Association of copy number polymorphisms at the promoter and translated region of COMT with Japanese patients with schizophrenia
Abstract
Chromosome 22q11.2 deletion syndrome and genetic variations including single-nucleotide polymorphism (SNP) and copy number variation (CNV) in catechol-O-methyltransferase (COMT) situated at 22q11.2 remains controversial. Here, the genetic relationship between COMT and Japanese patients with schizophrenia was investigated by examining whether the SNPs correlated with schizophrenia based on a common disease–common variant hypothesis. Additionally, 22q11.2DS were screened based on a common disease–rare variant hypothesis; low-frequency CNVs situated at two COMT promoters and exons were investigated based on the low-frequency variants with an intermediate effect; and positive findings from the first stage were reconfirmed using a second-stage replication study including a larger sample size. Eight SNPs and 10 CNVs were investigated using Taqman SNP and CNV quantitative real-time polymerase chain reaction method. For the first-stage analysis, 513 unrelated Japanese patients with schizophrenia and 705 healthy controls were examined. For the second-stage replication study, positive findings from the first stage were further investigated using a larger sample size, namely 1,854 patients with schizophrenia and 2,137 controls. The first-stage analysis showed significant associations among schizophrenia, intronic SNP rs165774, CNV6 situated at promoter 1, CNV8 at exon 6, and CNV9 at exon 7. The second-stage study showed that intronic SNP rs165774 (χ2 = 8.327, P = 0.0039), CNV6 (χ2 = 19.66, P = 0.00005), and CNV8 (χ2 = 16.57, P = 0.00025) were significantly associated with schizophrenia. Large and rare CNVs as well as low-frequency CNVs and relatively small CNVs, namely <30 kb in COMT, may be genetic risk factors for schizophrenia. © 2016 Wiley Periodicals, Inc.
INTRODUCTION
Schizophrenia is a debilitating disease with a prevalence of approximately 0.5–1% within a given population. The dopamine hypothesis of schizophrenia has been one of the most enduring ideas in psychiatry [Howes and Kapur, 2009]; however, the apparent pathophysiology has not yet been identified from a genetic, neurotransmissional, neurodevelopmental, and environmental point of view. From the genetic point of view and with regard to dopamine hypothesis, catechol-O-methyltransferase (COMT), an enzyme that catalyzes the O-methylation of catecholamine neurotransmitters, is a candidate gene of most concern for schizophrenia; several genetic case-control studies for COMT have been performed using single-nucleotide polymorphism (SNP) based on the common disease–common variant hypothesis. Among these, the most important SNP is Val->Met polymorphism (rs4680) that resulted in a change in the enzyme activity [Chen et al., 2004a], and haplotypes including this SNP were reported as a genetic risk factor of schizophrenia [Karayiorgou et al., 1998; Ohmori et al., 1998; Shifman et al., 2002; Inada et al., 2003; Chen et al., 2004b; Wang et al., 2010]. The heritability of rs4680 SNP is nominal and could not show the abundant risk for schizophrenia in all replication studies [Okochi et al., 2009] (also see SZGene section of the Schizophrenia Research Forum; http://www.szgene.org/).
Based on the findings of rare deletions of 1.5–3 MB of chromosome 22q11, chromosome 22q11.2 deletion syndrome (22q11.2DS) frequently showed psychotic symptoms and contributed toward genetic risk of schizophrenia [Karayiorgou et al., 1995; Lindsay et al., 1995a, 1995b]. Because the abovementioned COMT was situated at this region, the region was considered to be associated with schizophrenia to a substantially higher degree in the past than it is now [Arinami et al., 2001; Ivanov et al., 2003; Bassett et al., 2008; Kirov et al., 2009; Grozeva et al., 2010; Karayiorgou et al., 2010; Levinson et al., 2011]. In addition, these rare copy number variations (CNVs) could be one of the candidate genetic markers, thereby providing a strong effective genetic risk factor based on the common disease–rare variant hypothesis. Rare CNVs in 1q21.1, 15q11.2, and 15q13.3 showed associations with schizophrenia [International-Schizophrenia-Consortium, 2008; Stefansson et al., 2008]; thereafter, large comprehensive CNV studies were performed to survey de novo CNV in schizophrenia, and they revealed an association between CNVs in 22q11, COMT, and schizophrenia [Bassett et al., 2008; Guilmatre et al., 2009; Kirov et al., 2009; Saus et al., 2010; Buizer-Voskamp et al., 2011; Levinson et al., 2011; Grozeva et al., 2012]. However, genetic CNV studies of schizophrenia could not always show the abundant risk at 22q11 region [Grozeva et al., 2010; Ikeda et al., 2010].
Within genome-wide rare CNV studies, the probe for detecting rare CNVs would be set up to be as sensitive as possible. However, evidence for this rare variant hypothesis is limited, whereas the genetic influence of low-frequency variants with intermediate effect and their combination with rare variants were noteworthy in some common diseases [Manolio et al., 2009]. For CNVs, these were defined as copy number polymorphisms (CNPs) [minor allele frequencies (MAF) of >5%] [Manolio et al., 2009]. Researchers including ourselves previously reported certain positive associations between schizophrenia and relatively common CNPs in genes situated at 22q11 such as glutathione S-transferase (GSTs) theta 1 (GSTT1) [Saadat et al., 2007; Gravina et al., 2011; Raffa et al., 2013], theta 2 (GSTT2) [Rodriguez-Santiago et al., 2010], and d-dopachrome tautomerase-like protein (DDTL) [Nakamura et al., 2015].
