | 2M/1F | 13.1 | 17.4 |
| 5 | 7 | 3M/4F | 30.4 | 47.8 |
| 4 | 9 | 4M/5F | 39.2 | 87.0 |
| 3 | 2 | 1M/1F | 8.7 | 95.7 |
| 2 | 1 | 1M | 4.3 | 100 |
- F = female; M = male.
3.4 Comparison of neuropsychiatric features between group 1 and group 2
According with the preliminary evidence of an apparent clinical overlap between children with a primary diagnosis of GJH or JHS/hEDS and those with DCD (Kirby & Davies 2007; Kirby et al., 2005), a comparison was carried out between groups 1 and 2. Table 3 summarizes comparison data for the cognitive and motor features. Significant differences were noted in all VMI scales, in the total score of MABC-2 and in two MABC-2 subcategories. Results for the executive-attentive domain are presented in Table 4. Writing fluency was the only sub-item showing significant results. Table 5 summarizes the comparison for the attentive and emotional-behavioral domains. Relevant differences emerged for the somatic complaints and problems subscales of CBCL and opposition scale of CRS-R:S. Quality of life parameters were compared in Table 6. Significant p-values were found for the activity and health item of the PedsQL 4.0 self-report version, and for the total, activity and health, school activity, and psychosocial functioning items of the parent-report version.
| Items | Group 1 | Group 2 | p-value* | p-value** | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Mean | SD | Median | Range | Mean | SD | Median | Range | |||
| WISC-IV-tot IQ | 105.91 | 12.93 | 104.00 | 88–133 | 98.74 | 14.04 | 99.00 | 73–121 | .078 | .129 |
| WISC-IV-VCI | 108.09 | 12.39 | 108.00 | 86–132 | 109.22 | 12.32 | 110.00 | 80–130 | .758 | .488 |
| WISC-IV-PRI | 106.70 | 14.18 | 108.00 | 80–130 | 99.39 | 16.44 | 100.00 | 76–130 | .114 | .111 |
| WISC-IV-WMI | 99.84 | 11.68 | 100.00 | 82–124 | 89.42 | 13.28 | 91.00 | 64–115 | .015 | .028 |
| WISC-IV-PSI | 100.57 | 15.15 | 97.00 | 76–135 | 89.83 | 12.79 | 88.00 | 65–115 | .013 | .023 |
| M-ABC-2-Tot | 45.22 | 29.66 | 37.00 | 2–99 | 5.39 | 4.19 | 5.00 | 1–16 | <.001 | <.001 |
| M-ABC-2-MD | 51.13 | 30.79 | 50.00 | 5–99 | 14.15 | 17.45 | 5.00 | 1–75 | <.001 | <.001 |
| M-ABC-2-BS | 40.43 | 29.30 | 37.00 | 1–98 | 21.00 | 20.42 | 16.00 | 1–63 | .012 | .015 |
| M-ABC-2-BA | 42.00 | 26.92 | 35.00 | 5–91 | 8.15 | 9.25 | 5.00 | 1–37 | <.001 | <.001 |
| VMI | 58.74 | 26.31 | 61.00 | 5–59 | 30.00 | 26.03 | 23.00 | 3–82 | .001 | .001 |
| VMI-VP | 71.17 | 26.03 | 75.00 | 5–99 | 45.57 | 31.82 | 50.00 | 1–99 | .005 | .007 |
| VMI-MC | 50.04 | 30.46 | 45.00 | 5–98 | 31.82 | 23.85 | 23.00 | 1–95 | .007 | .008 |
- Significant p-values are in bold (*Student test; **Mann-Whitney test). Group 1: 19 patients ascertained for hypermobility spectrum disorder/hEDS, 3 for cEDS, and 1 for Loeys-Dietz syndrome type 1. Group 2: 23 patients ascertained for DCD. BA = balance; BS = ball skills; M-ABC-2 = movement assessment battery for children, 2nd edition; MC = motor coordination; MD = manual dexterity; PRI = perceptual reasoning index; PSI = processing speed index; SD = standard deviation; Tot = total score; Tot IQ = total intelligence quotient; VCI = verbal comprehension index; VMI = developmental test of visual-motor integration; VP = visual perception; WISC-IV = Wechsler intelligence scale for children- fourth edition; WMI = working memory index.
