Comparative mRNA analysis of behavioral and genetic mouse models of aggression
Abstract
Mouse models of aggression have traditionally compared strains, most notably BALB/cJ and C57BL/6. However, these strains were not designed to study aggression despite differences in aggression-related traits and distinct reactivity to stress. This study evaluated expression of genes differentially regulated in a stress (behavioral) mouse model of aggression with those from a recent genetic mouse model aggression. The study used a discovery-replication design using two independent mRNA studies from mouse brain tissue. The discovery study identified strain (BALB/cJ and C57BL/6J) × stress (chronic mild stress or control) interactions. Probe sets differentially regulated in the discovery set were intersected with those uncovered in the replication study, which evaluated differences between high and low aggressive animals from three strains specifically bred to study aggression. Network analysis was conducted on overlapping genes uncovered across both studies. A significant overlap was found with the genetic mouse study sharing 1,916 probe sets with the stress model. Fifty-one probe sets were found to be strongly dysregulated across both studies mapping to 50 known genes. Network analysis revealed two plausible pathways including one centered on the UBC gene hub which encodes ubiquitin, a protein well-known for protein degradation, and another on P38 MAPK. Findings from this study support the stress model of aggression, which showed remarkable molecular overlap with a genetic model. The study uncovered a set of candidate genes including the Erg2 gene, which has previously been implicated in different psychopathologies. The gene networks uncovered points at a Redox pathway as potentially being implicated in aggressive related behaviors. © 2016 Wiley Periodicals, Inc.
INTRODUCTION
Aggression is a behavior towards another individual with the intention either to intimidate or cause harm, and is consequently associated with violence. This trait is a substantial cause of mortality and morbidity globally [Anderson and Bushman, 2002; Veroude et al., 2015].
Figures pertaining to aggression-related mortality continue to rise, with a major predicted increase over the next 20 years [Bartolomeos et al., 2007]. Aggression also constitutes the sixth highest cause of disability in young people worldwide and contributes to approximately 5% of all disability [Vassos et al., 2014]. A study of the burden of crime on USA services found that ∼3% of expenses were secondary to violent crime driven by aggression. This highlights the social and economic costs incurred by aggressive behaviors. Hence, there is a need to understand the causes and develop potential management strategies of aggression, which currently lack the desired efficacy [McGuire, 2008].
Behavior-genetic studies have systematically reported moderate heritability estimates of aggression, with different measures of aggression resulting in estimates varying from 22% to 51% [Miles and Carey, 1997; Seroczynski et al., 1999; Vassos et al., 2014]. Consequently, current research aims to identify genetic variants associated with aggressive behaviors, which may help to inform candidate gene selection and the identification of gene pathways to gain further understanding of the genetic substrate of this complex trait [Kudryavtseva et al., 2015; Waltes et al., 2015; Zhang-James and Faraone, 2015]. Gene selection has predominantly been driven by inspecting genes and neurobiological pathways with an established association with aggression, including the serotonin (5-HT) gene, levels of which were found to be low in more aggressive individuals. Different polymorphisms of the gene encoding the 5-HTT-serotonin transporter have been associated with aggression in specific populations including children and cocaine–dependent individuals [Nelson and Trainor, 2007; Siever, 2008]. Other neurotransmitters also have been implicated, including excitatory neurotransmitters: catecholamines, vasopressin, cholecystokinin, and substance P, together with inhibitory neurotransmitters, including enkephalins, GABA, and serotonin acting via 5-HT1 receptors [Siegel et al., 2007]. Studies have investigated candidate genes from further pathways including those responsible for the regulation and catabolism of catecholamines such as monoamine oxidase A (MAOA) and catechol-O-methyl-transferase (COMT) [Brunner et al., 1993; Caspi et al., 2002; Moffitt, 2005]. Increased aggression and neurotransmitter levels have been observed in mice with either the MAOA or COMT genes deleted [Volavka et al., 2004].