The aim of the present study was to investigate the genetic relationship between COMT and Japanese patients with schizophrenia using the following steps: (i) examining the association of the aforementioned SNPs with schizophrenia in Japanese patients based on a common disease–common variant hypothesis by performing a case-control genetic study using Japanese common tag SNPs and candidate SNPs, which showed replicative significant associations with schizophrenia (e.g., rs4680 Val/Met); (ii) screening 22q11.2DS based on a common disease–rare variant hypothesis; (iii) investigating low-frequency CNPs situated at two COMT promoters and exons that could cause a change in transcript levels and a substitution of amino acids based on the low-frequency variants with intermediate effect; and (iv) reconfirming positive findings from the first stage using a second-stage replication study including a larger number of patients and controls. Finally, the contributions of the combination of these genetic mechanisms of COMT to cause the onset of schizophrenia were assessed.
MATERIALS AND METHODS
Participants
For the first stage of the study, a case-control genetic association was performed using 513 unrelated Japanese patients with schizophrenia (273 males and 240 females; mean age, 39.2 years; standard deviation [S.D.], ±13.5). All patients met the criteria for schizophrenia based on structured clinical interviews according to the Diagnostic and Statistical Manual of Mental Disorders-IV (DSM-IV). A total of 705 healthy controls (343 males and 362 females; mean age, 46.1 years; S.D., ±17.9) were additionally included and examined. Healthy controls did not meet the current or past criteria for any Axis I disorders (from the DSM-IV). In addition, all participants met the following criteria: (i) no evidence of systemic or neurological diseases; (ii) no prior head trauma with loss of consciousness; and (iii) no lifetime history of alcohol or substance dependency. Patients and controls for the first-stage study were recruited from two geographic regions in eastern Japan, namely Saitama and Tokyo. For the first-stage case-control genetic study, the mean age for the patients with schizophrenia was significantly younger than that of the controls (Student's t-test: t = 7.33, P < 0.001). The distribution between males and females within the two groups was not significantly different (χ2 = 3.03, P = 0.08).
The positive findings obtained from the first stage were further investigated by a second-stage case-control genetic association study as a multicenter study from four geographic regions within Japan, namely Saitama, Tokyo, Osaka, and Aichi. Second-stage study subjects were performed using a total of 1,854 (928 males and 924 females; age, 44.0 ± 15.1 years) patients with schizophrenia and 2,137 (1,084 males and 1,052 females; age, 41.6 ± 16.1 years) normal controls (the information for age and sex of two patients and one control subject were missing). The sex distribution was not different between the groups; however, the mean age of patients with schizophrenia was significantly higher than that of the controls (t = 4.81, P < 0.001). Written informed consent was obtained from all subjects after the procedures had been fully explained. The present study was conducted in compliance to the World Medical Association's Declaration of Helsinki and was approved by the Research Ethical Committees of Juntendo University, Osaka University, Fujita Health University, and Nagoya University.
SNP Selection and Genotyping
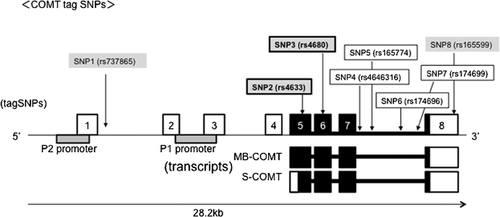
Genomic DNA was extracted from peripheral white blood cells using a QIAamp® DNA Blood Maxi kit (Qiagen, Courtaboeuf, France). For the selection of SNPs, tag SNPs for each gene (r2 > 0.8; MAF > 0.05] were chosen from the International HapMap Project database (release 27 Phase II + III, Feb 2009, on NCBI B36 assembly; dbSNP b126) using the TAGGER algorithm with a successful TaqMan probe design; rs4633, rs4680, rs4646316, rs165774, rs174696, and rs174699 were selected (the “rs” notation in front of each SNP represents the identification from the US National Center for Biotechnology Information SNP cluster within the dbSNP database; http:/www.ncbi.nlm.nih.gov/SNP/). In addition, five SNPs with an MAF of >0.05 within a Japanese population that have shown genetic associations with schizophrenia in its individual association or haplotype association, namely rs737865, rs4633, rs4680, and rs165599, were added. Among these, two SNPs, namely rs4633 and rs4680, were additionally selected as tag SNP for a final number of eight SNPs used in the present study, that is, rs737865, rs4633, rs4680, rs4646316, rs165774, rs1746946, rs174699, and rs165599. rs4680 (G > A Val/Met) was a missense mutation at exon 6, rs4633 was a nonsense mutation at exon 5, rs165599 was situated at 3′ UTR in exon 8, and all other SNPs were intronic SNPs. The locations of these SNPs are shown in Figure 1.
All investigated SNPs were typed by TaqMan® technology using an ABI7500 system (Applied Biosystems, Foster City, CA). All probes and primers were designed by the Assay-by-Design™ service for Applied Biosystems. The polymerase chain reaction (PCR) was performed using the standard PCR MasterMix reagent kit with a volume of 4 μl. Detailed information on PCR conditions is available upon request.