| Items | Group 1 | Group 2 | p-value* | p-value** | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Mean (#) | SD | Median | Range | Mean (#) | SD | Median | Range | |||
| NEPSY-II-VA | 10.30 (23) | 2.40 | 11.00 | 5–15 | 9.35 (23) | 2.89 | 10.00 | 4–15 | .228 | .176 |
| NEPSY-II-DF | 9.57 (23) | 2.01 | 9.00 | 6–13 | 7.48 (23) | 2.52 | 8.00 | 3–12 | .003 | .006 |
| NEPSY-II-IN-A | 9.22 (23) | 2.04 | 9.00 | 6–13 | 8.00 (23) | 2.17 | 8.00 | 3–11 | .057 | .117 |
| NEPSY-II-IN-B | 8.35 (23) | 2.72 | 8.00 | 2–13 | 7.26 (23) | 3.16 | 8.00 | 1–13 | .219 | .223 |
| NEPSY-II-IN-C | 8.75 (16) | 2.02 | 9.00 | 3–13 | 6.67 (17) | 3.12 | 6.00 | 1–13 | .030 | .015 |
| NEPSY-II-AS | 10.25 (16) | 3.36 | 11.00 | 4–15 | 9.88 (17) | 3.10 | 10.00 | 4–15 | .746 | .757 |
| NEPSY-II-AA | 4.04 (23) | 1.22 | 4.00 | 2–6 | 3.74 (23) | 1.68 | 3.00 | 1–6 | .487 | .512 |
| NEPSY-II-RS | 4.37 (16) | 1.26 | 4.00 | 2–6 | 3.35 (17) | 1.69 | 3.00 | 1–7 | .059 | .055 |
| NEPSY-II-CL | 4.25 (16) | 1.48 | 4.50 | 2–6 | 2.88 (17) | 1.54 | 3.00 | 1–6 | .014 | .018 |
| NEPSY-II-ST | 10.43 (7) | 0.97 | 10.00 | 9–12 | 9.33 (6) | 2.34 | 9.50 | 6–13 | .280 | .210 |
- Significant p-values are in bold (*Student's t test; **Mann–Whitney test). Group 1: 19 patients ascertained for hypermobility spectrum disorder/hEDS, 3 for cEDS, and 1 for Loeys-Dietz syndrome type 1. Group 2: 23 patients ascertained for DCD.AA = auditory attention; AS = animal sorting; CL = clocks; DF = design fluency; IN-A = inhibition-subtest A; IN-B = inhibition-subtest B; IN-C = inhibition-subtest C; NEPSY-II = developmental neuropsychological assessment, 2nd edition; RS = response set; SD = standard deviation; ST = statue; VA = visual attention.
| Items | Group 1 | Group 2 | p-value* | p-value** | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Mean (#) | SD | Median | Range | Mean (#) | SD | Median | Range | |||
| ABAS-II-GAC | 86.30 (23) | 12.82 | 86.00 | 62–116 | 91.00 (23) | 14.79 | 90.00 | 51–124 | .256 | .138 |
| ABAS-II-CON | 87.26 (23) | 13.19 | 88.00 | 57–127 | 89.61 (23) | 13.05 | 90.00 | 57–110 | .547 | .516 |
| ABAS-II-SO | 89.83 (23) | 13.21 | 95.00 | 61–114 | 92.70 (23) | 17.09 | 92.00 | 50–128 | .527 | .574 |
| ABAS-II-PR | 88.00 (23) | 12.56 | 91.00 | 69–123 | 93.39 (23) | 14.97 | 94.00 | 58–128 | .193 | .108 |
| CBCL-INT | 63.83 (23) | 10.59 | 63.00 | 37–86 | 56.87 (23) | 9.09 | 58.00 | 34–73 | .021 | .019 |
| CBCL-EXT | 53.37 (23) | 7.69 | 53.00 | 40–67 | 50.74 (23) | 8.98 | 52.00 | 34–72 | .258 | .257 |