Deciphering the neurobiology of aggression has been supported not only by an increased understanding of neurophysiology and the complex interplay of neurotransmitters and substrates, but also by well-established relationships with other psychological phenomena such as stress, implying the involvement of variations in the hypothalamic-pituitary-adrenal (HPA) axis, serotoninergic, and vasopressin systems. Indeed, it has been shown that aggressive behavior can be associated with stressors including early life trauma and childhood maltreatment [Widom, 1989; Lewis, 1992; Loeber and Stouthamer-Loeber, 1998; Hildyard and Wolfe, 2002; Ethier et al., 2004; Fonagy, 2004; Veenema et al., 2006]. Furthermore, these behavioral processes are also associated with numerous psychopathologies including major depressive disorder, schizophrenia, personality disorders, anxiety disorders, and post-traumatic stress disorder [Agid et al., 1999; Veenema, 2009]. Nevertheless, the aetiology of these pathologies is poorly understood.
Animal models offer intrinsic advantages for exploring the genetics of the molecular mechanisms underpinning complex disorders such as aggression and violent behavior. Importantly, environmental variables can be more precisely controlled in ways that is not possible in human studies. Furthermore, the creation of replicable inbred strains reduces the potentially confounding effects of genetic variation. Finally, genetic models can be created by means of artificial selection to display specific behaviors at easily observable extremes, which is not reproducible in human studies.
Several studies on mouse aggression have used the BALB/c and C57BL/6 strains to identify candidate genes involved in its aetiology [Potter, 1985]. BALB/c mice are very susceptible to stress, resulting in anhedonia and increased aggression [Mineur et al., 2003; Isingrini et al., 2010; Belzung and Griebel, 2001]. This contrasts with the C57BL/6C57BL/6 strain, which is resistant to stress [Crawley et al., 1997; Isingrini et al., 2010]. Both strains were among the earliest inbred strains developed, in a time when many doubted this could be even be done, but were not selectively bred to for their aggressive characteristics. Nevertheless, given the established relationship between stress and aggression, there is likely to be significant overlap between many of the genes involved in modulating aggressive behavior and the current pool of candidate genes for sensitivity to stress uncovered using the BALB/c and C57BL/6C57BL/6 strains.
A recent study reported the first transcriptomic characterisation three mouse models developed specifically for the study of aggression [Malki et al., 2014]. These models consisted of three pairs of lines: Turku Aggressive (TA) and Turku Non-Aggressive (TNA) mice; North Carolina Aggressive (NC900) and Non-Aggressive (NC100) mice, and short attack (SAL) and long attack latency (LAL) mice, in which one of each pair was bred for high aggression and the other for low aggression.
This study aims to identify genetic overlap between the stress study and the selection lines study by assessing the differential gene expression dependent on stress exposure, and subsequently comparing results with those obtained from an independent genetic mouse model of aggression. Meta-analysis of the hypothesized genetic overlap between the BALB/c/C57BL/6C57BL/6 model and three aggression specific mouse models will provide greater insight into the aetiology of aggressive behavior and identify a set of gene and gene networks implicated in aggression.
METHODS
Study Design
Two independent animal studies were used in a discovery-replication design. The discovery study was based on a behavioral model of aggression using 16 animals from two inbred mouse strains (BALB/cJ and C57BL/6J), half of which were exposed to a chronic mild stress protocol. The discovery took advantage of the stress sensitivity characteristic of the BALB/cJ strain and the resilience of the C57BL/6J strain to model individual variation in response to stress and associated aggressive behaviors in humans. The replication study was based on a genetic model of aggression and consisted of 18 animals from three pairs of lines, artificially selected for high or low aggressive behaviors (Turku Aggressive [TA] and Turku Non-Aggressive [TNA] mice, North Carolina Aggressive [NC900] and North Carolina Non-Aggressive [NC100] mice, and the short attack latency [SAL] and long attack latency [LAL] mice). Within each mouse line, half the animals were in high aggression and half in the low aggression group. Probe sets differentially expressed in response to stress and strain interaction uncovered in the stress mouse model were intersected with mRNA differences uncovered by comparing high and low aggressive strain subtypes in an independent genetic mouse model of aggression.
Animals
Eighteen animals from three different pairs of lines bred specifically to exhibit high and low aggressive behaviors were used for the genetic mouse model of aggression. We used the Turku Aggressive (TA) and Turku Non-Aggressive (TNA) mice, North Carolina Aggressive (NC900) and North Carolina Non-Aggressive (NC100) mice, and the short attack latency (SAL) and long attack latency (LAL) mice. All animals were bred in the laboratory of Sietse de Boer in the Netherlands and separated by sex until weaning in Perspex cages. At 3 and 4 weeks of age, after the mice had been weaned, male mice were paired with a 6–8 week old female of the same line and each pair was housed in a Makrolon Type II cage with similar internal environments.