CNV Selection and Determination of Relative Copy Numbers by Quantitative Real-Time (QRT)-PCR
Screening of 22q11.2DS using CNV
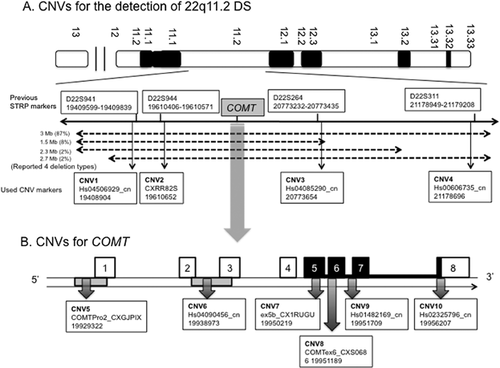
To discuss the relationship of COMT with schizophrenia, 22q11.2DS should not be disregarded and need to be screened. To screen the known four types of 22q11.2DS, we modified the previous screening methods using short tandem-repeat polymorphic (STRP) markers [Morrow et al., 1995; Arinami et al., 2001; Toyosima et al., 2011]. We used CNV markers instead of microsatellite markers because in the next step, we additionally performed CNV analysis for COMT situated in the 22q11 region. Thus, performing CNV analysis for both screening 22q11.2DS and COMT using the same reagents simultaneously could result in a much more cost- and time-effective method. Instead of the reported four microsatellite markers (D22S941, D22S944, D22S264, and D22S311) that could be used to accurately screen the known four types of 22q11.2DS (detailed principal was described in two papers [Morrow et al., 1995; Ivanov et al., 2003]), we selected validated CNV assays from designed probes and primers by TaqMan® Copy Number Assay or Custom TaqMan® Copy Number Assays service for Applied Biosystems. Primers and TaqMan probes for duplex QRT-PCR were designed to specifically amplify the target gene and to avoid paralogous or allelic sequence variants, which were proximate to the neighboring STRP markers mentioned above, respectively. The CNV markers used instead of STRP markers were Hs04506929 (CNV1) for D22S941 (distance from STRP marker; 695bp), Custom_CXRR82S (CNV2) for D22S944 (81 bp), Hs04085290 (CNV3) for D22S264 (219 bp), and Hs00606735 (CNV4) for D22S311 (253 bp). Locations and detailed information for these markers and the relationships with known 22q11.2DS are listed in Supplementary Table SI and Figure 2A.
CNV analysis in COMT
From the genomic structure of COMT, CNVs were selected based mainly on the “position effect” or “gene interruption” of CNVs for the disease [Lupski and Stankiewicz, 2005; Lee et al., 2007] according to the following criteria: (i) CNVs situated at promoter 2 (CNV5; COMTPro2_CXGJPIX, chr22:19929322) and 1 regions (CNV6; Hs04090456, chr22.19938973) overlap from exon 2 to exon 3 that might cause the change in transcript levels; (ii) CNVs situated at the exons of translated regions, namely exon 5 (CNV7; ex5b_CX1RUGU, chr22:19950219), exon 6 (CNV8; COMTex6_CXS0686, chr22:19951189), exon 7 (CNV9; Hs01482169, chr22 19951709), and exon 8 (CNV10; Hs02325796_cn, Chr22:19956207), which could change the amino acids; and (iii) a comprehensive study showed that CNVs had already showed positive association with schizophrenia [Saus et al., 2010]. The probe position for detecting COMT CNVs in a previous study [Saus et al., 2010], namely chr22 18,335,516–18,335,575 in GRC36hg18, could be converted to the position in the new assembly at chr22, namely 19,929,309–19,956,530 in GRCh37hg19. Therefore, this CNV overlapped into the aforementioned CNV5. Finally, six validated CNVs for COMT were additionally selected from the designed probes and primers by TaqMan® Copy Number Assays or Custom TaqMan® Copy Number Assays service for Applied Biosystems (Supplementary Table SI and Fig. 1B).
Determination of Relative Copy Numbers by QRT-PCR
The aforementioned CNVs were determined using QRT-PCR with TaqMan® Copy Number Assays [Covault et al., 2003] and validated probes and primers. All QRT-PCR reactions were performed on an ABI Prism 7500 Instrument (Applied Biosystems) with Sequence Detection Software version 1.3.1 with Ribonuclease P (RNase P) as a single copy number (CN), as reported previously [Nakamura et al., 2015]. More than four CNs were not reliable for the expression of actual gained CNs; therefore, these CNs were revealed as “3+,” that is, gained CNs. The detailed protocol can be provided upon request.
Statistical Analyses
Differences in mean age and sex ratios between healthy controls and patients were identified using two-tailed Student's t-tests and chi-square (χ2) tests, respectively, using SPSS Statistics Version 21 (IBM, Chicago, IL). For the case-control association study, Hardy–Weinberg equilibrium (HWE) tests for SNPs were performed using SNPAlyze Ver. 7.0 Pro (Dynacom, Yokohama, Japan). The HWE tests were performed for all loci in patients and controls. Differences in genotypic and allelic frequencies were evaluated using χ2 difference tests. Linkage disequilibrium (LD) denoted as D′ was calculated from haplotype frequencies using an expectation–maximization algorithm. The LD block was additionally identified using SNPAlyse Ver. 7.0 Pro when D′ was greater than 0.9. Case-control haplotype analyses were additionally performed using SNPAlyse software. Permutation analyses were used to determine empirical significance, and calculations for the P values were based on 10,000 replications. Global P values represented the overall significance for the χ2-difference tests when both the observed versus expected frequencies for all haplotypes were simultaneously considered. In addition, individual haplotypes were tested for associations by grouping all other haplotypes together and running χ2-tests using 1 df. Power calculations were conducted using the Power Calculator for Two Stage Association Studies (http://www.sph.umich.edu/csg/abecasis/CaTS/). Differences in CNs between study groups were identified using Fisher's exact tests with Yates’ continuity correction in SPSS Statistics Version 21. When the results from Fisher's exact tests showed statistical significance, the cells showing a value of standardized residual of >±1.96 were considered as significantly effective factors from residual analysis. All reported P values are two tailed.