| CBCL-Tot | 59.61 (23) | 7.74 | 60.00 | 48–74 | 55.22 (23) | 8.85 | 55.00 | 38–70 | .080 | .143 |
| CBCL-SOM-co | 68.04 (23) | 11.61 | 68.00 | 50–88 | 56.52 (23) | 7.87 | 53.00 | 50–74 | <.001 | <.001 |
| CBCL-SOM-pr | 69.04 (23) | 12.77 | 68.00 | 50–93 | 55.30 (23) | 7.12 | 50.00 | 50–70 | <.001 | <.001 |
| CPRS-R:S-OPP | 64.48 (23) | 17.66 | 58.00 | 42–98 | 49.74 (23) | 12.15 | 49.00 | 37–80 | .002 | .002 |
| CPRS-R:S-C pr/I | 69.61 (23) | 16.28 | 69.00 | 45–95 | 62.61 (23) | 14.35 | 59.00 | 41–90 | .129 | .180 |
| CPRS-R:S-HYPER | 60.74 (23) | 16.98 | 56.00 | 40–93 | 51.61 (23) | 14.91 | 47.00 | 38–93 | .059 | .026 |
| CPRS-R:S-ADHD-I | 70.13 (23) | 15.94 | 70.00 | 45–97 | 61.78 (23) | 15.17 | 57.00 | 41–93 | .076 | .091 |
| MASC-Tot | 56.79 (14) | 11.15 | 55.50 | 41–80 | 48.71 (23) | 9.69 | 49.50 | 34–64 | .051 | .062 |
| MASC-Phy-S | 58.07 (14) | 9.33 | 56.50 | 48–81 | 48.93 (23) | 7.27 | 51.50 | 37–57 | .008 | .017 |
| MASC-HA | 45.50 (14) | 10.95 | 45.00 | 30–64 | 37.50 (23) | 10.22 | 34.50 | 25–61 | .056 | .044 |
| MASC-SA | 57.21 (14) | 11.35 | 58.50 | 40–74 | 53.71 (23) | 12.27 | 50.50 | 38–80 | .440 | .447 |
| MASC-SP | 56.57 (14) | 12.21 | 55.00 | 37–82 | 53.43 (23) | 7.54 | 54.50 | 41–62 | .420 | .678 |
| CDI | 13.29 (14) | 11.17 | 11.00 | 2–44 | 10.07 (23) | 5.30 | 10.00 | 2–17 | .339 | .673 |
- Only CBCL items with values above the cutoff or with significant p-values were reported. Significant p-values are in bold (*Student's t test; **Mann–Whitney test). Group 1: 19 patients ascertained for hypermobility spectrum disorder/hEDS, 3 for cEDS, and 1 for Loeys-Dietz syndrome type 1. Group 2: 23 patients ascertained for DCD. ABAS-II = adaptive behavior assessment system, 2nd edition; ADHD-I = attention deficit-hyperactivity disorder index; C pr/I = cognitive problems/inattention; CBCL = child behavior checklist; CDI = children's depression inventory; CON = conceptual adaptive domain; CPRS-R:S = Conner's parent rating scale revised: short form; EXT = externalizing problems; GAC = general adaptive composite; HA = harm avoidance; HYPER = hyperactivity; INT = internalizing problems; MASC = multidimensional anxiety scale for children; OPP = oppositional problems; PR = practical adaptive domain; SA = social anxiety; SO = social adaptive domain; SOM-co = somatic complaints; SD = standard deviation; SP = separation panic; Phy-S = physical symptoms; SOM-pr = somatic problems; Tot = total score.