The TA/TNA strains were bred from Swiss albino mice in Turku, Finland. Aggressive traits were assessed in males at the age of 60 days using a seven-point scale, in a standard 7 min dyadic test against non-aggressive mice, for which they were placed in a neutral container. The male mice with high aggression scores were bred together with the sisters of other aggressive mice in order to avoid inbreeding.
The NC900/NC100 strains were bred from an outbred population of NCR mice in North Carolina. Aggressive mice were selected based on increased aggression and reactivity to stimulation, the former having been assessed via a standard 10 min dyadic test with attack frequency recorded.
The SAL/LAL strains were bred from a feral Mus musculus domesticus population captured in Groningen, The Netherlands. Mice were selected for breeding at the age of 14 weeks with the use of the resident-intruder test, in which the experimental mice were the residents and had their attack latencies (time taken for the first attack by the resident) recorded against the naïve albino intruders. The attack latencies determined the SAL and LAL mice.
The BALB/cJ and C57BL/6J strains were purchased from the Jackson Laboratory in Bar Harbor, ME, and bred at UMass Medical School, Worchester, for a minimum of two generations. Weaning occurred at 28 ± 1 days and mice were housed in same sex groups in groups of two to four until the age of 2 months ± 1 week, at which point they were assigned to the control or unpredictable chronic mild stress (UCMS) group. The UCMS treatment began at the same time, continuing during the testing phase, although precautions were taken to ensure that stressors were not applied just before a test. Throughout these phases, all mice were kept in similar rooms [Malki et al., 2015].
Unpredictable Chronic Mild Stress Protocol
The UCMS model leads to depressive traits in mice due to repeated administration of unavoidable stressors at unpredictable moments. This model emulates well-documented research showing a strong association between stress and major depressive disorders. Stressors used here included: predator sounds, cage tilting, placement in an empty cage with and without water on the bottom, damp sawdust, reversal of the light/dark cycle, and switching on the lights transiently during the dark phase. Food or water deprivation was not included in the procedure. Two stressors on average were randomly applied on a daily basis for 2 weeks. This schedule was carried out for 4 weeks before animals were sacrificed. Animals used for this study did not undergo behavioral testing.
mRNA Extraction Protocol
Male mice from each of the two lines in the three artificially selected mouse models were sacrificed by cervical dislocation at the age of 13 and 14 weeks old. The prefrontal cortices (PFC) of all 18 mice were extracted via dissection and snap frozen. RNA was extracted using the Trizol RNA isolation method and RNA quality was measured via gel electrophoresis. After the Affymetrix One-Cycle Target labelling protocol, 3 µg of RNA was processed and hybridized to Affymetrix MOE430v2 Gene Expression Arrays.
The stress-induced model of aggression using the BALB/cJ and C57BL/6J strains involved the extraction of RNA from 16 frozen dissected hippocampi using the guanidium thiocyanate method with TRIzol (Invitrogen Life Technologies, UK). With the use of reverse transcriptase and poly-T primer, the mRNA was subsequently converted to cDNA, which was amplified using T7 RNA polymerase with biotin-UTP and biotin-CTP. The cDNA was fragmented in a fragmentation buffer and then hybridized on the GeneChip Mouse Genome 430A 2.0 to allow scanning and data analysis.
Statistical Analysis
Mean probe set intensities from C57BL/6J and BALB/cJ animals obtained from Affymetrix MOE 430 arrays were normalized and summarized using Robust Multichip Average (RMA) method [Irizarry et al., 2003]. Probe sets that were systematically absent (based on the MAS 5.0 detection present/absent call) across all the arrays were removed leaving 22,690 probe sets. Raw intensity measures from the genetic mouse study were also processed using RMA based upon an additive background-multiplicative model, returning log2-transformed intensities. RMA was performed using the Affymetrix package for R (http://cran.r-project.org/). The Affymetrix software package, Microarray Suite (MAS) 5.0, was used to identify missing values in the data using the detection function.
Principal component analysis (PCA) analysis was first performed on the BALB/c/C57BL/6 data using the “prcomp” function in R in order to identify main sources of variation. Prior to analysis, the data was scaled using Pareto transformation using the “prep” function available in the Bioconductor package “pcaMethods” for R.