RESULTS
First-Stage Genetic Case-Control Analyses for SNPs on COMT
Genotyping call rates for the eight SNPs during the first-stage study were 99.0% (SNP1), 98.9% (SNP2), 99.4% (SNP3), 98.3% (SNP4), 99.2% (SNP5), 98,4% (SNP6), 99.7% (SNP7), and 99.1% (SNP8). In addition, to ensure the quality of the results, we confirmed the SNPs from 380 randomly chosen subjects for each SNP using the same method to check for errors using the TaqMan method. All genotypes determined by replicative TaqMan methods were in agreement with the genotypes obtained from the first TaqMan method for all investigated SNPs. No deviation from HWE in the examined SNPs was detected in the patients with schizophrenia or healthy controls (Table I; P > 0.05). Power estimates were based on allelic frequencies for associated markers ranging from 0.156 (rs165774) to 0.439 (rs156699), with odds ratios ranging from 1.002 (rs4646316) to 1.418 (rs165774) for the investigated SNPs with an alpha level of 0.05/8. Values of power were calculated using a prevalence rate below 0.01 with an additive or a multiplicative model, assuming various degrees of allelic frequencies and the odds ratios for the SNPs. Results of power analysis showed the power to range from 1% (rs4646316) to 70% (rs165774).
| Genotype frequency (%) | P | HWE c/s | Allele frequency (%) | χ2 | P | Odds ratio (95%CI) | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| rs737865 | A/A | A/G | G/G | 0.484 | 0.618/0.671 | A | G | 1.419 | 0.234 | 0.896 |
| Schizophrenia | 263 (52.4) | 204 (40.6) | 35 (7.0) | 730 (72.7) | 274 (27.3) | (0.748–1.074) | ||||
| Controls | 340 (49.2) | 294 (42.5) | 57 (8.2) | 974 (70.5) | 408 (29.5) | |||||
| rs4633 | C/C | C/T | T/T | 0.182 | 0.765/0.716 | C | T | 3.046 | 0.081 | 1.17 |
| Schizophrenia | 228 (45.4) | 224 (44.6) | 50 (10.0) | 680 (67.7) | 324 (32.3) | (0.981–1.395) | ||||
| Controls | 351 (50.8) | 280 (40.5) | 60 (8.7) | 982 (71.1) | 400 (28.9) | |||||
| rs4680 | G/G | G/A | A/A | 0.078 | 0.252/0.585 | A | G | 3.466 | 0.063 | 0.848 |
| Schizophrenia | 215 (42.8) | 232 (46.2) | 55 (11.0) | 342 (34.1) | 662 (65.9) | (0.713–1.009) | ||||
| Controls | 341 (49.3) | 279 (40.4) | 71 (10.3) | 421 (30.5) | 961 (69.5) | |||||
| rs4646316 | C/C | C/T | T/T | 0.901 | 0.887/0.486 | C | T | 2.757 × 10−4 | 0.987 | 1.002 |
| Schizophrenia | 259 (51.6) | 198 (39.4) | 45 (9.0) | 716 (71.3) | 288 (28.7) | (0.837–1.199) | ||||
| Controls | 353 (51.1) | 280 (40.5) | 58 (8.4) | 986 (71.3) | 396 (28.7) | |||||
| rs165774 | G/G | G/A | A/A | 0.0056 | 0.822/0.595 | G | A | 10.616 | 1.121 × 10−3 | 1.418 |
| Schizophrenia | 318 (63.3) | 160 (31.9) | 24 (4.8) | 796 (79.3) | 208 (20.7) | (1.149–1.751) | ||||
| Controls | 494(71.5) | 179 (25.9) | 18 (2.6) | 1167 (84.4) | 215 (15.6) | |||||
| rs174696 | C/C | C/T | T/T | 0.06 | 0.565/0.378 | T | C | 4.300 | 0.038 | 0.841 |
| Schizophrenia | 135 (26.9) | 261 (52.0) | 106 (21.1) | 473 (47.1) | 531 (52.9) | (0.714–0.990) | ||||
| Controls | 230 (33.3) | 330 (47.8) | 131 (19.0) | 592 (42.8) | 790 (57.2) | |||||
| rs174699 | T/T | T/C | C/C | 0.037 | 0.210/0.179 | T | C | 2.964 | 0.085 | 0.863 |
| Schizophrenia | 197 (39.2) | 246 (49.0) | 59 (11.8) | 640 (63.7) | 364 (36.3) | (0.730–1.021) | ||||
| Controls | 260 (37.6) | 313 (45.3) | 118 (17.1) | 833 (60.3) | 549 (39.7) | |||||
| rs165599 | A/A | A/G | G/G | 0.113 | 0.036/0.734 | G | A | 0.600 | 0.439 | 1.067 |
| Schizophrenia | 155 (30.9) | 269 (53.6) | 78 (15.5) | 425 (42.3) | 579 (57.7) | (0.906–1.257) | ||||
| Controls | 220 (31.8) | 335 (48.5) | 136 (19.7) | 607 (43.9) | 775 (56.1) | |||||