| Items | Group 1 | Group 2 | p-value* | p-value** | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Mean | SD | Median | Range | Mean | SD | Median | Range | |||
| PedsQL-SR-Tot | 59.52 | 17.58 | 62.00 | 29–88 | 67.61 | 11.99 | 67.00 | 44–92 | .076 | .126 |
| PedsQL-SR-PF | 55.35 | 22.21 | 59.00 | 12–91 | 73.87 | 15.23 | 75.00 | 37–100 | .002 | .004 |
| PedsQL-SR-EF | 61.52 | 23.23 | 65.00 | 10–100 | 63.26 | 20.37 | 65.00 | 30–100 | .788 | .921 |
| PedsQL-SR-SF | 71.09 | 20.94 | 75.00 | 20–100 | 66.09 | 20.22 | 65.00 | 25–100 | .415 | .309 |
| PedsQL-SR-Phy-H | 53.48 | 21.81 | 55.00 | 10–90 | 65.43 | 18.40 | 65.00 | 25–100 | .051 | .056 |
| PedsQL-SR-Psy-H | 62.09 | 16.58 | 63.00 | 32–87 | 65.00 | 14.73 | 63.00 | 33–95 | .532 | 0.636 |
| PedsQL-PR-Tot | 52.96 | 16.70 | 51.00 | 22–84 | 72.74 | 13.43 | 70.00 | 35–100 | <.001 | <.001 |
| PedsQL-PR-PF | 43.04 | 20.55 | 40.00 | 6–84 | 75.35 | 17.76 | 75.00 | 40–100 | <.001 | <.001 |
| PedsQL-PR-EF | 60.00 | 20.11 | 60.00 | 15–95 | 73.70 | 17.46 | 70.00 | 50–100 | .018 | .027 |
| PedsQL-PR-SF | 62.61 | 19.41 | 60.00 | 30–100 | 71.09 | 21.16 | 80.00 | 30–100 | .164 | .104 |
| PedsQL-PR-Phy-H | 52.74 | 22.68 | 50.00 | 10–100 | 69.57 | 17.70 | 70.00 | 35–100 | .007 | .009 |
| PedsQL-PR-Psy-H | 58.35 | 15.98 | 57.00 | 25–88 | 71.35 | 14.03 | 72.00 | 46–95 | .006 | .009 |
- Significant p-values are in bold (*Student's t test; **Mann–Whitney test). Group 1: 19 patients ascertained for hypermobility spectrum disorder/hEDS, 3 for cEDS, and 1 for Loeys-Dietz syndrome type 1. Group 2: 23 patients ascertained for DCD. EF, emotional functioning; PedsQL, pediatric quality of life inventory; PF, physical functioning; Phy-H, physical health summary score; PR, parents report; Psy-H, psychosocial health summary score; SD, standard deviation; SF, school functioning; SR, self-report; Tot, total score.
An analysis was carried out by comparing the values at WISC-IV, M-ABC-2, and VMI of the seven patients with HCTD and DCD (from Group 1), and a representative subgroup of DCD patients (from Group 2) matched for age and sex (Mann–Whitney test). All p-values were below the cutoff of significance (Table 7).
| Items | HCTD + DCD (from Group 1) | DCD only (from Group 2) | p-value | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Mean | SD | Median | Range | Mean | SD | Median | Range | ||
| WISC-IV-tot IQ | 102.86 | 13.25 | 99.00 | 88–125 | 102.71 | 13.74 | 101.00 | 84–121 | .949 |
| WISC-IV-VCI | 111.71 | 13.57 | 112.00 | 90–132 | 114.00 | 12.44 | 114.00 | 92–130 | .654 |
| WISC-IV-PRI | 103.71 | 20.09 | 98.00 | 80–130 | 103.57 | 19.36 | 102.00 | 76–130 | .949 |
| WISC-IV-WMI | 97.00 | 9.80 | 94.00 | 88–115 | 94.43 | 13.57 | 94.00 | 79–115 | .699 |
| WISC-IV-PSI | 91.00 | 11.49 | 88.00 | 76–109 | 90.57 | 6.58 | 88.00 | 82–100 | 1.000 |
| M-ABC-2-Tot | 19.29 | 12.42 | 25.00 | 2–37 | 3.57 | 2.94 | 2.00 | 1–9 | .016 |
| M-ABC-2-MD | 26.00 | 22.80 | 25.00 | 5–63 | 12.79 | 27.50 | 2.00 | 1–75 | .045 |
| M-ABC-2-BS | 17.71 | 21.55 | 9.00 | 1–63 | 23.00 | 22.18 | 16.00 | 1–50 | .847 |
| M-ABC-2-BA | 45.00 | 34.55 | 37.00 | 5–91 | 4.64 | 3.4 | 5.00 | 1–9 | .006 |
| VMI | 31.71 | 19.85 | 27.00 | 5–61 | 14.29 | 15.20 | 7.00 | 3–45 | .083 |
| VMI-VP | 48.14 | 26.79 | 58.00 | 5–75 | 27.26 | 26.28 | 27.00 | 4–73 | .140 |
| VMI-MC | 20.29 | 12.77 | 16.00 | 5–39 | 13.14 | 18.33 | 3.00 | 1–45 | .140 |
- Significant p-values are in bold (Mann–Whitney test). BA = balance; BS = ball skills; DCD = developmental coordination disorder; M-ABC-2 = movement assessment battery for children, 2nd edition; HCTD = hereditary connective tissue disorder; MC = motor coordination; MD = manual dexterity; PRI = perceptual reasoning index; PSI = processing speed index; SD = standard deviation; Tot = total score; Tot IQ = total intelligence quotient; VCI = verbal comprehension index; VMI = developmental test of visual-motor integration; VP = visual perception; WISC-IV = Wechsler intelligence scale for children-fourth edition; WMI = working memory index.