General linear models were conducted in R, using strain and stress as fixed effects and normalized probe intensity as dependent variable. The model was used to uncover probe sets differentially expressed in response to stress × strain interaction. False Discovery Rate (FDR) adjusted P-values at a threshold of 0.05 were used to identify probe sets differentially expressed in response to stress x strain controlling for the number of multiple non-independent tests.
The RankProd package for R from Bioconductor was used to uncover genes differentially expressed between high and low aggressive lines in the three selected mouse models. RankProd is a non-parametric method derived from biological reasoning that identifies up and down-regulated genes across replicates dependent on different experimental conditions (origins). RankProd offers several advantages compared to more traditional parametric models [Malki et al., 2013]. In this study, RankProd was used to rank probe sets by fold change in expression between aggressive and non-aggressive sub-strains. The RankProd algorithm allowed the evaluation of mRNA expression differences between high and low groups within each strain independently and then to combine the results in a meta-analysis approach. This allowed circumventing the issue of the predominant strain effects given the use of mice of three different genetic backgrounds. A percentage of false positive predictions (pfp) of 0.05, determined using 100,000 permutations, was used to control for the number of multiple, non-independent tests. Lastly, only expression differences that showed consistency in the directionality of expression change across all strains were reported. Probe sets that varied in directionality across the three different strains point at interaction effects which were not looked at in the genetic mouse model; given that the genetic mouse model was used as a replication study, we were only interested in uncovering differences related to main effects of aggression.
Probe sets uncovered in the BALB/c/C57BL/6 model were intersected with those uncovered in the selected mouse models of aggression using nominal P-values. We then selected the most highly differentially regulated probes across both mouse models using FDR corrected values. These were then carried forward for networks analysis using the Ingenuity Pathway Analysis software (IPA): IPA provides integrated data from different experimental platforms to aid in the identification of key biological and molecular networks in “omics” data. IPA constructs pathways using previously identified associations between genes and proteins stored in the Ingenuity Pathways Knowledge Base (IPKB), independent of established canonical pathways. The parameters were determined using the probe set list uploaded to the IPA platform. IPA then returns generated networks ranked by order of mean significance according to P-value. Significance is determined using “right-tailed Fisher Exact Test” considering the number of focus genes (matched to probe-sets uploaded) that are involved in the process, and the total number of genes known to be associated with that process in the IPKB.
RESULTS
PCA was first used to explore strain and stress components in the stress model of aggression. A clear separation of both stress and strain was visible, suggesting that stress effects caused by the CMS paradigm are sufficient to cause a pervasive effects across the transcriptome. This is likely driven by the stress sensitivity of the BALB/c strain and replicates previously published findings [Tannenbaum et al., 2003; Priebe et al., 2005].
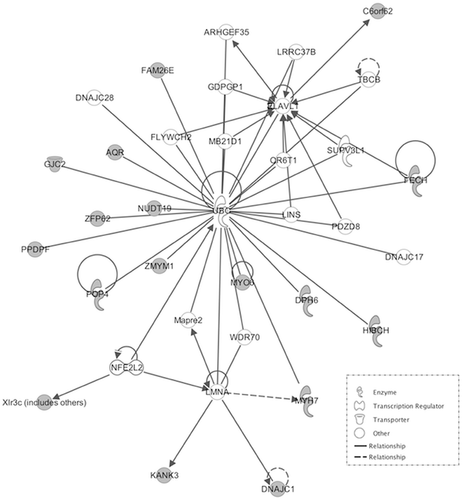
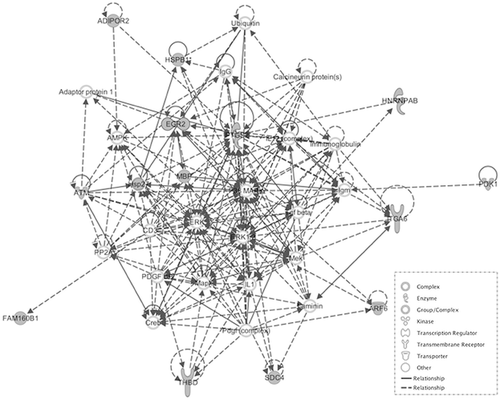