| Haplotype analysis (global P-value) | ||||||||
|---|---|---|---|---|---|---|---|---|
| Two SNP-based haplotype analysis | Three SNP-based haplotype analysis | |||||||
| Haplotype frequency (%) | P | Haplotype frequency (%) | P | |||||
| A–C | A–T | G–C | 0.164 | |||||
| 420 (41.8) | 309 (30.8) | 259 (25.8) | ||||||
| 607 (43.9) | 368 (26.6) | 376 (27.2) | 0.339 | A–C–G | A–T–A | G–C–G | 0.67 | |
| C–G | T–A | 402 (40.0) | 304 (30.3) | 250 (24.9) | ||||
| 651 (64.8) | 314 (31.3) | 574 (41.5) | 368 (26.6) | 373 (27.0) | ||||
| 947 (68.5) | 386 (27.9) | C–G–C | T–A–C | C–G–T | ||||
| G–C | A–C | G–T | 0.242 | 365 (36.4) | 311 (31.0) | 285 (28.4) | 0.277 | |
| 377 (37.5) | 340 (33.9) | 285 (28.4) | 557 (40.3) | 384 (27.8) | 390 (28.2) | |||
| 572 (41.4) | 415 (30.0) | 388 (28.1) | G–C–G | G–T–G | A–C–A | A–C–G | ||
| C–G | T–G | C–A | 3.00 × 10−3 | 375 (37.4) | 284 (28.3) | 208 (20.7) | 132 (13.1) | 0.045 |
| 507 (50.5) | 289 (28.8) | 208 (20.7) | 567 (41.0) | 388 (28.1) | 211 (15.3) | 203 (14.7) | ||
| 773 (55.9) | 395 (28.6) | 214 (15.5) | C–G–C | T–G–T | C–A–T | |||
| G–C | G–T | A–T | 0.02 | 488 (48.6) | 269 (26.8) | 187 (18.6) | 0.055 | |
| 507 (50.5) | 288 (28.7) | 186 (18.5) | 738 (53.4) | 366 (26.5) | 193 (14.0) | |||
| 766 (55.4) | 401 (29.0) | 191 (13.8) | G–C–C | G–T–T | G–C–T | A–T–T | ||
| T–T | C–C | C–T | 9.00 × 10−3 | 323 (32.2) | 252 (25.1) | 184 (18.3) | 187 (18.6) | 0.015 |
| 438 (43.6) | 327 (32.6) | 203 (20.2) | 464 (33.6) | 326 (23.6) | 301 (21.8) | 187 (13.5) | ||
| 513 (37.1) | 470 (34.0) | 319 (23.1) | T–T–A | C–C–G | C–T–A | T–C–G | ||
| T–A | C–G | T–G | 0.124 | 394 (39.2) | 319 (31.8) | 180 (17.9) | 36 (3.6) | 0.037 |
| 572 (57.0) | 355 (35.4) | 68 (6.8) | 467 (33.8) | 449 (32.5) | 286 (20.7) | 79 (5.7) | ||
| 753 (54.5) | 528 (38.2) | 79 (5.7) | ||||||
- P-values reached statistical significances (corrected significant levels of P-values with means of Bonferroni correction were as follows: single SNP of <0.006; two SNP-based haplotype analysis of <0.007, and three SNP-based haplotype analysis of <0.008) and are indicated in bold.
- aHWE, Hardy–Weinberg equilibrium P-value; c/s, controls/schizophrenia.
- bMinor haplotypes with frequencies less than 3% in either group were omitted.
A single SNP, namely rs165774, showed significant association with schizophrenia in its genotypic and allelic analyses (Table I). The two SNP-based haplotype analysis between this SNP and the adjacent SNP rs4646316 additionally showed a positive association with schizophrenia after conducting strict tests for multiple comparisons; it was assumed that the C–G haplotypes were significantly lower in patients with schizophrenia (50.5%) than in controls (55.9%), whereas C–A haplotypes were significantly higher in patients (20.7%) than in controls (15.5%) (Table I). Single SNP analysis with rs174699 and two SNP-based haplotype analysis between rs165774-rs174696 and rs174696-rs174699 as well as three SNP-based haplotype analysis among rs4680-rs4646316-rs165774, rs165774-rs174696-174-699, and rs174696-rs174699-rs165599 showed marginal association with schizophrenia; however, none of these survived under the corrected P value.
D′ of >0.9 was assumed to represent a strong LD, and results indicated that SNP2 to SNP5 and SNP7 to SNP8 displayed a strong LD block in both controls and patients with schizophrenia (Supplementary Fig. S1). Furthermore, there were no larger LD blocks than those in the aforementioned three-window haplotype blocks; thus, additional haplotype block analyses were not performed.