4 DISCUSSION
This is the first study exploring in depth the neurodevelopmental profile of children with HCTDs and JH, by using a full repertoire of psychometric tools for motor, cognitive, executive-attentive and emotional-behavior features in a child neurology and psychiatry setting. The work was limited to children aged between 4 and 13 years in order to obtain homogeneous data. Only two previous researches tried to study the rate and spectrum of neurodevelopmental disorders in patients with EDS. In 1998, Hunter, Morgan, and Bird performed a simple questionnaire study on hearing, speech, voice, and swallowing in 414 members of the UK national EDS support group and various ages. They found a 48% rate of language and speech difficulties in preschool and school age children. In a subsequent study, Adib et al. (2005) carried out a retrospective study in 125 children with JHS/EDS (old criteria) in a pediatric rheumatology setting and found a rate of 48%, 36%, 14%, 7% and 2% for clumsiness, poor coordination, learning difficulties, dyspraxia, and dyslexia, respectively.
The present work is in continuity with our previous observation of features compatible with DCD and ADHD in 55% and 34.8% of the cases, respectively, in a segregation study on JHS/EDS (old criteria) (Castori et al., 2014). Kirby et al. (2005) compared 68 children with JHS/EDS (old criteria) and 58 with DCD for various motor coordinator abilities and found comparable difficulties, except for an excess of difficulties in writing, reading, and ball skills in the latter group. Here, we defined DCD as the most common neurodevelopmental disorder (7/23; 30%) in 23 children with HCTDs after an assessment by a battery of psychometric tests. The rate of DCD in the sample is approximately fivefold higher than in the general population, as DCD has a prevalence of 4–5% in children aged between 5 and 11 years (Lingam et al., 2009). Three patients also featured dysgraphia and this might amplify the clinical impact of such a comorbidity in these subjects. The observation of different neurodevelopmental outcomes in children with HCTDs and the recurrence of multiple diagnoses in the same individual is in line with the concept of “atypical brain development” by Gilger and Kaplan (2001), who introduced this term 17 years ago in order to initiate a debate on the nosology of neurodevelopmental disorders.
LDs were the second most common neurodevelopmental disorder (5/23; 22%) in our sample and presented with a mixture of reading and writing difficulties (i.e., dyslexia and dysorthographia). Also in this case, the rate seems higher than in general population, as a 2012 survey estimated a prevalence of 8% for LDs among children with ages 3–17 years (NSCH, 2015). ADHD was the third more common neurodevelopmental disorder in our sample as it occurred in 13% of the cases, compared to ∼5% in the pediatric population (APA, 2013). Our observation confirms the slight rate increase (7%) of ADHD in EDS adults reported by Hershenfeld et al. (2016). A recent nationwide population-based study in Sweden demonstrated an increased risk of ADHD in people with EDS or JHS, and their siblings (risk ratio 5.6 and 2.1, respectively) (Cederlӧf et al., 2016). Altogether, these data show a high rate of neurodevelopmental disorders in children with HCTDs contrasting with a normal IQ in all subjects. This suggests the existence of selective dysfunctions linking defective connective tissue with abnormal neurodevelopmental outcome possibly excluding central pathways that impact on cognitive development.