We then explored interaction effects in the behavioral model of aggression using strain (BALB/c and C57BL/6) as one factor and stress (CMS and Control) as the other. The results from the linear model uncovered 2,375 probe-sets out of 22,690 as significantly dysregulated in response to stress-strain interaction at a nominal P-value of 0.05. We then evaluated differences in the genetic model of aggression. Probe sets in the genetic mouse model of aggression were analyzed using the RankProd algorithm, which evaluated differences between high and low aggressive sub strains for each of the three strains and combined the results using a meta-analysis approach. Probe sets showing consistency in the directionality of fold change across the three stains and reaching a nominal P-value of 0.05 were uncovered and intersected with those found in the behavioral model of aggression. Out of the 2,375 probe sets found in the stress model of aggression, 1,916 were also found in the genetic model. The results show an important overlap across the two models of aggression in mouse (81%). We then selected those probe sets showing the greatest expression difference across the two studies. In the stress model of aggression we adjusted for multiple testing using FDR <0.05 while in the genetic model of aggression we used PfP < 0.05 and consistency of directionality of fold change. Fifty-one highly implicated probe sets were identified across the two models, 30 of which were down-regulated (Table I) and 21 of which were up-regulated (Table II). Probe sets were uploaded to IPA for networks analysis. Fifty of these probe sets were found on Ingenuity's reference list and mapped to known genes. IPA returned two significant pathways. The first pathway, with a score of 40, is centred on the Ubiquitin C (UBC) gene hub (Fig. 1). The second pathway, with a score of 28, is centred on the P38 mitogen–activated protein kinase (MAPK) gene hub, which is a key redox-signaling pathway (Fig. 2). Both pathways are biologically plausible and consistent with literature on the neurobiology of aggression.
| Down-regulated genes | |||||
|---|---|---|---|---|---|
| Probe set ID | Gene symbol | Gene title | Fold change | P-value | FDR |
| 1417262_at | Ptgs2 | prostaglandin-endoperoxide synthase 2 | 5.48 | 1.000E-06 | 1.000E-06 |
| 1423748_at | Pdk1 | pyruvate dehydrogenase kinase, isoenzyme 1 | 3.78 | 1.000E-06 | 1.000E-06 |
| 1424607_a_at | Gm4354 | predicted gene 4354 | 2.41 | 1.000E-06 | 1.000E-06 |
| 1425408_a_at | 2610034M16Rik | RIKEN cDNA 2610034M16 gene | 3.31 | 1.000E-06 | 1.000E-06 |
| 1427683_at | Egr2 | early growth response 2 | 4.88 | 1.000E-06 | 1.000E-06 |
| 1428052_a_at | Zmym1 | zinc finger, MYM domain containing 1 | 3.88 | 1.000E-06 | 1.000E-06 |
| 1449875_s_at | H2-T10 /// H2-T22 /// H2-T9 | histocompatibility 2, T region locus 10 /// .. locus 22 /// .., locus 9 | 3.86 | 1.000E-06 | 1.000E-06 |
| 1450779_at | Fabp7 | fatty acid binding protein 7, brain | 2.88 | 1.000E-06 | 1.000E-06 |
| 1448793_a_at | Sdc4 | syndecan 4 | 3.15 | 1.043E-06 | 2.222E-04 |
| 1421205_at | Atm | ataxia telangiectasia mutated homolog (human) | 2.71 | 1.252E-05 | 2.400E-03 |
| 1420500_at | Dnajc1 | DnaJ (Hsp40) homolog, subfamily C, member 1 | 2.43 | 1.000E-06 | 3.091E-03 |
| 1426896_at | Zfp191 | zinc finger protein 191 | 2.49 | 4.799E-05 | 7.667E-03 |
| 1448839_at | Kank3 | KN motif and ankyrin repeat domains 3 | 2.56 | 6.364E-05 | 8.714E-03 |
| 1448554_s_at | Myh6 /// Myh7 | myosin, heavy polypeptide 6, cardiac muscle, alpha /// .., beta | 2.32 | 6.051E-05 | 8.923E-03 |
| 1435755_at | 1110001A16Rik | RIKEN cDNA 1110001A16 gene | 2.33 | 8.555E-05 | 1.093E-02 |
| 1425263_a_at | Mbp | myelin basic protein | 2.35 | 1.085E-04 | 1.300E-02 |