Second-Stage Genetic Case-Control Study With Large Samples for Extending Results From the First-Stage SNPs Study
Positive associations for a single SNP, rs165774, and two SNP-based haplotype analysis between this SNP and the adjacent SNP rs4646316 were re-investigated using second-stage replication samples (1,854 patients with schizophrenia and 2,137 normal controls). Power estimates were based on allelic frequencies for the associated markers 0.258 (rs4646316) and 0.159 (rs165774), with odds ratios of 1.024 (rs4646316) and 1.190 (rs165774) for the investigated SNPs and with an alpha level of 0.05/2. Results of power analysis using this large number of subjects showed the power ranging from 6% (rs4646316) to 100% (rs165774). A single SNP, rs165774, again showed significant association with schizophrenia during genotypic and allelic analysis (Table II). In addition, the two SNP-based haplotype analysis between this SNP and the adjacent SNP rs4646316 once again showed a positive association with schizophrenia; hence, it is was assumed that the C–G haplotypes were significantly lower in patients with schizophrenia (53.0%) than in controls (55.9%), whereas C–A haplotypes were significantly higher in patients (18.3%) than in controls (15.9%) (Table II).
| Genotype frequency (%) | P | HWEa c/s | Allele frequency (%) | χ2 | P | Odds ratio (95%CI) | Two SNP-based haplotype analysis (global P-value) Haplotype frequencyb (%) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| rs4646316 | C/C | C/T | T/T | 0.816 | 0.511/0.305 | C | T | 0.324 | 0.569 | 1.029 | ||||
| Schizophrenia | 956 (51.3) | 743 (39.9) | 163 (8.8) | 2655 (71.3) | 1069 (28.7) | (0.932–1.133) | C–G | T–G | C–A | 0.006 | ||||
| Controls | 1112 (52.0) | 852 (39.8) | 176 (8.2) | 3076 (71.9) | 1204 (28.1) | 1973 (53.0) | 1065 (28.6) | 681 (18.3) | ||||||
| rs165774 | G/G | G/A | A/A | 0.016 | 0.610/0.795 | G | A | 8.327 | 0.0039 | 0.842 | 2393 (55.9) | 1203 (28.1) | 681 (15.9) | |
| Schizophrenia | 1243 (66.8) | 554 (29.8) | 65 (3.5) | 3040 (81.6) | 684 (18.4) | (0.750–0.947) | ||||||||
| Controls | 1516 (70.8) | 566 (26.4) | 58 (2.7) | 3598 (84.1) | 682 (15.9) | |||||||||
- P-values reached statistical significances (corrected significant levels of P-values with means of Bonferroni correction were as follows: single SNP of <0.025; two SNP-based haplotype analysis of <0.05) and are indicated in bold.
- a HWE, Hardy–Weinberg equilibrium P-value; c/s, controls/schizophrenia.
- b Minor haplotypes with frequencies less than 3% in either group were omitted.
A Comparison of the Distribution of CNVs at the 22q11.2 Region and the COMT Gene Between Patients With Schizophrenia and Controls
CNVs in the 22q11.2 region (for 22q11.2 DS) and COMT were additionally studied in 513 patients with schizophrenia and in 705 controls by means of QRT–PCR assay. As shown in Table IV, CNVs varied both in patients and in controls. CNVs for the 22q11.1 DS (showing all single copies from CNV1 to CNV10) were observed in one (0.2%) individual with schizophrenia and one (0.1%) normal control (Table III). The frequencies of subjects in each CNV for 22q11.2 DS, CNV1, CNV2, CNV3, and CNV4 were not significantly different between the groups (Table III). In contrast, for the CNVs of the COMT, the frequencies of subjects in CNV6, CNV8, and CNV9 were significantly different between the groups after conducting strict tests for multiple comparisons (P < 0.005, Table III). In these three CNVs, loss and gain CNs were significantly higher, and normal two CNs were lower in patients with schizophrenia than in controls (standardized residual of >±1.96; Table III).
| Rate; n (%) | |||||
|---|---|---|---|---|---|
| CNVs | CN | Controls (705) | Schizophrenia (513) | Standardized residual | Fisher's exact test χ2 value (P-value) |
| CNVs for 22q11.2 DS | |||||
| CNV1 | 1 | 8 (1.1%) | 14 (2.7%) | 5.66 (0.059) | |
| 2 | 690 (97.9%) | 490 (95.5%) | |||
| 3+ | 7 (1.0%) | 9 (1.8%) | |||
| CNV2 | 1 | 40 (5.7%) | 17 (3.3%) | 7.07 (0.070) | |
| 2 | 544 (77.2%) | 424 (82.7%) | |||
| 3+ | 120 (17.0%) | 72 (14.0%) | |||
| CNV3 | 1 | 8 (1.1%) | 10 (1.9%) | 2.41 (0.30) | |
| 2 | 678 (96.2%) | 484 (94.3%) | |||
| 3+ | 19 (2.7%) | 19 (3.7%) | |||
| CNV4 | 1 | 6 (0.9%) | 12 (2.3%) | 7.06 (0.029) | |
| 2 | 697 (98.9%) | 496 (96.7%) | |||
| 3+ | 2 (0.3%) | 5 (1.0%) | |||
| 22q11.2 DSa | 1 (0.14%) | 1 (0.2%) | 0.05 (0.822) | ||
| CNVs in COMT | |||||
| CNV5 | 1 | 9 (1.4%) | 11 (6.8%) | 9.04 (0.011) | |
| 2 | 669 (95.4%) | 466 (84.2%) | |||
| 3+ | 24 (3.2%) | 35 (9.0%) | |||
| CNV6 | 1 | 6 (0.9%) | 14 (2.8%) | ±2.6 | 12.96 (0.002) |
| 2 | 692 (98.6%) | 481 (95.1%) | ±3.6 | ||
| 3+ | 4 (0.6%) | 11 (2.2%) | ±2.5 | ||
| CNV7 | 1 | 7 (1.0%) | 15 (3.0%) | 9.02 (0.011) | |
| 2 | 668 (95.4%) | 463 (91.5%) | |||
| 3+ | 25 (3.6%) | 28 (5.5%) | |||
| CNV8 | 1 | 4(0.6%) | 12 (2.4%) | ±2.7 | 12.64 (0.002) |
| 2 | 689 (98.1%) | 478 (94.5%) | ±3.5 | ||
| 3+ | 9 (1.3%) | 16 (3.2%) | ±2.3 | ||
| CNV9 | 1 | 2 (0.3%) | 8 (1.6%) | ±2.3 | 12.21 (0.002) |
| 2 | 691 (98.4%) | 481 (95.1%) | ±3.4 | ||
| 3+ | 9(1.3%) | 17 (3.4%) | ±2.5 | ||
| CNV10 | 1 | 3 (0.4%) | 9 (1.8%) | 10.54 (0.005) | |
| 2 | 669 (95.4%) | 461 (91.1%) | |||
| 3+ | 29 (4.1%) | 36 (7.1%) | |||
- The rates in each population (columns 1 and 2) are presented as the number of CNV carriers/number of all tested individuals.