In a review paper, Ghibellini et al. (2015) proposed that defective proprioception and/or impaired vestibular system might be the “missing” link between GJH, DCD and related neurodevelopmental disorders. Knee proprioception is commonly impaired in children and adults with JH or JHS/EDS (old criteria) (Fatoye, Palmer, Macmillian, Rowe, & van der Linden, 2009; Hall, Ferrell, Sturrock, Hamblen, & Baxendale, 1995; Pacey, Adams, Tofts, Munns, & Nicholson, 2014; Rombaut, De Paepe, Malfait, Cools, & Calders, 2010; Sahin et al., 2008). While similar results were not confirmed at the shoulders (Rombaut et al., 2010), Mallik, Ferrell, McDonald, and Sturrock (1994) found impaired position sense at the finger proximal interphalangeal joints. Poor position sense at the hands can affect fine motor skills and handwriting, and could result in poor manipulation competences and dysgraphia. Poor proprioception at the knees may trigger a different pattern of muscle activation of the lower limbs (Greenwood, Duffell, Alexander, & McGregor, 2011) and significantly affect balance and lateral trunk stability (Rombaut et al., 2011; Celletti et al., 2011; Falkerslev et al., 2013), thus leading to poor gross motor competence. A single study demonstrated vestibular deficiency and/or insufficient proprioceptive capabilities of the neck in adults with JHS/EDS (old criteria) (Iatridou, Mandalidis, Chronopoulos, Vagenas, & Athanasopoulos, 2014). An impaired vestibular system may affect visual competences with visual tracking issues and reading disorders. Neurodevelopment may be further hampered by the need of concentrating more attention on maintaining posture due to poor balance control (Rigoldi et al., 2013), with significant consequences on executive-attentive skills. Hence, a congenital and widespread “laxity” of the connective tissue can directly affect proprioception and vestibular function, and this might generate abnormal neurodevelopmental patterns without affecting cognition.
While neurodevelopmental disorders were highly represented in group 1, a complementary excess of full-blown HCTDs was not found among children with a primary diagnosis of DCD (group 2). Previous studies emphasized a positive association between GJH and DCD (Celletti et al., 2015; Jelsma et al., 2013; Morrison, Ferrari, & Smillie, 2013). According to the most recent recommendations fixing to six the BS cutoff for GJH in children (Juul-Kristensen et al., 2017), we found GJH in 4 out of 23 (17%) DCD subjects only. Our finding of such a “low” rate of GJH in DCD children is in contrast with previous studies (Ghibellini et al., 2015). A likely explanation is the high inter-observer variability of BS and the lack of consensus in its application among different centers (Remvig, Flycht, Christensen, & Juul-Kristensen, 2014). It is also possible that the high rate of GJH found by other authors mirrors the application of different cutoffs. For example, in the 2015 paper by Celletti et al., the authors followed the recommendations by van der Giessen et al. (2001), who established a cutoff of five for children aged 3 to 9 years, and of four for older children. With different cutoffs, our findings are in line with previous studies, as we found a BS ≥5 and ≥4 in 47.8% and 87.0%, respectively, among children with DCD. Although epidemiological data on GJH in children are scanty (Remvig et al., 2007), reinterpretation of available data suggests that children with DCD usually present JH in a high number of joints and this might be defined GJH according to the applied cutoff. The fact that such a presumed excess of JH in children with DCD might contribute to the pathogenesis of selected features and/or represent a phenotypic discriminator for a subset of patients is intriguing. Anyway, we failed to find relevant differences between DCD children with or without GJH, and our sample was too small for definitively supporting this evidence.
Further insights come from the comparison between children with HCTDs (group 1) and those with a primary diagnosis of DCD (group 2). Worsen scores in the visual integration test (VMI) and in many motor performance and handwriting skills (M-ABC-2) were found in the DCD group, while an excess of somatic complaints and problems at CBCL, physical symptoms at MASC, and opposition behaviors at CRS-R:S occurred in the HCTDs group. Although the DCD group presented more motor and coordination troubles, quality of life of children with HCTDs resulted more deteriorated due to somatic manifestations and, possibly, behavioral traits. This did not reflect differences in cognitive and motor abilities in these two groups (Table 7). Hence, HCTDs children present a more complex overall clinical picture than nonsyndromic DCD patients with coordination troubles intermingling with the other pleiotropic manifestations of the syndrome.
In conclusion, our work showed a wide array of neurodevelopmental comorbidities (i.e., DCD, LDs, and ADHD) in HCTDs, and compared the neurodevelopmental profile of HCTDs and DCD children. The results suggest the opportunity of prompt neurodevelopmental assessment in all children with a definite diagnosis of HCTD for early diagnosis and intervention. More research is needed for elucidating the link between connective tissue, JH and nonsyndromic DCD. Although HCTDs are apparently rare among children primarily assessed for DCD, the presence of poor quality of life and satellite somatic symptoms could prompt the exclusion of an underlying HCTD by referring the patient to specialized clinical genetics centers.
ACKNOWLEDGMENTS
The authors wish to thank the patients and parents for their kind availability and support in this study.
CONFLICT OF INTEREST
All authors have no conflict of interest to declare.