| 1452378_at | Malat1 | metastasis associated lung adenocarcinoma transcript 1 | 2.03 | 2.890E-04 | 2.916E-02 |
| 1452234_s_at | Tmem191c | transmembrane protein 191C | 2.43 | 2.619E-04 | 2.953E-02 |
| 1425964_x_at | Hspb1 | heat shock protein 1 | 1.64 | 3.109E-04 | 2.980E-02 |
| 1426114_at | Hnrnpab | heterogeneous nuclear ribonucleoprotein A/B | 1.98 | 2.848E-04 | 3.033E-02 |
| 1422444_at | Itga6 | integrin alpha 6 | 2.21 | 3.965E-04 | 3.455E-02 |
| 1422943_a_at | Hspb1 | heat shock protein 1 | 1.55 | 4.413E-04 | 3.525E-02 |
| 1423154_at | BC005537 | cDNA sequence BC005537 | 1.88 | 3.933E-04 | 3.590E-02 |
| 1420982_at | Rbm39 | RNA binding motif protein 39 | 1.93 | 4.340E-04 | 3.617E-02 |
| 1428381_a_at | Ppdpf | pancreatic progenitor cell differentiation and proliferation factor | 1.89 | 5.175E-04 | 3.968E-02 |
| 1426607_at | Gm7120 /// LOC101055764 | predicted gene 7120 /// transmembrane protein C5orf28 homolog | 1.93 | 5.561E-04 | 4.100E-02 |
| 1416632_at | Me1 | malic enzyme 1, NADP(+)-dependent, cytosolic | 2.31 | 5.822E-04 | 4.133E-02 |
| 1435559_at | Myo6 | myosin VI | 2.11 | 6.521E-04 | 4.464E-02 |
| 1424270_at | Dclk1 | doublecortin-like kinase 1 | 2.04 | 7.470E-04 | 4.773E-02 |
| 1448529_at | Thbd | thrombomodulin | 1.35 | 7.334E-04 | 4.848E-02 |
- The table shows Affymetrix's probe set name, gene symbol, gene title, fold change, P-value, and fdr value.
| Up-regulated genes | |||||
|---|---|---|---|---|---|
| Probe set ID | Gene symbol | Gene title | Fold change | P-value | FDR |
| 1419034_at | Csnk2a1 | casein kinase 2, alpha 1 polypeptide | 6.97 | 1.000E-06 | 1.000E-06 |
| 1419035_s_at | Csnk2a1 /// LOC100047957 | casein kinase 2, alpha 1 polypeptide /// casein kinase II subunit alpha-like | 7.29 | 1.000E-06 | 1.000E-06 |
| 1419344_at | Tcte1 | t-complex-associated testis expressed 1 | 3.94 | 1.000E-06 | 1.000E-06 |
| 1426548_a_at | Atpbd4 | ATP binding domain 4 | 5.03 | 1.000E-06 | 1.000E-06 |
| 1433497_at | Aqr | aquarius | 2.93 | 1.000E-06 | 1.000E-06 |
| 1434216_a_at | Nudt19 | nudix (nucleoside diphosphate linked moiety X)-type motif 19 | 6.92 | 1.000E-06 | 1.000E-06 |
| 1449181_at | Fech | ferrochelatase | 3.81 | 1.000E-06 | 1.000E-06 |
| 1428224_at | Hnrpdl | heterogeneous nuclear ribonucleoprotein D-like | 2.65 | 4.173E-06 | 1.000E-03 |
| 1425495_at | Zfp62 | zinc finger protein 62 | 2.34 | 8.346E-06 | 1.778E-03 |
| 1420357_s_at | Xlr3a /// Xlr3b /// Xlr3c | X-linked lymphocyte-regulated 3A /// X-linked lymphocyte-regulated 3B /// X-linked lymphocyte-regulated 3C processing of precursor 4, ribonuclease P/MRP family, (S. cerevisiae) | 2.47 | 1.043E-05 | 2.000E-03 |
| 1448419_at | Pop4 | 2.42 | 1.252E-05 | 2.182E-03 | |
| 1422564_at | Actl6b | actin-like 6B | 2.14 | 7.512E-05 | 1.200E-02 |
| 1423875_at | Fam160b1 | family with sequence similarity 160, member B1 | 1.73 | 9.181E-05 | 1.257E-02 |
| 1436684_a_at | Riok2 | RIO kinase 2 (yeast) | 2.26 | 8.659E-05 | 1.277E-02 |
| 1424680_at | Fam26e | family with sequence similarity 26, member E | 2.09 | 1.075E-04 | 1.373E-02 |
| 1418822_a_at | Arf6 | ADP-ribosylation factor 6 | 1.97 | 1.231E-04 | 1.475E-02 |
| 1426159_x_at | Tcrb-J | T cell receptor beta, joining region | 2.14 | 1.315E-04 | 1.482E-02 |
| 1450483_at | Gjc2 | gap junction protein, gamma 2 | 1.99 | 2.984E-04 | 2.860E-02 |
| 1423413_at | Ndrg1 | N-myc downstream regulated gene 1 | 1.44 | 2.963E-04 | 2.989E-02 |
| 1434329_s_at | Adipor2 | adiponectin receptor 2 | 1.54 | 2.911E-04 | 3.100E-02 |
| 1451511_at | Hibch | 3-hydroxyisobutyryl-Coenzyme A hydrolase | 2.03 | 4.705E-04 | 4.295E-02 |
- The table shows Affymetrix's probe set name, gene symbol, gene title, fold change, P-value, and fdr value.