- P-values reached statistical significance (corrected significant levels of P-values of <0.005) and are indicated in bold. 22q11.2 DS, 22q11.2 deletion syndrome.
- a 22q11.2 DS were categorized as subjects who showed one copy for investigated CNV1 to CNV4 (accompanied with one copy for CNV5 to CNV10).
Second-Stage Genetic Case-Control Study With Large Samples for Extending Results From the First-Stage CNV Study
The second-stage CNV replication study was additionally performed for focusing on CNV6, CNV8, and CNV9 in second-stage subjects (1,854 patients with schizophrenia and 2,137 controls). Results showed significant differences in the frequencies of subjects with regard to CNV6 and CNV8 between the groups. In addition, loss and gain CNs were significantly higher, and normal two CNs were lower in patients with schizophrenia than in controls (Table IV). The positive findings in CNV9 from the first-stage study disappeared with a restricted corrected P value of <0.0167. The case-control analysis of three CNV combinations is shown in Table V. There were significant differences in subject frequencies in combination CNs between groups. On comparison with standardized residuals, normal CNs throughout the three CNVs (six CNs; 2-2-2) were lower in patients with schizophrenia than in controls (standardized residual; −3.1); in addition, the smallest loss CNs (three CNs; 1-1-1) and largest gain CNs (eight CNs and nine CNs) were higher in patients with schizophrenia than in controls (standardized residual; 2.8, 2.3, and 2.6, respectively). Fewer alterations in CNs, four CNs, five CNs, and seven CNs were not altered between the groups (standardized residual of <1.96).
| Rate; n (%) | |||||
|---|---|---|---|---|---|
| CNVs | CN | Controls (2,137) | Schizophrenia (1,854) | Standardized residual | Fisher's exact test χ2 value (P-value) |
| CNV6 | 1 | 6 (0.3%) | 16 (0.9%) | 2.5 | 19.66 (0.00005) |
| 2 | 2126 (99.5%) | 1816 (98.0%) | −4.4 | ||
| 3+ | 5 (0.2%) | 22 (1.2%) | 3.7 | ||
| CNV8 | 1 | 4 (0.2%) | 14 (0.8%) | 2.7 | 16.57 (0.00025) |
| 2 | 2122 (99.3%) | 1813 (97.8%) | −4.0 | ||
| 3+ | 11 (0.5%) | 27 (1.5%) | 3.1 | ||
| CNV9 | 1 | 3 (0.1%) | 11 (0.6%) | 7.7 (0.021) | |
| 2 | 2111 (98.8%) | 1814 (97.8%) | |||
| 3+ | 23 (1.1%) | 29 (1.6%) | |||
- P-values reached statistical significances (corrected significant levels of P-values of <0.0167) and standardized residuals of >|1.96| and are indicated in bold.
| CNV6-CNV8-CNV9 | Fisher's exact test | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Total CNs | 3 | 4 | 5 | 6 | 7 | 8 | 9 | Total | χ2 value (P-value) | |
| Combinationsa | 1-1-1 | 2-1-1b | 2-2-1c | 2-2-2 | 2-2-3d | 2-3-3e | 3-3-3 | |||
| Schizophrenia | n | 9 (0.5%) | 5 (0.3%) | 4 (0.2%) | 1,793 (96.7%) | 18 (1.0%) | 15 (0.8%) | 10 (0.5%) | 1,854 | |
| Controls | n | 1(0.0%) | 2 (0.1%) | 6 (0.3%) | 2,099 (98.2%) | 21 (1.0%) | 6 (0.3%) | 2 (0.1%) | 2,137 | 27.61 (0.001) |
| Total | 10 (0.3%) | 7 (0.2%) | 10 (0.3%) | 3,892 (97.5%) | 39 (1.0%) | 21 (0.5%) | 12 (0.3%) | 3,991 | ||
| Standardized residual | 2.8 | 1.3 | −0.4 | −3.1 | 0 | 2.3 | 2.6 | |||
- Standardized residual of >|1.96| is indicated in bold.
- a Actual CNV combination.
- b 1-1-2 = 5; 1-2-1 = 0; 2-1-1 = 6.
- c 1-2-2 = 10; 2-1-2 = 4, 2-2-1 = 5.
- d 2-2-3 = 85, 2-3-2 = 12, 3-2-2 = 18, 1-3-3 = 2.