DISCUSSION
In this study, we presented a comparative analysis of mouse transcriptome from disease relevant brain regions, between a behavioral model of mouse aggression using a chronic stressor and a genetic mouse model of aggression. Over 81% of the probe sets uncovered by exploring stress x strain interaction were also differentially expresses across high and low aggressive sub strains. The results validate the use of the BALB/cJ and C57BL/6 as a good model to explore the neurobiology of aggression and reinforce the known association between stress and aggression. The most strongly differentially expressed probe sets across both the behavioral and genetic model of aggression, point at genes and gene networks with higher probability of association with aggressive behaviors and may provide additional insight into neuogenetic factors that may be modulating violent behavior in humans. Networks analyses of these genes revealed two networks with a plausible association with aggression; the first network is centred on the UBC gene hub while the second is a redox-associated network.
Ubiquitination Pathway
The association of ubiquitination and the UBC gene with aggression is poorly documented. However, there are features of ubiquitination which may apply to aggressive behavior and may help further understanding of its neurobiology.
UBC is one of the four genes which encodes ubiquitin, the other three being UBB, UBA52, and RPS27A [Board et al., 1992]. More specifically, UBA52 and RPS27a encodes the single copy of ubiquitin while UBB and UBC encode for polyubiquitin. Ubiquitin is responsible for post-translational cellular protein ubiquitation, which is a reversible modification involving the regulation of several processes, including: protein degradation, protein trafficking, DNA repair, apoptosis, cell-cycle regulation, and signal transduction. Out of these functions, ubiquitin is best known for tagging proteins for proteolysis via the 26S proteasome.
Existing literature suggests that UBC is extremely susceptible to oxidative stress, which may provide some insight into the neurobiology of aggression as the significance of oxidative stress in aggression has also been documented [Kim et al., 2015; Coccaro et al., 2016]. Research has found that reduced antioxidant function and increased levels of oxidative stress markers are associated with aggressive behavior. Given that UBC is central to one of the neurobiological pathways derived from the ingenuity pathway analysis and has multiple functions, it may be a possibility that oxidative stress to UBC impairs normal cellular processes involving other related substrates, thus contributing to aggressive traits. This may be plausible due to the aforementioned documentation of increased markers of oxidative stress, which could possibly be due to impaired functioning of proteolysis via the 26S proteasome. However, it is uncertain whether oxidative stress contributes to aggression or aggression contributes to oxidative stress and further research is needed to explore this.
P38 MAPK Redox Signaling Pathway
The MAPK signaling pathway can be activated by a series of stimuli, including cytokines and stress, which have an inflammatory component, and are involved in cellular processes such as cell differentiation and apoptosis [Wada and Penninger, 2004]. Interestingly, elevated levels of inflammatory markers in the plasma of individuals exhibiting aggressive behavior has been documented. These markers include C-reactive protein (CRP) and interleukin 6 [Coccaro et al., 2015]. Therefore, there may be an association between this pathway and aggression, a hypothesis, which is further backed up by the implication of interleukin-1 in this pathway derived from ingenuity. This is supported by previous research which has also shown that interleukin-1b increases the response to environmental stressors [Anisman and Merali, 2003]. Raised inflammatory cytokines have also been associated with major depressive disorder, which may provide another link with aggression as genetic, and behavior links have been previously made between the two, as well as with several other psychopathologies [Capuron and Miller, 2004].
The pathway itself involves a three-tiered phosphorylation cascade of MAP kinases, in which activated MAP3Ks (MAPK kinase kinases) phosphorylate and stimulate MAP2Ks (MAPK kinases) to phosphorylate MAPKs. The stimulated MAPKs subsequently regulate various cellular processes including those mentioned above, via methods such as the activation of gene transcription. The MAPKs include extracellular signal-regulated kinases 1 and 2 (ERK1 and ERK2), ERK5, p38 kinase, and c-Jun N-terminal kinase (JNK), which each play specific roles in this pathway [Seger and Krebs, 1995]. Interestingly, the ingenuity analysis has also implicated ERK1 and ERK2 in the aetiology of aggression, which further supports the involvement of the MAPK pathway.
Redox signaling may also be involved with other signaling pathways such as nuclear factor kappa-light-chain-enhancer of activated B cells (NF-kB), which has previously been implicated in aggression. Both pathways are postulated to be connected via p150, an nf-kb1 gene product which has a role in regulating MAPK signalling. The NF-kB pathway is activated by phosphorylation of inhibitory kBα (IkBα) proteins which normally keep it quiescent, allowing the release of the NF-kB dimer and translocation to nuclei where it is able to regulate gene expression. This pathway is essential in regulating cellular processes such as apoptosis, differentiation, immunity, and stress responses. Activating stimuli of the NF-kB pathway include interleukin 1 and tumor necrosis factor (TNF), both of which are associated with aggression [Patel et al., 2010]. Previous studies on rat have reported an association between MAPK and increased aggression scores in rat [DeMar et al., 2006; Frey et al., 2006]. Given that NF-kB regulates a large variety of cells, the interaction of this pathway with MAPK is likely to be very complex and requires further research to fully understand it in the context of aggression.
A Link Between Ubiquitin and the P38 MAPK Redox Signaling Pathway
An interesting finding is that ubiquitin and redox signaling appear to be connected, adding more layers of complexity to the neurobiology of aggression as it is likely that the two ingenuity pathways are not only connected, but influenced by other factors such as the aforementioned NF-kB pathway.
Ubiquitin and redox signaling appear to be linked through the phosphorylation of the ubiquitination pathway; therefore, the MAPK phosphorylation cascade may influence ubiquitination, hence protein degradation. This has implications regarding the level of cellular proteins, which would presumably have a role in influencing aggressive behavior. The effect that these varying levels would have on aggressive traits is likely to depend on the specific proteins affected; hence, further unravelling and analysis of our results is the essential first step in understanding these interactions.
Although this study presents a number of strengths, including the discovery replication design using two independent mouse studies and accessibility to data from the first genetic mouse model of aggression, there are also a number of limitations. First, due to the complex connectivity of different brain regions, the analysis of the same brain regions from these model organisms could have provided further insight into the mechanisms underlying variation in aggressive behavior. Several genes may have been missed as identification of highly expressed genes across studies is further restricted by the correlation structure of gene-expression across two different brain regions: hippocampus and prefrontal cortex. However, choices on brain tissues were restricted to what was available. Only data from prefrontal cortex was available from the genetic mouse model of aggression. Second, mice are not miniature human beings and therefore there are number of behavioral characteristics in human that cannot be modelled in mouse. While the results can serve to inform the molecular mechanisms that may be relevant in aggression in human, these will have to be replicated in human studies. Moreover, the chronic mild stress protocol used is generally considered a mild stress; it is possible that a more severe stress protocol may cause stronger and more pervasive dysrgulation at the level of the transcriptome. However, stress effects were visible simply through PCA, which highlights the stress sensitivity of the BALB/cJ and suitability of the CMS protocol for this study. Lastly, although the sample size was sufficiently powered to detect a number of important differences, microarray data tends to be high dimensional by nature. It is possible that a meta-analysis approach using a clustering method could be used to gain additional into the neurobiology of this complex trait.
CONCLUSIONS
As hypothesized, significant overlap between genes differentially regulated in response to a stressor were also differentially regulated in a genetic mouse model of aggression, both validating the stress model of aggression and identifying genes with a higher probability of being implicated in modulating aggressive behaviors. These results are a promising start to the unravelling of the complex neurobiology behind aggression and provide clues on the candidate genes that may be relevant in human.