- e 2-3-3 = 17, 3-2-3 = 4, 3-3-2 = 2.
DISCUSSION
In the present study, we performed a genetic case-control study focusing on the 22q11.2 region, particularly on the most notable gene in schizophrenia, that is, COMT, using susceptibility SNPs and CNVs. In the first genetic case-control study, we failed to show the significant associations with previously reported positive-association SNPs, namely rs737865 [Shifman et al., 2002; Chen et al., 2004b], rs4633 [Wang et al., 2010], and the most noteworthy miss-sense SNP rs4680 [Karayiorgou et al., 1998; Ohmori et al., 1998; Shifman et al., 2002; Inada et al., 2003; Chen et al., 2004b; Wang et al., 2010]. It is not surprising that a case-control study comprising the largest number of subjects (schizophrenics 1,118, controls 1,100) at present showed negative findings between COMT and Japanese patients with schizophrenia using 19 SNPs, including the aforementioned regions [Okochi et al., 2009]. Although the present study found that rs165774 showed a positive association with schizophrenia in its genotypic, allelic, and two window-haplotype analysis throughout the first and final second-stage replication study, the aforementioned previous study did not show the association with the same SNP [Okochi et al., 2009]. We could not conclude the results from two-window haplotype analysis, including that of rs4646316, during the second-stage replication study because the power analysis for this SNP is low (6%), which is derived from its small allelic odds ratio (1.029). In addition, although rs165774 showed a positive association, it was the neighboring CNV8 and CNV9 that showed positive associations with schizophrenia; thus, this SNP might affect the statuses of those CNVs (Figs. 1 and 2B). These were some of the limitations of the present findings with regard to the SNP study. We would like to refer this finding to the CNV findings mentioned below because the situation of this SNP was included in the positive finding regions of CNVs that are mentioned below.
As expected, in the CNV study, 22q11.2DS were rare and were found in 1 of 502 patients with schizophrenia (0.2%) and 1 of 691 controls (0.1%). Although further investigation for large deletion is required for confirmation, this result suggests that 22q11.2DS did not appear to be related to the common disease–rare variant hypothesis, although with larger number of subjects (1,854 vs. 2,157) in the second study. Thus, a further replication study was not performed during the second-stage study.
The most interesting result of the present findings was that low-frequency CNVs, namely CNV6 situated at promoter 1 and CNV8 at exon 6, showed significant associations with schizophrenia with regard to their frequencies of loss and gain CNs in the second-stage analysis (Table V). Furthermore, in these three CNV combinations of each subject, the effect of genetic risk factor appeared to be higher in the cases simultaneously showing two or three altered CNs through the three CNVs (e.g., loss-loss-loss [1-1-1], gain-gain-gain [3-3-3]; Standardized residuals in Table V) than in the cases with only one CNV (e.g., 2-2-1 and 2-2-3). Although large (>100 kb) and rare (<1%) CNVs were the focus in the genome-wide study, it was reported that the number of CNs per patient was higher in schizophrenia [International-Schizophrenia-Consortium, 2008]. In each gene, the number of CNs per patient appeared to be influential as the genetic risk factors for schizophrenia. In addition, known 22q11.2 DS were relatively large (1.5–3 Mb; Fig. 2A); however, the lengths of the CNVs in the present study were particularly large. For example, seven cases during the second-stage study showed 2-3-2 CNV combinations (footnote c of Table V), and the location of the amplicon center was 19,938,973 in CNV6 and 19,951,709 in CNV9 (Supplementary Table SI; Chr.22 location, NCBI build 37.3). This indicates that the length of sandwiched one copy of CNV8 was approximately 12 kb at the maximum. Nevertheless, by considering the 1-1-1 (or 3-3-3) CNV combination (CNV6–CNV9), the maximum length of loss (or gain) CNV was approximately 27 kb (Supplementary Table SI). Although these were small CNVs and their combinations, the location of these CNVs, promoter, and exons could be more influential for transcriptional levels and structure of amino acids than SNPs. There were some limitations to the present study. We could not determine whether CNVs showing significant association with schizophrenia were de novo or were inherited CNVs because samples from parents were not available. The actual length and structure of present CNVs and how the altered function of transcript caused by the present CNVs could be involved in the pathophysiology of schizophrenia were problems to be solved. Finally, we have to refer to the discrepancy shown specifically in some genome-wide CNV analyses focusing on rare CNVs showing negative association with COMT in schizophrenia. The differences were that although the present selected CNVs were not rare (e.g., for CNV8, loss or gain CNs were 2.3% in cases; Table V), array probes for genome-wide CNV analysis were designed only for rare CNV; thus, present sequences from 10 CNV amplicons did not have any coinciding probe sequences with dense arrays (e.g., SurePrint G3 Human CGH microarray 1 × 1 M eArray (https://earray.chem.agilent.com/earray/).
In conclusion, not only large and rare CNVs but also additionally low-frequency CNVs and relatively small CNVs (e.g., 30 kb) and their combination in COMT could be involved as a genetic risk factor for schizophrenia.
ACKNOWLEDGMENTS
Funding for this study was provided by the Juntendo Institute of Mental Health from 2011 to 2014 (201101, 201201, 201301, and 201401), and research grants from the “Integrated research on neuropsychiatric disorders” performed under the Strategic Research Program for Brain Sciences from Japan Agency for Medical Research and development, AMED and the Brain Mapping by Integrated Neurotechnologies for Disease Studies from Japan Agency for Medical Research and development, AMED.